Full Length Research Paper
ABSTRACT
Effect of smoking technologies on nutritional quality and concentration levels of polycyclic aromatic hydrocarbons (PAHs) in Mormyrus caschive and Oreochromis niloticus were determined to enable adoption and utilization of suitable facility that maintains good quality for sustainable supply of nutritious fish products in South Sudan. A total of 72 fresh M. caschive and O. niloticus were purchased, of which 24 were iced for reference and the other 48 were divided into two groups for pit and chorkor smoking. Experimental smoking of fish samples was conducted twice in a randomized design. Fish samples were analyzed for nutritional values using standard methods of the association of official analytical chemists and levels of PAHs using gas chromatography-mass spectrometry. Results revealed; the two smoking methods concentrated crude protein, fat and ash contents. However, fish smoked using chorkor had significantly higher nutritional values than fish smoked using pit kiln. About seven types of PAHs comprising of low and medium molecular weight were recorded from the two smoked fish species. Naphthalene and fluorene were the two dominant PAHs with fish smoked using pit kiln having significantly higher concentration levels of naphthalene (5.86±4.16 µg/kg) and fluorene (3.83±0.10 µg/kg) than fish smoked using chorkor oven. The study concluded that chorkor oven has better quality smoked fish than pit. Hence, its utilization and adoption for artisanal fisheries in South Sudan is recommended.
Key words: Nutritional values, food quality and safety, smoking techniques, polycyclic aromatic hydrocarbons (PAHs).
INTRODUCTION
Fish post-harvest handling provides livelihoods and income to many people in the world, especially countries gifted with aquatic resources (FAO, 2016). Fish processing and preservation are important because fish is perishable and prone to quality decline immediately after harvest and to deter economic, quality and nutrient losses (Msusa et al., 2017). Proper handling of fresh fish is vital to achieve the best quality and highest profits (Muhame et al., 2020). Regardless of these immense contributions, the fishing industry suffers from considerable post-harvest losses which are valued at 30% in dry season and 40% in rainy season (FAO, 2015). Fish post-harvest losses have intense adverse effect on rural fishing population whose income and wellbeing often depend on fisheries activities (Adeyeye and Oyewole, 2016). Lack of appropriate infrastructure for fish post-harvest handling is a major challenge facing rural fishing communities of South Sudan. As such better preservation facilities and techniques are required to reduce the effects of fish post-harvest losses (Famurewa et al., 2017).
Amongst the customary preservation techniques of fermenting, salting and sun drying, fish smoking is the leading type of fish preservation method used in Terekeka County. This is due to the fact that most consumers prefer smoked fish to fermented, sun dried and salted fish. Besides, lack of suitable processing and preservation infrastructures have made fisher-folks to extensively use smoking to preserve their catches which could not be delivered to far markets in fresh form. Hygienic and efficient fish smoking indeed, preserve the nutritional quality and safety of smoked fish due to antioxidants and preservatives; phenol and formaldehyde contained in the smoke (Magawata and Musa, 2015; Pemberton-Pigott et al., 2016). However, fish smoking may lead to deposition and accumulation of carcinogenic PAHs in the products as a result of fuel source and the method used in processing fish.
Concisely, PAHs are a class of organic compounds that are typically formed and released during incomplete pyrolysis of organic matter such as waste or food, during food preservation, industrial processes and other human activities (Abdel-Shafy and Mansour, 2016; Lee et al., 2019). They are a large group of organic compounds with two or more fused aromatic rings (Erawaty-Silalahi et al., 2021). Although PAHs have a relatively low solubility in water, they are extremely lipophilic. Besides, most of the PAHs with low vapor pressure in the air are readily adsorbed on some particles (Erawaty-Silalahi et al., 2021). There are more than 100 PAHs, of which 16 of them are categorized as PAHs of health concern (Yusuf et al., 2015; Tran-Lam et al., 2018), and are used to determine the presence and levels of carcinogens in food. However, the European Food Safety Authority (EFSA) recommended that the concentrations of benzo[a]pyrene (BaP), and the sum of concentrations of four PAHs: benzo[a]pyrene (BaP), benz[a]anthracene (BaA), benzo[b]?uoranthene (BbF), and chrysene (Chry) (PAH4), be considered as a reference for determination of PAHs in food (Puljic´ et al., 2019). According to the regulations on maximum limits for PAHs contamination in food (EFSA, 2008), which complied with the European Commission (EU) regulation no.835/2011 (EC, 2011) as amended, the maximum permissible limits for concentrations of BaP in meat and fish products is set at 2 µg/kg and the sum of PAH4 concentrations set not to exceed 12 µg/kg (Wet weight). As such, the use of PAH4 was adapted in 2014 to be the most suitable indicators of occurrence of PAHs in food (Zelinkova and Wenzl, 2015; Puljic´ et al., 2019). Due to different techniques of food preservation, a lot of studies have been done to determine the levels of PAHs in food using PAH4 as indictors of carcinogenic potency. This is done to ensure safety of preserved food and protection of consumers’ health. However, none has been done for preserved fish products of South Sudan.
It is noted that, due to variation in smoking parameters, PAHs levels in smoked fish may exceed the maximum acceptable limit of 2 µg/kg for benzo[a]pyrene, 12 µg/kg for the sum of PAH4 recommended by the European Commission (EC, 2011). During smoking, the concentration levels of PAHs may increase. Besides, the levels of PAHs contaminants are associated with detrimental human health effects (cancer causing effects). Additionally, poor heat control during processing may lead to increased temperature that degrade nutrients hence, reducing nutritional values of smoked fish products. The present study therefore, assessed the effect of smoking technologies on nutritional quality and concentration levels of PAHs in smoked Mormyrus caschive (Family: Mormyridae, Common name: Elephant snout) and Oreochromis niloticus (Family: Cichlidae, Common name: Nile tilapia) with the aim of adopting and utilizing suitable smoking facility that maintains the nutritional values and safety of smoked fish for sustainable supply of fish products in South Sudan.
MATERIALS AND METHODS
Study area
The study was done in June, 2018 in Terekeka County of Central Equatoria State, South Sudan. The area is located approximately, 52 miles north of the capital city, Juba on the western part of River Nile (Benansio, 2013). Its geographical position is within latitudes of 5?23'N and longitudes of 31?48'E (FAO and WFP, 2019). The county occupies an estimated area of 10,538.232 km2 with population projection of 246,483 (South Sudan Centre for Census, Statistics and Evaluation, 2018). Terekeka has tropical climate with relatively small periodic variations in humidity, temperature and wind the whole year (Climate-data.org., 2018). It receives rainfall from the months of March to May and August to November. Annually, an average rainfall of 907 mm is received in the area. Terekeka is having two dry periods; between the months of December to February and June to July with average annual temperature of 27.7°C. It is in the dry season where most fisher-folks are actively participating in fishing and other support activities. However, abundant periods of fish harvest occur in the months of June, July and August mostly after flood inundation recedes. It is within this period that majority of fish processors mainly women and children are actively involved in fish processing and preservation particularly, smoking of their catches.
Study design
Experimental smoking of the two species; M. caschive (Family: Mormyridae; common name: Elephant snout) and O. niloticus (Family: Cichlidae; common name: Nile Tilapia) using pit and chorkor facilities was done twice using a complete randomized design. The chorkor facility used for the experiment measured 2 m long, 1 m wide and 1m high. It has three chambers with trays attached in each compartment. This smoking oven is made of unburnt bricks, interior plastered with clay soil and perforated hard iron sheets as roof. The base has two inlets for aeration and smoke production. Its smoking chamber has flexible door that remains shut except during checking periods. A customary pit erected beside chorkor oven performance. The pit kiln measured; 1 m long, 0.5 m wide and 0.5 m high as used by fisher-folks in Terekeka. Logs are usually put at the edges for wire mesh to sit. In the process of smoking the fish, flat iron sheet was placed on top of the mesh to cover the samples (Figure 1).

Sampling, processing and analytical procedures
In total, seventy two (72) fresh M. caschive and O. niloticus were bought at Sur-num fish landing site located about one kilometer East of Terekeka Town. Fresh fish samples were preserved in ice-cold containers immediately after harvesting at the fishing grounds. Fresh samples procured for experimental smoking were processed following traditional procedures. A purposeful smoking of fish samples using chorkor and pit ovens was done at Terekeka fish landing station. Fish smoking took an average time of 8 h in chorkor and 20 h in pit kiln. From the total fresh fish, 12 samples of each species were stored at 4°C in ice-cold containers before being transported by bus to the laboratory of which, 6 specimens from each species were destined for proximate chemical composition and PAHs analyses, respectively. The other 48 samples were divided into two groups for pit and chorkor experimental smoking using Acacia seyal, the main tree species used by fisher-folks for smoking fish in the area. All fishes were carefully washed to remove slime, descaled, eviscerated and rewashed thoroughly with clean water to remove blood. Cleaned samples of fish were immersed in freshly prepared salt solution (a mixture of 100 g salt in 10 L of clean water) for 15 min followed by draining for 15 min. Fire was set in pit and chorkor ovens to produce smoke heat. The cured fish samples were randomly loaded on the trays and wire mesh in a chorkor and pit ovens, respectively. The preferred temperature of 60 - 80°C was maintained manually by the help of a thermometer until all the fish samples were smoke dried. During smoking, the position of fish samples in the trays were changed in chorkor to attain uniformity and turned upside down in pit kiln for uniformity of the smoking process. Thereafter, the smoke dried samples were cooled for 12 h at ambient temperature of 20°C. Smoke dried fish were enclosed in an aluminum foil, labeled, packed in carton boxes, and transported by bus to the laboratory in Makerere University, Uganda for analyses.
Sample preparation for analyses
From each sample, one hundred grams (100 g) of smoked fish muscle was removed from the edible parts, grinded to powder form using blender, and the weighed samples were labeled and stored in deep freezers at -18°C for proximate chemical analyses. One hundred grams of fresh fish muscles from each sample were taken as control and prepared following the same procedures as the treatment samples.
Chemical composition analyses
After homogenization of the weighed edible portion of each specimen, the following determinations were performed in triplicate by following the standard methods for the Association of Official Analytical Chemists (AOAC, 2005); moisture content determined by the weight reduction method, total crude protein determined by digestion, distillation and titration using micro-Kjeldahl method, crude fat determined by solvent extraction procedures in a Soxhlet system an ash content determined by incineration procedures in a muffle furnace using weight difference.
Polycyclic aromatic hydrocarbons analysis
Chemical reagents
All chemical reagents procured for the PAHs analysis were of high performance liquid chromatography grade; acetone, dichloromethane and hexane were obtained from Sigma Aldrich (Steinheim, Germany). The standard mixture of PAH was obtained from Augsburg, Germany.
PAHs extraction and purification in fish samples
The Wretling et al. (2010)method was used with minor modifications. Briefly, to the 10 g homogenized fish muscles per sample in a 250-mL round bottomed Erlenmeyer flask, 40 mL of dichloromethane (99.8% pure) extraction solvent was added and the flask was thoroughly sealed with aluminum foil for 30 min to prevent evaporation. To mixture in the flask, 5 g of anhydrous sodium sulphate was added. The content was shaken vigorously in a reciprocating shaker for 15 min. The content was further homogenized using auto vertex mixer for 5 min and allowed to settle for 10 min to ensure that the layers separate. The liquid layer was filtered to another 250-mL round bottomed Erlenmeyer flask by passing it through a separatory funnel packed with a glass wool to a height of 2 cm. The flask was washed with methanol/water (20 ml; 4:1 v/v) and content added to the funnel. The extract was then dehydrated by passing it through anhydrous sodium sulphate in a separatory funnel packed with florisil to a height of 5 cm and conditioned with 20 mL dichloromethane. The flask was rinsed with 10 mL dichloromethane and rinsing added to the separatory funnel. The clean and clear aqueous content was decanted into a 50-mL spherical flask and concentrated to 1 mL using a rotary evaporator at 35°C. The extracts were further purified as described by Mottier et al. (2000). A chromatographic column (1 cm internal diameter, id) was plugged with glass wool at the base. Activated silica gel was put in the column to a height of 5 cm. Additional 2 g sodium sulphate was added to the column. The packed column was then conditioned with 10 mL dichloromethane, thereafter 1 mL concentrate was loaded into the column and eluted with 20 mL dichloromethane. The eluate was transferred to a conical flask and concentrated to 1 mL in a vacuum rotary evaporator at 35°C. The eluate was further concentrated under nitrogen flow at 37°C to near dryness. To the concentrate, 1 mL of cyclohexane (containing 99.5% purity) was added and the content transferred into 2-mL amber glass vials with Teflon lined screw cap using pipettes for gas chromatography-mass spectrometry (GC-MS) analysis. To minimize PAHs volatilization, vials were kept in deep freezer at -20°C prior to analysis.
Chromatographic analysis of fish samples
Samples were analyzed with an Agilent 6890N gas chromatography instrument coupled with a 5975-mass spectrometry (Agilent Technologies, Santa Clara, CA, USA) following standard procedures (Lee et al., 2015). To separate the compounds, 1 µL sample solution was injected in the pulsed split-less mode onto a HP-5MS column with 30 m × 0.25 mm id. The column was programed as follows: injector temperature; 300°C, oven temperature at 80°C and held for 1 min, 245°C (6°C/min), 270°C (30°C/min) and held for 10 min, and 310°C held for 10 min. Other operating conditions were pulse pressure 45 psi, pulse time 0.9 min, purge flow 50 mL, purse time 1 min. Helium gas at constant flow rate of 1.1 mL/min was used as the carrier gas. The mass spectrometry was operated in electron impact ionization mode at 70eV with a solvent delay of 3.75 min. Identi?cation and quantification of individual PAHs was con?rmed by comparing the mass spectra and the peak retention time with those of the PAHs standards in the equivalent conditions. A retention time match of ±1% was considered for confirmation (Samuel et al., 2010).
Identification and quantitation of PAHs in fish samples
External standard was used for the identification and quantitation of PAHs. A stock solution of 20 µL/mL obtained from Sigma Aldrich (Steinheim, Germany) was prepared by diluting 0.2 mL of a 1000 µg/mL PAHs standard to 10 mL with cyclohexane. 1 µg/mL standard working solution was prepared by diluting 0.5 mL of a 20 µg/mL PAHs standard stock solution to 10 mL with cyclohexane. PAHs in the samples were identified and quantified by a combination of a retention time and mass spectral match against the calibration standards. Sample peak areas were compared to peak areas of the standard. A minimum of three concentration levels of the standards ranging from 0.1 to 5 ppm were injected into the GC-MS and calibration curve for each standard was obtained by plotting peak area against concentration of the standards. Each PAH in the sample was then quantified using the formula;
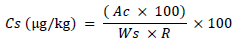
In which, Cs is the concentration of PAH in the sample in µg/kg, Ac is the concentration (ng/ml) relative to the highest part in the injection volume (µl), Ws is the mass (in grams) of the sample extracted and R is the recovery. For control purpose, solvent blanks were included in every run during the analysis. Arithmetic means and standard deviations were computed from quantifiable samples only. A computer program XLSTAT (version 7.5.2) was used for the calculation.
Percent recoveries (%R)
To determine the average percent recoveries, a set of three samples (30 g) of the homogenized fish muscles were spiked with 50 µl mixture of the 16 PAH standards of concentration ranging from 2 to 10 µg/L. Another set of three samples (30 g) were set as controls. Both the test and the control samples were allowed to stand for 24 h to allow absorption of the added PAHs into the matrix. The two sets of the samples were then taken through the same analytical procedures. The percent recoveries were calculated using the formula;
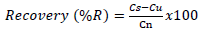
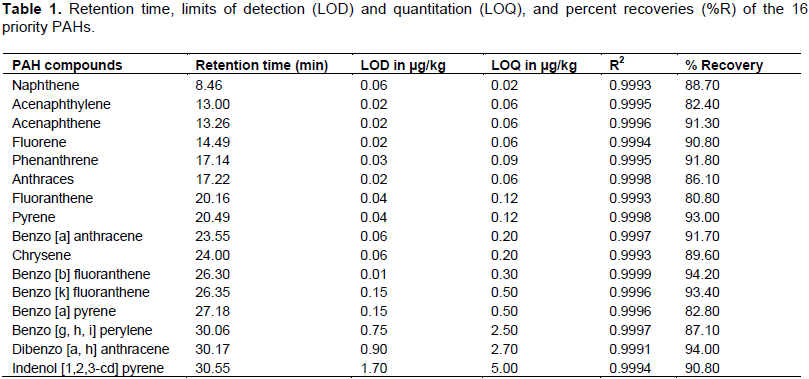
In which, Cs is the concentration of PAHs in the test sample, Cu is the concentration of PAHs in the control sample, Cn is the (nominal) theoretical concentration increase that results from spiking the samples. The mean percent recoveries obtained were within the range (70 to 130%) with an average of 89.28% (Table 1). These values were relatively quantitative and were therefore used without any correction. The limits of detection (LODs) and limits of quantification (LOQs) ranged from 0.01 to 1.7 µg/kg and 0.02 to 5 µg/kg, respectively (Table 1).
Statistical analysis
Data obtained from the study was analyzed using R (R Core Team, 2018). A two-way analysis of variance was used to test the difference in proximate nutritional values and concentration levels of PAHS in fish samples smoked using chorkor and pit ovens with fresh samples as reference. Tukey’s Honest Significant Difference Test was done where means of the two groups under comparison were significantly different. Level of significance was measured at P≤ 0.05.
RESULTS AND DISCUSSION
Proximate chemical composition of M. caschive and O. niloticus
As expected, the mean moisture content in fresh M. caschive (56.69±1.01%) was significantly higher than pit (15.3±0.57%), and chorkor (10.0±0.83%) smoked fish, P<0.05. In regards to the technologies, chorkor oven significantly reduced moisture content in M. caschive (10.0±0.83%) more than pit (15.3±0.57%), P<0.05. Similarly, the mean moisture content in fresh O. niloticus (64.34± 0.66%) was significantly higher than pit (17.23±0.42%) and chorkor (15.08±0.48%) smoked fish while chorkor oven reduced moisture to 15.08±0.48%, pit reduced moisture to 17.23±0.42%. This finding showed a significant reduction of moisture content in fish smoked using chorkor than pit. Indeed, the ultimate aim of fish smoking is to reduce moisture content that supports bacterial activities, oxidation or rancidity leading to spoilage (Adeyeye and Oyewole, 2016). Studies noted that high moisture provides conducive environment for spoilage microorganisms to thrive (Muhame et al., 2020). Heat application during smoking thus, breaks the hydrogen bond resulting to free molecules which eventually evaporate on the surface of fish (Akintola, 2015). Excessive loss of moisture leads to decreased water activity in fish tissues (Olukayode and Paulina, 2017). Effective method of fish preservation should thus reduce moisture to the recommended level of less than 20% depending on the purpose and the desired products (FAO, 2016).
Concerning performance of the two technologies; chorkor effectively reduced moisture to 10 and 15%, 15 and 17% for M. caschive and O. niloticus smoked using pit, respectively. In line with other studies (Adeyeye et al., 2015; Omodara et al., 2016; Katola and Kapute, 2017; Olukayode and Paulina, 2017), chorkor is an efficient technology for reducing moisture in fish muscle. Effective removal of moisture is attributed to heat concentration linked with the construction materials and enclosed characteristics of chorkor oven. While moisture content of chorkor smoked fish products in this study was within the level considered acceptable for smoked fish to inhibit both bacterial and fungal growth (Msusa et al., 2017), relatively higher values recorded in fish smoked using pit facility signify susceptibility of products to microbial spoilage particularly during storage.
Regarding nutritional quality, fresh M. caschive contained; 22.20±0.40% crude protein, 18.23±0.30% fat content and 9.13±0.14% ash. M. caschive smoked using pit kiln had 22.21±0.50% crude protein, 18.30±0.31% fats and 9.13±0.14% ash content. M. caschive smoked using chorkor had; 22.23±0.77% crude protein, 18.44±0.12% fat content and 9.24±0.08% ash. The results revealed chorkor oven concentrated the nutrients more than pit kiln. Equally, fresh O. niloticus contained; 24.24±0.27% crude protein, 8.87±0.34% fat and 9.28±0.22% ash content. O. niloticus smoked using pit had 24.24±0.25% crude proteins, 8.90±0.35% fat, 9.30±0.23% ash and O. niloticus smoked using chorkor contained; 24.30±0.27% crude protein, 8.92±0.35% fat and 9.35±0.21% ash. Similarly, chorkor concentrated nutrients more than pit technology. Results of this study supports the findings of other scholars regarding nutrient retention (Akintola, 2015; Abraha et al., 2017). However, the slight difference in the nutritional values of the two smoking facilities could be attributed to higher heat retention capacity of the construction materials used in chorkor oven that efficiently break down hydrogen bond in water molecules leading to water evaporation from the surface of fish.
PAHs levels in M. caschive and O. niloticus smoked using pit and chorkor kilns
During the study, seven types of PAHs consisting of chrysene, benzo [a] anthracene, anthracene, phenanthrene, fluorene, acenaphthene and naphthalene were recorded from the smoked fish samples (Table 2). The PAHs types recorded in pit and chorkor smoked fish products were mostly low and medium molecular weight (LMW, e.g. naphthalene, 2-ringed; anthracene, fluorene, acenaphthene and phenanthrene, 3-ringed and MMW, benzo [a] anthracene and chrysene; 4- ringed) compounds. In terms of abundance, naphthalene was the most occurring PAH followed by fluorene, benzo [a] anthracene, chrysene, phenanthrene, anthracene and acenaphthene. Fluorene and naphthalene were detected in fresh samples of M. caschive (Table 2). The high molecular weight (HMW, benzo [b] fluoranthene, benzo [k] fluoranthene, benzo [a] pyrene and dibenzo [a, h] anthracene, 5-ringed; Benzo [g, h, i] perylene and indenol [1, 2, 3-c, d] pyrene, 6-ringed) compounds were not detected and quantified in all the samples analyzed. As such, the ?PAH4 (benzo [a] anthracene, chrysene, benzo [b] fluoranthene, benzo [a] pyrene) was employed to determine the carcinogenic potency of PAHs measured in the smoked fish samples.
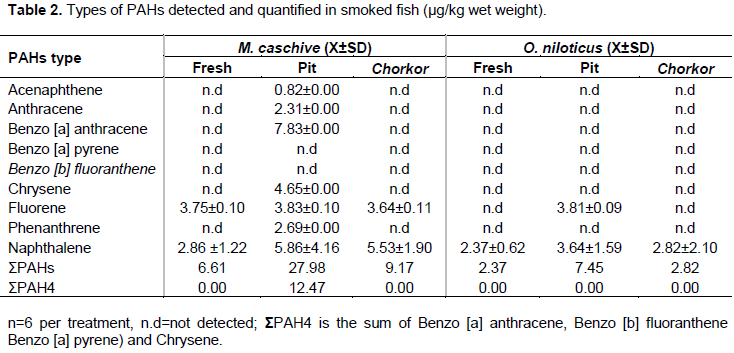
The concentration levels of each type of PAH in smoked samples of M. caschive and O. niloticus are presented in Table 2. The concentration levels of fluorene (3.75±0.10 µg/kg) and naphthalene (2.86 ±1.22 µg/kg) measured in fresh samples of M. caschive were significantly lower than those recorded in smoked samples. Pit smoked M. caschive had higher concentrations of fluorene (3.83±0.10 µg/kg) and naphthalene (5.86±4.16 µg/kg) than chorkor smoked samples (n.d and 5.53±1.90 µg/kg), P<0.05. Besides, the sum of PAH4 in pit smoked M. caschive (12.47 µg/kg) exceeded the maximum limit set by the European Union regulation (Table 2). Regarding O. niloticus, naphthalene was the only PAHs detected in fresh samples with a concentration level of 2.37±0.62 µg/kg. About the effect of smoking facilities, chorkor smoked O. niloticus had significantly lower naphthalene concentration (2.82±2.10 µg/kg) than pit smoked products (3.64±1.59 µg/kg), P<0.05. Fluorene (3.81±0.09 µg/kg) was only detected in pit smoked O. niloticus but not in the fresh and chorkor smoked fish samples.
Findings on the concentration levels of PAHs revealed that fluorene and naphthalene quantified in fresh fish samples were significantly lower than that recorded in smoked fish products. This is in conformity with reports that naturally, fish and aquatic invertebrates contain small and sometimes undetected quantity of PAHs absorbed from aquatic environment (Ongwech et al., 2013; Yusuf et al., 2015). Low PAHs measured in fresh samples could be due to degradation of compounds into other components during metabolic processes in fish body. Most of the PAHs (about 85%) were below the limit of detection in the fresh samples. This shows that, PAHs recorded in smoked fish samples were solely attributed to smoking processes as observed by earlier studies (Olabemiwo et al., 2013; Forsberg et al., 2013). Concerning samples, low molecular weight and medium molecular weight PAHs compounds were identified. This may be linked to the type of firewood used during the smoking process. Pagliuca et al. (2003) showed that smoke produced by hard woods usually generates high concentrations of less molecular weight PAHs. Although the individual PAHs of less molecular weights were detected in quantifiable amount, their levels were much lower than the sum of PAH4 (chrysene and benzo [a] anthracene). This could be attributed to the fact that, medium molecular weight compounds (e.g. benzo [a] anthracene, pyrene, chrysene and fluoranthene; 4-ringed) and high molecular weight PAHs (e.g. benzo [a] pyrene, benzo [b] fluoranthene, benzo [k] fluoranthene and dibenzo [a,h] anthracene; 5-ringed; benzo [g,h,i] perylene and indeno [1,2,3-c,d] pyrene; 6-ringed) compounds are more resistant to degradation both in aquatic organisms including fish and the environment. Similar observation was made by Anyakora and Coker (2007), who assessed PAHs content in four fish species from the Niger Delta. Their study attributed the difference to the fact that fish samples used were procured in an environment heavily polluted by petroleum products. Linda et al. (2011)noted higher levels of HMW than LMW PAHs when they categorized PAHs in smoked fish products from Ghana. They accredited the difference to residues of former combustion processes that might have occurred in the facility. Ongwech et al. (2013)also reported higher concentration levels of HMW than LMW PAHs when they investigated PAHs in smoked Lates niloticus collected in some markets of Gulu District, Uganda. They endorsed such a difference to exposure of fish markets to heavy air pollution from automobiles as markets are situated along Gulu-Kampala highway. Ongwech et al. (2013)further explained the higher levels of HMW compounds in relation to the mechanism of PAHS formation in that, during extended smoking, chances are that LMW PAHs formed are subsequently converted to HWM compounds through addition of combustion products from continued wood pyrolysis. Likewise, further burning of aromatic hydrocarbons’ residues may lead to formation of additional HMW PAHs and subsequently increase their concentrations in smoked fish products.
In this study, fish species smoked using pit kiln have higher ?PAH4 concentration levels than chorkor smoked fish samples. This difference could be explained in relation to the design and standardization of the smoking facility and processes (time of fish exposure to the smoke heat and regulation of the facility’s temperature). Chorkor oven has standard structural design and materials; perforated iron sheet, wood trays, enclosed system that will help in the control and normalization of the smoking process (oxygen and temperature). Besides, the difference could be associated with the time spent smoking; 8-12 h in a chorkor oven and 18-24 h in pit kiln. Excessive burning of firewood due to free air supply in pit facility could have resulted into increase in temperature that consequently led to variation in the rate of fat exudation. High rate of fat exudation may lead to modification of fish surface due to the presence of oils (Linda et al., 2011). The oily surface allows deposition of PAHs and other smoke chemicals on fish surface with subsequent penetration into fish muscles (Lee et al., 2019). Additionally, difference in fat exudation created by elevated temperature may have increased the rate of PAHs deposition and penetration in the muscles of pit smoked fish.
The presence of individual PAHs in higher levels in pit smoked fish samples on the other hand could be related to excessive heat treatment and proximity of fish products to heat that might have increased the penetration of smoke chemicals into fish flesh (Forsberg et al., 2013). However, presence of low molecular weight individual PAHs in higher level could be as a result of average wood combustion temperature (60-80°C) during the smoking process that indeed, could not degrade PAHs of higher molecular weight (Yusuf et al., 2015; Slámová et al., 2017).
It also suggests that the wood type; Acacia seyal used for smoking fish may probably contain smoke chemicals of low and medium molecular weight (LMW, 3 and MMW, 4-ringed PAHs). Besides, the presence of low molecular weight PAHs in fresh samples analyzed could be due to the occurrence of the compounds in the environment as a result of anthropogenic processes including forest burnings which could have been washed into water bodies and finally got their ways to fish. In regards to the use of sum of PAH4 as indicator for carcinogenicity, chorkor smoking can be adopted as a safer processing technology than pit kiln. Although the results of the current study revealed, smoked fish samples from the two technologies could be deemed safe for human consumption, chances are that consumption of pit smoked fish may pose health risk due to cumulative effect. This therefore, calls for the need to regularly monitor the concentration levels of PAHs especially the higher molecular weight (HMW, PAHs) compounds in smoked fish products of South Sudan.
CONCLUSION
The effect of smoking ovens on nutritional quality and concentration levels of PAHs in smoked M. caschive and O. niloticus were investigated and the study revealed, both smoking kilns retained the nutritional values of smoked fish. However, chorkor oven concentrated the chemical components of fish; protein, fats and ash more than pit resulting to better nutrients retention. The structural design of chorkor oven and standardization of its smoking process significantly reduced the rate of PAHs deposition, accumulation and migration into fish muscles. As such, fish smoked using chorkor oven were associated with less PAHs than pit smoked fish products. The present study recommends chorkor oven acceptance and utilization for artisanal fisheries of South Sudan. However, regular monitoring of PAHs concentration levels; tree species survey and profile of smoke to minimize use of species with high risk of PAHs for smoking fish should be undertaken.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
Authors are grateful to the editors and reviewers whose critiques and comments have greatly improved and enriched this manuscript. This study was financially supported by Sudd Ecology and Management project. Makerere University is appreciated for hosting this study.
REFERENCES
|
Abdel-Shafy HI, Mansour MSM (2016). "A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum 25(1):107-123. |
|
|
Abraha B, Samuel M, Mohammud A, Admassu H, Al-hajj NQM (2017). A comparative study on quality of dried anchovy (Stelophorus heterolobus) using open sun rack and solar tent drying methods. Turkish Journal of Fisheries and Aquatic Sciences 17(6):1107-1115. |
|
|
Adeyeye SAO, Oyewole OB (2016). An overview of traditional fish smoking in Africa. Journal of Culinary Science and Technology 14(3):198-215. |
|
|
Adeyeye SAO, Oyewole OB, Obadina AO, Omemu AM, Oyedele HA, Adeogun SO (2015). A survey on traditional fish smoking in Lagos State, Nigeria. African Journal of Food Science 9(2):59-64. |
|
|
Akintola SL (2015). Effects of smoking and sun-drying on proximate, fatty and amino acids compositions of Southern pink shrimp (Penaeus notialis). Journal of Food Science and Technology 52(5):2646-2656. |
|
|
Anyakora C, Coker H (2007). Assessment of polynuclear aromatic hydrocarbon content in four species of fish in the Niger Delta by gas chromatography/mass spectrometry. African Journal of Biotechnology 6(6):737-743. |
|
|
Association of Official Analytical Chemists (Eds.) (2005). Official methods of analysis 18th edition. Association of official analytical Chemists, Washington, Arlington, Virginia, USA. |
|
|
Benansio JS (2013). Consultancy Report for Fisheries and Livelihood. United Nations Development Programme, South Sudan Diagnostic Trade Integration Study. |
|
|
Climate-data.org (2018). National centers for environmental information (online). Retrieved from View |
|
|
Commission Regulation (EU) No 835/2011. Available online: View (accessed on 15 July 2019). |
|
|
European Food Safety Authority (EFSA) (2008). Scienti?c opinion of the panel on contaminants in the food chain on a request from the European Commission onpolycyclic aromatic hydrocarbons in food. EFSA Journal 724:1-114. |
|
|
Erawaty-Silalahi ETM, Anita S, Teruna HY (2021). Comparison of extraction techniques for the determination of polycyclic aromatic hydrocarbons (PAHs) in soil. Journal of Physics: Conference Series 1819(2021):012061. IOP publishing. |
|
|
Famurewa JAV, Akise OG, Ongubodede T (2017). Effect of storage methods on the nutritional quality of African catfish (Clarias gariepinus, Burchell, 1822). African Journal of Food Science 11(7):223-233. |
|
|
Food and Agriculture Organization of the United Nations (FAO) (2015). Resilient Livelihoods for Sustainable Agriculture, Food Security and Nutrition. Country Programming Framework for Sudan Plan of Action (2015-2019). |
|
|
Food and Agriculture Organization of the United Nations (FAO) (2016). The state of world fisheries and aquaculture. Contributing to food security and nutrition for all. Rome 200 p. |
|
|
Food and Agriculture Organization/World Food Programme of the United Nations (FAO/WFP) (2019). Crop and Food Security Assessment Mission to South Sudan. Special Report, Rome, 2019, 80 pages. |
|
|
Forsberg ND, Dave S, Harding A, Barbara H, Stuart H, Melissa MM, Andres C, Katrina MW, Anderson KA, (2013). Effects of Native American fish smoking methods on dietary exposure to polycyclic aromatic hydrocarbons and possible risks to human health. Journal of Agriculture and Food Chemistry 60(27):6899-6906. |
|
|
Katola A, Kapute F (2017). Nutrient composition of solar dried and traditionally smoked Oreochromis mossambicus, International Food Research Journal 24(5):1986-1990. |
|
|
Lee SY, Lee JY, Shin HS (2015). Evaluation of chemical analysis method and determination of polycyclic aromatic hydrocarbons content from seafood and dairy products. Toxicological Research 31(3):265-271. |
|
|
Lee SY, Lee JY, Shin HS (2019). Chemical analysis techniques and investigation of polycyclic aromatic hydrocarbons in fruits, vegetables and meats and their products. Food Chemistry 277(2019):156-161: Elsevier. |
|
|
Linda MN, Carboo PD, Quasie WJ, Mordecai A, Gorleku MA, Darko A (2011). Characterization of polycyclic aromatic hydrocarbons (PAHs) present in smoked fish from Ghana. Advanced Journal of Food Science and Technology 3(5):332-338. |
|
|
Magawata I, Musa T (2015). Quality characteristics of three hot-smoked fish species using locally fabricated smoking chorkor. International Journal of Fisheries and Aquatic Studies 2(5):88-92. |
|
|
Mottier P, Parisod V, Turesky R (2000). Quantitaive determination of polycyclic aromatic hydrocarbons in barbecued meat sausages by gas chromatography coupled to mass spectrometry. Journal of Agriculture and Food Chemistry 48(4):1160-1166. |
|
|
Muhame AW, Mugampoza E, Lubuulwa LL, Bazirake GWB, Mutambuka M (2020). Microbiological quality and safety assessment of Sun-dried Rastrineobola argentea (Mukene) sold at selected landing sites of Lake Victoria and Peri-urban Kampala City Markets. African Journal of Food Science 14(6):154-160. |
|
|
Msusa N, Likongwe J, Kapute F, Mtethiwa A, Sikawa D (2017). Effect of processing method on proximate composition of gutted fresh Mcheni (Rhamphochromis species) (Pisces: Cichlidae) from Lake Malawi. International Food Research Journal 24(4):1513-1518. |
|
|
Olabemiwo OM, Tella AC, Omodara NB, Esan AO, Oladapo A (2013). Polycyclic aromatic hydrocarbons in three local snacks in Ogbomoso. American Journal of Food and Nutrition 3(2):90-97. |
|
|
Olukayode AS, Paulina I (2017). Assessment of smoked fish quality using two smoking Chorkors and hybrid solar dryer on some commercial fish species in Yola, Nigeria. Journal of Animal Research and Nutrition 2(1):1-7. |
|
|
Omodara MA, Olayemi FF, Oyewole SN, Ade AR, Olaleye OO, Abel GI, Peters O (2016). The drying rates and sensory qualities of African catfish, Clarias gariepinus dried in three NSPRI developed fish chorkors. Nigerian Journal of Fisheries and Aquaculture 4(1):42-49. |
|
|
Ongwech A, Nyakairu GW, Mbabazi J, Kwetegyeka J, Masette M (2013). Polycyclic aromatic hydrocarbons in smoked Lates niloticus from selected markets, Gulu District, Uganda. African Journal of Pure and Applied Chemistry 7(4):164-172. |
|
|
Pagliuca G, Gazzotti T, Zironi E, Serrazanetti GP, Mollica D, Rosmini R (2003). Determination of high molecular mass polycyclic aromatic hydrocarbons in a typical Italian smoked cheese by HPLC-FL. Journal of Agriculture and Food Chemistry 51(17):5111-5115. |
|
|
Pemberton-Pigott C, Robinson J, Kwarteng E, Boateng L (2016). Low PAH improved Fish Smoking Stove Design Development Report. The USAID/Ghana Sustainable Fisheries Management Project (SFMP). Narragansett, RI: Coastal Resources Center, Graduate School of Oceanography, University of Island and Netherland Development Organization. HH2014 _ACT063_SNV 46 p. |
|
|
Puljic L, Mastanjevic K, Kartalovic B, Kovac?evic D, Jelena Vraneševic J Mastanjevic K (2019). The in?uence of di?erent smoking procedures on the content of 16 PAHs in traditional dry cured smoked meat "Hercegovac?ka pec?enica". Foods 8(12):690. |
|
|
R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. |
|
|
Samuel G, Angela M, Bryan G, Jill G, David J, John R, Laura C, Healther M, Gerry M, David C, Walter K, Terri A, Jason W, Michele F, Yoko J, Douglas H, Fred F (2010). Screen f or the presence of polycyclic aromatic hydrocarbons in select sea foods using LC-Fluorescence. Minnesota: 1 Forensic Chemistry Centre (FCC), Food and Drug Administration 4475:1-39. |
|
|
Slámová T, Fra?ková A, Hubá?ková A, Banout J (2017). Polycyclic aromatic hydrocarbons in Cambodian smoked fish. Food Additives and Contaminants: Part B Surveillance 10(4):248-255. |
|
|
South Sudan Centre for Census, Statistics and Evaluation (2018). Statistical year Book for South Sudan. South Sudan Centre for Census. |
|
|
Wretling S, Eriksson A, Eskhult GA, Larsson B (2010). Polycyclic aromatic hydrocarbons (PAHs) in Swedish smoked meat and fish. Journal of Food Composition and Analysis 23(3):264-272. |
|
|
Yusuf KA, Ezechukwu LN, Fakoya KA, Akintola SL, Julius I, Omoleye TO (2015). Influence of fish smoking methods on polycyclic aromatic hydrocarbons content and possible risks to human health, African Journal of Food Science 9(3):126-135. |
|
|
Zelinkova Z, Wenzl T (2015). The occurrence of 16 EPA PAHs in food - a review. Polycyclic Aromatic Compounds 35(2-4):248-284. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0