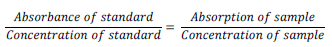ABSTRACT
Kokoro, a local maize snack was made from white maize (W) flour and supplemented with Moringa oliefera leaf (M) and defatted soybean (S). WMS0 (100:0:0%), WMS1 (90:10:0%), WMS2 (90:0:10%), WMS3 (90:5:5%), WMS4 (90:7.5:2.5%), and WMS5 (90:2.5:7.5%) were carried out in triplicates. Kokoro was produced by deep frying in hot refined vegetable oil. The proximate composition, vitamin content and phytochemicals composition were determined. Kokoro formulated with 90% maize flour and 10% deffated soybean (WMS2) had the highest moisture, crude protein, fat, oxalate, phytic acid and alkaloid, while Kokoro formulated with 90% maize flour and 10% moringa (WMS1) had the highest crude fibre, vitamin A, B3 (niacin), C and flavonoid. On the other hand, Kokoro formulated with only 100% maize flour (WMS0) had the least phytochemical composition and vitamins A, B3, C contents. Although, the addition of soybean had the highest positive effect on the protein and crude fibre contents of Kokoro, it was the addition of moringa that had the highest positive effect on the vitamins contents. On the other hand, moringa also raised the phytochemical contents significantly (p≤0.05). Overall, sample WMS4 (90% Maize + 7.5% Moringa + 2.5% Soybean) had substantial proximate and minerals composition in addition to having the least phytochemical could be considered the best formulation for Kokoro formulation.
Key words: Moringa, phytochemicals, proximate composition, soybean, Kokoro.
The traditional maize (Zea mays L. Poaceae) snack (Kokoro) is a popular local snack in Southwestern Nigeria and is made solely from maize flour that contains primarily carbohydrates (Awolu et al., 2016a). Snack containing 100% maize do not meet nutritional requirements of the body, hence the need to fortify with crops rich in protein, fibre, minerals and antioxidants (Awolu et al., 2016a, 2015; Omueti and Morton, 1996). Specifically, toasted had been produced by optimum blends using response surface methodology (Awolu et al., 2016b).
Maize,the primary material for making Kokoro snack is a cereal and is deficient in the essential amino acids such as lysine and tryptophan (Omueti et al., 1992) that are essential for human nutrition (Lasztity, 1984). Research effort has been concentrated on supplementing cereal food with legumes and has successfully enhanced the nutritional value and/or functionality of staple foods (Awolu et al., 2016a).
Moringa oleifera is a highly valuable plant, distributed in many tropical and subtropical countries. It has an impressive range of medicinal uses with high nutritional value. Different parts of this plant contain a profile of important minerals, and are a good source for protein, vitamins, β-carotene, amino acids and various phenolics. Moringa plant provides a rich and rare combination of zeatin, qurcetin, kaempferol and many other phytochemicals. It is also very important plant for its medicinal value. Various parts of the plant such as leaves, roots, seeds, bark, fruit, flowers and immature pods, etc., as cardiac and circulatory stimulants, possess antitumour, antipyretic, antitiepileptic, anti-inflammatory, antiulcer, antispasmodic, antihypertensive, cholesterol lowering, antioxidant, antidiabetic, antibacterial and antifungal (Bukar et al., 2010). M. oleifera leaves are highly nutritious. In 100 g dry matter, they contain 29±6 g of protein, 28±6 mg of iron, 1,924±288 mg of calcium, 15,620±6,475 IU of vitamin A and 773±91 mg of vitamin C. This is at least twice the protein in milk and half the protein in egg, and has more iron than in beef, more calcium than in milk, equal vitamin A to carrot and more vitamin C than in orange (Wangcharoen and Gomolmanee, 2013). In addition, the leaves of this plant are reported to have various biological activities such as diuretic, immune boosting and hypotensive, anti-inflammatory, antiulcer, antihepatotoxic, antitumour, thyroid hormone status regulating, hypocholesterolaemic, radioprotective, hypolipidaemic, antiatherosclerotic, antidiabetic, and antioxidant (Jaiswal et al., 2009; Chumark et al., 2008; Singh et al., 2009; Sreelatha and Padma, 2008; Verma et al., 2009).
This study was to evaluate the addition of soybean and moringa into maize for the production of Kokoro with high nutritional and phytochemical properties.
Sample collection and preparation
Maize (Zea mays L., Poaceae) was sourced from the Teaching and Research Farm of the Federal University of Technology, Akure, Nigeria. It was shelled, winnowed and sorted to remove damaged grains before milling. Soybean (Glycine max (L.) Merrill) was sourced from Ondo State Agricultural Development Project (ADP), Akure. The seeds were cracked with hammer mill and seed coat winnowed. It was toasted, cooled and milled into flour. The flour was defatted with n-hexane in a Soxhlet apparatus. Moringa leaf was plucked from the branch of the tree raised in Akure, Nigeria. The leaves were cleaned, air dried under shade, dry milled and kept in airtight container. Considerable amount of chlorophyll in the leaves were removed by soaking in ethanol overnight, followed by draining and air-drying.
Composite flour formulation
White maize (W), moringa (M) and soybean (S) were mixed to make 180 g at different ratios and replicated three times in the following percentage: WMS0 (100:0:0%), WMS1 (90:10:0%), WMS2 (90:0:10%), WMS3 (90:5:5%), WMS4 (90:7.5:2.5%), and WMS5 (90:2.5:7.5%). Each of the mixture was replicated three times.
Formulation of Kokoro
Salt was added to 180 g composite flour to taste and was gently stirred into 0.5 L boiling water in a stainless steel pot. The mixture was cooked with continuous stirring until stiff dough was formed. The dough was cooled to a temperature (40°C) at which it could be kneaded by hand for 5 min. The kneaded dough was cut into pieces, rolled into cylindrical shapes and deep fried at 150°C in 1 L of hot refined vegetable oil for 3 min. The fried Kokoro was then cooled and packed in sealed polyethylene bags. The frying was carried out in triplicates.
Proximate analysis of Kokoro
Moisture and protein content were determined using the procedure described by AOAC (1990). The ash content was determined using the procedure described by Pearson (1976).
The fat content determination was carried out by soxhlet extraction method (AOAC, 2005). Oil in 5 g sample was extracted using hexane in Soxhlet extraction equipment for 2.5 h under reflux. The crude fibre content was determined using the procedure described by Kirk and Sawyer (1991). Total carbohydrate was estimated using the formula (Ouzouni et al., 2009):
Total carbohydrates (% f) = [100 - moisture (%) - protein content (%) - crude fat (%) - ash (%)].
Determination of vitamin A
Method of AOAC (2005) was used. Exactly 1 ml of the hydrophilic extracts from the sample was measured to the test-tube I (centrifugal) with a tight stopper and 1 ml of the KOH solution was added, the tube was plugged and shake vigorously for 1 min. The tube was heated in a water bath (60°C, 20 min), and was then cooled down in cold water. About 1 ml of xylene was added, the tube was plugged and shake vigorously again for 1 min. The tube was centrifuged (1500×g, 10 min), the whole of the separated extract (upper layer) was collected and transferred into the test tube II made of “soft” (sodium) glass. The absorbance A1 of the obtained extract was measured at 335 nm against xylene. The extract in the test tube II was irradiated to the UV light for 30 min, then the absorbance A2 was measured. The concentration (Cx) of vitamin A (μM) in the analyzed liquid was calculated using the equation:
Cx = [A1] – [A2]. 22.23
where 22.23 is the multiplier received on basis of the absorption coefficient of 1% solution of vitamin A (as the retinol form) in xylene at 335 nm in a measuring cuvette, 1 cm thickness.
Determination of vitamin B3 (Niacin)
Five grams of the sample was treated with 50 ml 1N H2SO4 and shaken for 30 min. About 3 drops of ammonia solution was added to the samples and filtered. The filtrate was pipette into a 50 ml volumetric flask and 5 ml potassium cyanide was added. This was acidified with 5 ml of 0.02 N H2SO4 and absorbance was measure using spectrophotometer at 470 nm (Okwu and Josiah, 2006)
Determination of vitamin C
The modified method of the method adopted by Awolu et al. (2013) was used. The vitamin C content of the hydrophilic extracts from the sample was determined by the spectrophotometric method using ascorbic acid as a reference compound. Exactly 10 ml of the juice sample was weighed into 10 ml of water and mixed together. 200 µl, that is, 0.2 ml of the extract was pipetted and mixed with 300 µl (0.3 ml) of 13.3% of trichloro-acetic acid (TCA) and 75 µl (0.075 ml) of dinitrophenylhydrazyl (DNPH). The mixture was incubated in water bath at 37°C for 3 h. After 3 h, 500 µl (0.5 ml) of 65% sulphuric acid was added and the absorbance was read with the spectrophotometer at 520 nm. The concentration of vitamin C was calculated as follows:
Determination of phytochemicals
Flavonoid was determined using the procedure of Boham and Kocipai (1994). From oxalate through Day and Underwood (1986) procedure, phytin-phosphorus was determined by the method of Wheeler and Ferrell (1971) as modified by Reddy et al. (1978). Phytic acid was calculated by multiplying Phytin-P by the factor of 3.55 (Enujiugha and Olagundoye, 2001). The tannin content was determined by the quantitative method of Makker and Goodchild (1996). Alkaloid was determined using the procedure of Harborne (1973).
Statistical analysis
All analyses were carried out in triplicates. The results obtained were subjected to analysis of variance (ANOVA) using the statistical package for social sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA). Means were separated using the Duncan multiple range test (DMRT) at 95% confidence level (p<0.05).
Proximate composition and vitamin content of formulated Kokoro
The results of proximate composition and vitamin contents of the formulated Kokoro are shown in Figure 1. Kokoro made from WMS0 formulation had the highest CHO (65.35%) and least crude protein (5.03%) and crude fibre (2.08%) content. The significantly (p<0.05) high crude carbohydrate and least crude protein found in Kokoro from WMS0 is consistent with the study of Omueti et al. (1992) and Miracle (1997). This study showed cereal based product to contain primarily carbohydrate and to be low in crude protein. Increased substitution of soybean and moringa flour for maize flour in the formulated Kokoro increased crude protein content and decreased carbohydrate content.
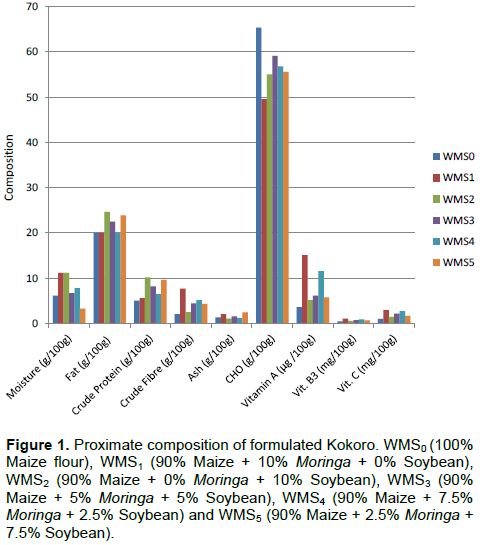
Kokoro (WMS2) had the highest moisture (11.20%) and crude protein (10.17%). Kokoro (WMS1) showed no significant (P>0.05) difference with WMS2 with regards to moisture content. The highest moisture and crude protein content observed in Kokoro (WMS2) was due to defatting effect on soybean flour. Defatting has been reported to increase flour water absorption capacity (Gonzalez-Agramon and Serna-Saldivar, 1988; Ogunsina et al., 2010) and crude protein (Alobo et al., 2009; Uzor-Peters et al., 2008). Serna-Saldivar et al. (1988) reported 35% more crude protein in wheat flour fortified with 11.1% defatted soybean meal (SBM).
The highest crude fibre (7.71%) content was recorded in WMS1. The highest crude fibre recorded in Kokoro (WMS1) is consistent with the study of Sanford (2000) and Oduro et al. (2008) that showed dried moringa leaf to contain high crude fibre concentration. Increase in substitution with dried moringa leaf powder from 0 to 15% has been reported to result in increase in dietary fibre (Dachana et al., 2010). Potential health benefits of dietary fibre have been documented in relation to the prevention of cardiovascular disease (Bazzano et al., 2003).
The highest fat content (24.67%) was obtained in WMS5. The fat content in WMS1 did not show significant difference (p<0.05) from those obtained in WMS4 and WMS5. Substituting white maize flour with defatted soybean flour in the formulation of Kokoro at WMS3, WMS5 and 10% level of defatted soybean flour substitution (WMS2) significantly (p<0.05) increased Kokoro fat content. This is consistent with the study of Ogunsina et al. (2010) revealing the ability of defatting of soybean in increasing fat absorption capacity of flour.
The highest value of vitamin A, B and C recorded in formulated Kokoro (WMS1) showed the ability of moringa leaf in improving vitamin composition of Kokoro when substituted for maize in its formulation. This is in agreement with earlier study on moringa leaf that showed it to be substantially rich in vitamin A, B and C (ascorbic acid) (Ramachandran et al., 1980; Sanford, 2000; Anwar et al., 2007). The ascorbic acid acts as antioxidant and hence enhance the shelf life of fat containing food (Dillard and German, 2000; Siddhuraju and Becker, 2003).
Though vitamin B3 (niacin and niacinamide) has been reported to possess antioxidant properties (Gliszczynska-Swiglo, 2006), and is therefore able to protect the body against free radical induced degenerative diseases due to its antioxidant properties. Dried moringa leaf has been reported to contain seven times more of the vitamin C found in orange and four times more the vitamin A obtained in carrot (Balbir, 2005; Fahey, 2005). Kokoro (WMS0) had the least value of vitamin A, B3 (niacin) and C. The highest value of vitamin A, B3 (niacin) and C were recorded in Kokoro (WMS1).
Substitution of soybean or moringa for maize in the formulated flour significantly (p<0.05) improved Kokoro vitamin A, B3 (niacin), and C. Likewise, the increase in the levels of substituting moringa for soybean in the formulated flour.
Phytochemical composition of formulated Kokoro
The results of the phytochemical composition of formulated Kokoro are presented in Table 1. The highest value for oxalate (0.59 mg/g) and phytic acid (30.49 mg/g) were recorded in Kokoro made from 10% level of defatted soybean flour substitution (WMS2). The presence of phytate has been established in Soybean (Ronald, 2000). Phytates are organic acid, present in the bran or hulls of seeds, which blocks the uptake of essential minerals, calcium, magnesium, iron and especially zinc in the intestinal tract (Ronald, 2000) due to its ability to chelate divalent cations (Nelson et al., 1968).
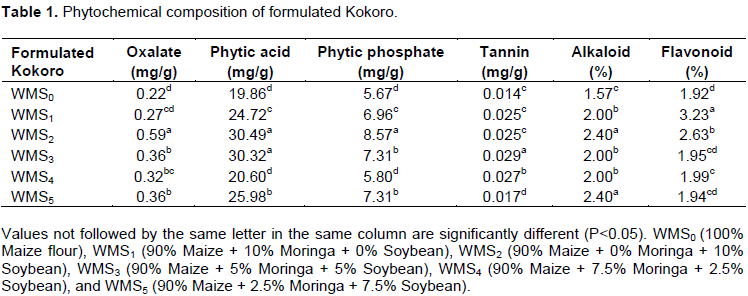
Phytate content is higher in the raw white variety of the maize than the raw yellow variety (Oboh et al., 2010). It is only a long period of fermentation that can significantly reduce the phytate content. The concentration of high level of oxalate, a salt or ester of oxalic acid in Kokoro formulated with 10% level of defatted soybean flour substitution (WMS2) was due to its presence in soybean seed. The high concentration of oxalate in Kokoro produced at all levels of moringa flour formulation may be as a result of large amounts oxalate found in the stems and leaves of M. oleifera (Dachana et al., 2010; Olson and Carlquist, 2001).
There were no significant differences (p<0.05) between 100% white maize flour (WMS0) formulation and WMS4 with regards to phytic acid and phytic phosphate. Kokoro (WMS0) had the least value for all anti-nutritional factors considered. The highest value for phytic phosphate and tannin occurred in WMS3. The low tannin in 100% white maize flour formulated Kokoro (WMS0) is consistent with the finding of Oboh et al. (2010) that reported low tannin content in white maize. Tannin has also been found negligible in all fractions of moringa plant (Reyes-Sánchez et al., 2006). Tannin is known to affect nutritive value of food products by forming complex with protein (both substrate and enzyme) thereby inhibiting digestion and absorption (Osuntogun et al., 1987). They also bind Fe making it unavailable (Aletor and Adeogun, 1995).
The highest values for alkaloids were recorded in WMS5, WMS2 and WMS3 formulated Kokoro. There were no significant differences (p<0.05) between the three formulations. The highest value for flavonoid (3.23%) was in Kokoro (WMS1). The least value (1.92%) was revealed in (WMS0). Flavonoid content in formulated Kokoro increases with increase in moringa flour substitution in the flour mixture. The observed rise in flavonoid content of formulated Kokoro with attendant rise in moringa flour substitution is consistent with earlier study that showed moringa leaf as a good source of natural antioxidants (Dillard and German, 2000; Siddhuraju and Becker, 2003; Anwar et al., 2005). Antioxidants are known for free radical scavenging ability (Scherer and Godoy, 2009) and therefore, neutralize free radicals that have the ability of stimulating reaction that make the cells more vulnerable to cancer causing chemicals, called carcinogen. Reddy et al. (2005) reported that the use of M. oleifera as source of natural antioxidant and it was found effective in controlling lipid oxidation during storage of biscuits.
The addition of defatted soybean and M. oleifera leaf flours to maize flour in the production enhanced the protein, crude fibre and vitamins contents of the snack (Kokoro). In addition, the flavonoid and alkanoids (antioxidants) contents were improved by the incorporation of deffated soybean and M. oleifera leaf flours. Soybean had a negative effect on the anti-nutrients at 5% and above incorporation. Therefore, sample WMS4 (90% maize + 7.5% moringa + 2.5% soybean) was the best formulation which guaranteed enough protein, crude fibre, vitamins and antioxidants, with considerably low anti-nutrients.
The authors have not declared any conflict of interests.
REFERENCES
|
Aletor VA, Adeogun OA (1995). Nutrient and anti-nutrient components of some tropical leafy vegetables. Food Chem. 53:375-379.
Crossref
|
|
|
|
Alobo AP, Agbo BN, Ilesanmi SA (2009). Physicochemical and functional properties of full fat and defatted cashew kernel flours. Int. J. Food Sci. Technol. 44:581-585.
Crossref
|
|
|
|
|
Anwar F, Ashraf M, Bhanger MI (2005). Inter-provenance variation in the composition of Moringa oleifera oilseeds from Pakistan. J. Am. Oil Chem. Soc. 82:45-51.
Crossref
|
|
|
|
|
Anwar F, Latif S, Ashraf M, Gilani AH (2007). Moringa oleifera: A Food Plant with Multiple Medicinal Uses. Phytother. Res. 21:17-25.
Crossref
|
|
|
|
|
AOAC (Association of Official Analytical Chemist) (1990). Official method of analysis, 14th Edition, Washington DC.
|
|
|
|
|
AOAC (Association of Official Analytical Chemist) (2005). Official method of analysis of the AOAC (W. Horwitz, Ed.) 18th Edition, Washington DC.
|
|
|
|
|
Awolu OO, Aderinola TA, Adebayo IA (2013). Physicochemical and rheological behavior of African Star apple (Chrysophyllum albidium) juice as affected by concentration and temperature variation. J. Food Process. Technol. 4:229.
|
|
|
|
|
Awolu OO, Oluwaferanmi PM, Fafowora OI, Oseyemi GF (2015). Optimization of the extrusion process for the production of ready-to-eat snack from rice, cassava and kersting's groundnut composite flours. LWT Food Sci. Technol. 64:18-24.
Crossref
|
|
|
|
|
Awolu OO, Omoba OS, Olawoye O, Dairo M (2016a). Optimization of production and quality evaluation of maize-based snack supplemented with soybean and tigernut (Cyperus esculenta). Food Sci. Nutr. 5:3-13.
Crossref
|
|
|
|
|
Awolu OO, Osemeke RO, Ifesan BOT (2016b). Antioxidant, functional and rheological properties of optimized composite flour, consisting wheat and amaranth seed, brewers' spent grain and apple pomace. J. Food Sci. Technol. 53(2):1151-1163.
Crossref
|
|
|
|
|
Balbir M (2005). Tree for life Moringa Book. [Bronchure] Balbir Mathur; Wichita, Kansas.
|
|
|
|
|
Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK (2003). Dietary fibre intake and reduced risk of coronary heart disease in US men and women: the National Health and Nutrition Examination Survey I epidemiologic follow-up study. Arch. Intern. Med. 163:1897-1904.
Crossref
|
|
|
|
|
Boham AB, kocipai AC (1994). Flavonoid and condensed tannins from leaves of Hawaiian vaccinium vaticulum. Pac. Sci. 48:458-463.
|
|
|
|
|
Bukar A, Uba A, Oyeyi TI (2010). Antimicrobial profile of Moringa oleifera Lam. extract against some food – borne microorganisms. Bayero J. Pure Appl. Sci. 3(1):43-48.
Crossref
|
|
|
|
|
Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-ngam L, Ratanachamnong P, Srisawat S, Pongrapeeporn KU (2008). The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. Leaves. J. Ethnopharmacol. 116:439-446.
Crossref
|
|
|
|
|
Dachana KB, Jyotsna RD, Indrani JP (2010). Effect of dried Moringa (Moringa Oleifera Lam.) leaves on rheological, microstructural, nutritional, textural and organoleptic characteristics of cookies. J. Food Qual. 33(5):225-300.
Crossref
|
|
|
|
|
Day RA Jr, Underwood AL (1986). Quantitative Analysis. 5th edition, Prentice-Hall publication 70 p.
|
|
|
|
|
Dillard CJ, German JB (2000). Phytochemicals: nutraceuticals and human health: A review. J. Sci. Food Agric. 80:1744-1756.
Crossref
|
|
|
|
|
Enujiugha VN, Olagundoye TV (2001). Comparative nutritional characteristic of raw, fermented and roasted African oil bean (Pentaclethra macrophylla Benth) seed. Rivista Italiana delle Sostanze Grasse 78:247-250.
|
|
|
|
|
Fahey J (2005) Moringa oleifera: A review of the medical evidence for its nutritional therapeutic, and prophylactic properties. Part 1. Tree Life J. 1(5):1-5.
|
|
|
|
|
Gliszczynska-Swiglo A (2006). Antioxidant activity of water soluble vitamins in the TEAC (Trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 96:131-136.
Crossref
|
|
|
|
|
Gonzalez-Agramon M, Serna-Saldivar S (1988). Effect of defatted soybean and soybean isolate fortification on the nutritional, physical, chemical and sensory properties of wheat flour tortillas. J. Food Sci. 53:793-797.
Crossref
|
|
|
|
|
Harborne JB (1973). Phytochemical Methods. London Chapman and Hall, Ltd. Pp. 49-188.
|
|
|
|
|
Jaiswal D, Rai PK, Kumar A, Mehta S, Watal G (2009). Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 123:392-396.
Crossref
|
|
|
|
|
Kirk RS, Sawyer R (1991). Pearson's Composition and Analysis of Foods. 9th edition. Longman Group. P 646.
|
|
|
|
|
Lasztity R (1984). Maize proteins. In: The Chemistry of Cereal Proteins, CRC Press, Inc., Boca Raton, FL. pp. 131-155.
|
|
|
|
|
Makker AOS, Goodchild AV (1996). Quantification of tannins. A laboratory manual. International Centre for Agricultural Research in Dry Areas (ICARDA), Aleppo Syria. 25 p.
|
|
|
|
|
Miracle MP (1997). Increasing efficiency in maize production in Tropical Africa. Longman, London. 233 p.
|
|
|
|
|
Nelson TS, Ferrara LW, Stover NL (1968). Phytate phosphorus content of feed ingredients derived from plants. Poult. Sci. 47: 1372-1378.
Crossref
|
|
|
|
|
Oboh G, Ademiluyi AO, Akindahunsi AA (2010). The effect of roasting on the nutritional and antioxidant properties of yellow and white maize varieties. Int. J. Food Sci. Technol. 45:1236-1242.
Crossref
|
|
|
|
|
Oduro I, Ellis WO, Owusu D (2008). Nutritional potentials of two leafy vegetables: Moringa oleifera and Ipomea batatas leaves. Sci. Res. Essays 3(2):57-60.
|
|
|
|
|
Ogunsina BS, Radha C, Singh RSG (2010). Physicochemical and functional properties of full-fat and defatted Moringa oleifera kernel flour. Int. J. Food Sci. Technol. 45:2433-2439.
Crossref
|
|
|
|
|
Okwu DE, Josiah C (2006). Evaluation of the chemical composition of two Nigerian medicinal plants. Afr. J. Biotechnol. 5:357-361.
|
|
|
|
|
Olson ME, Carlquist S (2001). Stem and root anatomical correlation with life form diversity, ecology, and systematics in Moringa (Moringaceae). Bot. J. Linn. Soc. 135(4):315-348.
Crossref
|
|
|
|
|
Omueti O, Morton ID (1996). Development by extrusion of soya Bari snack sticks: A nutritionally improved soya-Maize product based on the Nigerian snack (Kokoro). Int. J. Food Sci. Nutr. 47(1):5-13.
Crossref
|
|
|
|
|
Omueti O, Morton ID, Emery PW (1992). Nutritional characteristics of soybean seed flour after processing with sodium bicarbonate or trona. Int. J. Food Sci. Nutr. 43:147-53.
Crossref
|
|
|
|
|
Osuntogun BA, Adewusi SRA, Telek L, Oke OL (1987). Human nutrition. Food Sci. Nutr. 41(F):41-46.
|
|
|
|
|
Ouzouni PK, Petridis D, Koller WD, Riganakos KA (2009). Nutritional value and metal content of wild edible mushrooms collected from West Macedonis and Epirus, Greece. Food Chem. 115:1575-1580.
Crossref
|
|
|
|
|
Pearson D (1976). Chemical Analysis of Foods.7th edition, Churchill Livingston, Edinburgh, United Kingdom.
|
|
|
|
|
Ramachandran C, Peter KV, Gopalakrishnan PK (1980). Drumstick (Moringa oleifera): a multipurpose Indian vegetable. Econ. Bot. 34:276-283.
Crossref
|
|
|
|
|
Reddy NR, Balakrishman CV, Salunkhe DK (1978). Phytate phosphorus and mineral changes during germination and cooking of black gram (Phaseolu mungo L) seeds. J. Food Sci. 43(2):540-543.
Crossref
|
|
|
|
|
Reddy VP, Garett MR, Perry G, Smith MA (2005). Carnosine: A versatile antioxidant and anti-glycating agent. Sci. Aging Knowl. Environ. 5(2):119-125.
|
|
|
|
|
Reyes-Sánchez A, Nchez N, Ledin S and Ledin I (2006). Biomass production and chemical composition of Moringa oleifera under different management regimes in Nicaragua. Agroforestry Syst. 66:231-242.
Crossref
|
|
|
|
|
Ronald WH (2000). Use of soybean meals in diets of salmon and trout. Written in cooperation with the United Soybean Board and American Soybean Association. pp. 1-16.
|
|
|
|
|
Sanford H (2000). Moringa, Nature's Medicine Cabinet. Sierra Sunrise Publishing, Sherman Oaks, California. 122 p.
|
|
|
|
|
Scherer R, Godoy HT (2009). Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 112:654-658.
Crossref
|
|
|
|
|
Serna-Saldivar S, Lopez-Ahumada G, Ortega-Ramirez R, Dominguez RA (1988). Effect of sodium stearoyl-2-lactylate on the rheological and baking properties of wheat bread fortified with defatted soybean and sesame meal. J. Food Sci. 53:211.
Crossref
|
|
|
|
|
Siddhuraju P, Becker K (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.). J. Agric. Food Chem. 15:2144-2155.
Crossref
|
|
|
|
|
Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, Singh HB (2009). Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 47:1109-1116.
Crossref
|
|
|
|
|
Sreelatha S, Padma PR (2008). Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 64:303-311.
Crossref
|
|
|
|
|
Uzor-Peters PI, Arisa NU, Lawrence CO, Osondu NS, Adelaja A (2008). Effect of partially defatted soybean or groundnut cake flour on proximate and sensory characteristics of Kokoro. Afr. J. Food Sci. 2:98-101.
|
|
|
|
|
Verma AR, Vijayakumar M, Mathela CS, Rao CV (2009). In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 47:2196-2201.
Crossref
|
|
|
|
|
Wangcharoen W, Gomolmanee S (2013). Antioxidant activity changes during hot-air drying of Moringa oleifera leaves. Maejo Int. J. Sci. Technol. 7(3):353-363.
|
|
|
|
|
Wheeler EI, Ferrell RE (1971). A method of phytic acid determination in wheat fractions. Cereals Chem. 48:312-314.
|
|