ABSTRACT
In Senegal, sweet corn is produced for export market while the canned ones are imported to supply national market. This work was carried out to investigate the effect of different processing conditions such as heating temperature and sterilization time on the microbial quality, color, ascorbic acid and shelf life of canned sweet corn produced in Senegal. The hygiene level of sweet corn samples at different preliminary processing stages before canning processing was evaluated also. Aerobic mesophilic total counts were lowest at blanching (1.8 log10 CFU/g) and no microorganisms related to food spoilage and public health concerns were detected in all canned sweet corn regardless of treatment. However, treatment E (125°C/12 min) had the highest F-value (35.7 min) and the lowest C-value/F-value ratio (3.84 min). This treatment had also less impact on total color change (DE*=6.81) and ascorbic acid content. Canned sweet corn was shelf stable after 12 months of storage.
Key words: Sweet corn processing, canning processing, sterilization, thermal treatment, microbial quality, shelf life, color, vitamin C.
Sweet corn (Zea mays L.sppsaccharata), a crop that is planted worldwide, is one of the most common vegetables grown and consumed throughout the world (Siddiq and Pascall, 2011; Yu et al., 2016).According to More et al. (2018), it is a cultivated plant for human consumption and is a raw or processed material of the food industry throughout the world. For example, in the U.S. and Canada, sweet corn is considered to be a symbol of summer, being one of the most popular vegetables (Pacurar et al., 2019).Sweet corn is present in the market in fresh, frozen and canned forms (Alan et al., 2014). Recently introduced in Senegal (since 2004), sweet corn was identified by the Senegalese Government as a high value-added crop with potential for export markets (Sow and Lagnane, 2011). Production is increasing (up to 12,253 metrics tons in 2015), and more than 40 million ears of fresh sweet corn were sold each year by one of the big five Senegalese producers (SCL, 2019). However, the country still imports canned sweet corn to cover the national market while local sweet corn production is exported fresh to European Union markets (Ndiaye et al., 2017).According to FAOSTATS (2019), 417 tons of prepared or preserved sweet corn were imported into Senegal during 2015. Thus, the production of canned sweet corn could be an opportunity to create added value and new markets for the horticulture sub-sector andpromote the development of local food processing industry at different scale. Therefore, canned sweet corn could be a new food product made in Senegal. Furthermore, development of such processing units could contribute to reducing importations andpost-harvest losses. It could be also an opportunity to diversify their market.
Because of low acidity, sweet corn is susceptible to growth of spoilage and pathogenic organisms including Clostridium botulinum, mesophilic spore-forming bacteria and thermal tolerant bacteria (Liato et al., 2016; Mishra and Sinha, 2018). In the food industry, thermal processing is one of the oldest food processing technologies and the most common process to enable microbiologically safe food and extending the useful shelf life of foods (Simpson and Abakarov, 2009; Pankaj, 2016; Mishra and Sinha, 2018).Sterilization must take into account the microbiological characteristics of the product and the storage requirements after processing. Canning is the general term applied to packaging a food in a hermetically sealed container that avoid the passage of gas or microorganisms and subjecting it to a thermal process for the purpose of extending its useful life (Berry and Pflug, 2003; Erkmen and Bozoglu, 2016).Thermal treatment may also affect quality characteristics of the final product, such as color or vitamin C content.Therefore, the purpose of this work was to investigate the effect of different heat sterilization treatments on microbial quality, color, vitamin C and shelf life of canned sweet corn produced in Senegal. The most suitableprocessing conditions are proposed such as heating temperature and time, with the hope that results would guide future canned sweet corn Senegalese processors to producea safe and good quality of shelf stable canned sweet corn.
Freshyellow sweet cornears (super sweet varieties) were purchased from a local sweet corn grower in Saint Louis (northern region in Senegal).
Preliminary operation stages prior to canning processing
In this study, preliminary processing stages before canning were as follows: husking, blanching, cooling, cutting and washing. Three batches of one hundred fresh sweet corn ears per batch were used for sample preparation. For each batch, ears were husked and silks were removed manually. No water was used on whole sweet corn ears before husking to prevent contamination of kernels. Furthermore, two operators carried out husking so that there was no contact between sweet corn leaves and kernels. Ears were then steam blanched for 6 min. Blanched ears were cooled in fresh water for 3 min and drained. Fresh water was used because sterile water was not available in our laboratory. After cooling, kernels were cut from the cobs, washed and drained. The colony forming units CFU/g) of total aerobic mesophilic counts at 30°C were determined at different preliminary processing stages according to NF EN ISO 4833-1 (2013) to assess hygiene level of sweet corn samples.
Preparation of canned sweet corn kernels
Five batches (one batch for one combination of heating temperature and holding time) of canned sweet corn kernels are processed. For each batch, 100 fresh sweet corn ears were used to prepare canned sweet corn kernels. The unit operations were as follows: husking, cutting kernels from the cobs, washing, blanching (by steam exposure for 6 min), cooling, filling/weighting (230 g of prepared sweet corn kernels), exhausting (180 mL of hot water at 10° brix and 1% salt) and seaming (at atmospheric pressure using a semi-automatic seaming machine Sertinox S.C.I.M., Casteljoux, France). Easy open cans ref ½ haute T40 (73 mm x 109 mm) were used in this study.
Thermal sterilization of canned sweet corn kernels
After seaming, canned sweet corn kernels were sterilized to achieve microbial safety. Sterilization of canned sweet corn kernels was carried out using a vertical non-rotary retort (Techna FT 60/95E) consisting of a cylindrical storage vessel, a feeding and cooling water system, a digital thermo regulator, a temperature recording and control elements. An average number of 44 cans ofprepared sweet corn kernelswere implied in thermal sterilization. Canned sweet corn kernels were sterilized at the following five combinations of heating temperature and holding time: 121.1°C for 4 min (treatment A), 118°C for 40 min (treatment B), 121.5°C for 18 min (treatment C), 125°C for 8 min (treatment D) and 125°C for 12 min (treatment E). Each combination of temperature and time was tested in duplicate. A temperature data logger SL53T (0°C to +125°C ± 0.12°C accuracy) was inserted at the center point of the can for core temperature measurements. Data were analyzed with the TempIt software (Signatrol). To reduce length of coming-up time, hot water (> 53°C) was used to fill the retort. The initial temperature of the product was also up to 50°C. Sterilization values (F-values) were calculated at each temperature by Equation 1 using a reference temperature of 121.1°C.
In Equation 3, N represents the number of microorganisms expressed in CFU/g of sweet corn; ∑ Colonies is the sum of colonies in Petri dishes retained; Vmlis the volume inoculated into Petri dishes; n1 is the number of dishes considered at the first dilution retained; n2represents the number of dishes considered at the second dilution retained and d1is the factorof the first dilution retained.
Stability tests
Stability tests were carried out on all canned sweet corn samples processed at each thermal treatment according to AFNOR (NF V08-401, 1997). Two samples of sweet corn cans were incubated at 30 and 55°C respectively for seven and 21 days. The control was placed at ambient temperature (20 to 25°C). Macroscopic and microscopic analyses were done. Measurement of pH was done with 10 g of homogenized sample in 50 mL of distilled water. Difference of pH between incubated cans and control should not exceed to 0.5 units.Aerobic Mesophilic Total Count at 30°C, Yeasts and Molds, C.botulinum, Thermophilic and Mesophilic Bacillus were enumerated.
Shelf life study
Shelf life of canned sweet corn processed at five heat sterilization
treatmentswas evaluatedduring 12 month ofstorage at room temperature by following the evolution of pH, yeasts and molds, aerobic mesophilic total counts, sulfide-reducer spores of Clostridium, thermophilic and mesophilicBacillus.
Color analysis
Color measurements were made using a Minolta CR 410 Chroma Meter (Osaka, Japan) calibrated with a standard white plate. Color was evaluatedon fresh sweet corn and after each sterilization treatment in triplicate for each sample. CIE* values for color lightness (L*), greenness/redness (a*) andblueness/yellowness(b*) were used to express color characteristic of samples. Total color difference (Delta E*) was calculated using Equation 4, where subscript “0” refers to the color reading of fresh sweet corn. Fresh sweet corn was used as the reference.
Delta E*=((L0-L) 2 + (a0-a) 2 + (b0-b) 2) ½ (4)
Ascorbic acid analysis
Ascorbic acid was determined on fresh sweet corn samples and after each sterilization treatment using official methods of analysis (AOAC, 1990).
Statistical analysis
All statistical analyses were performed using SPSS 20.0. (IBM stats software). The Student-Newman-Keuls(SNK) test was used to determine difference at a=0.05.
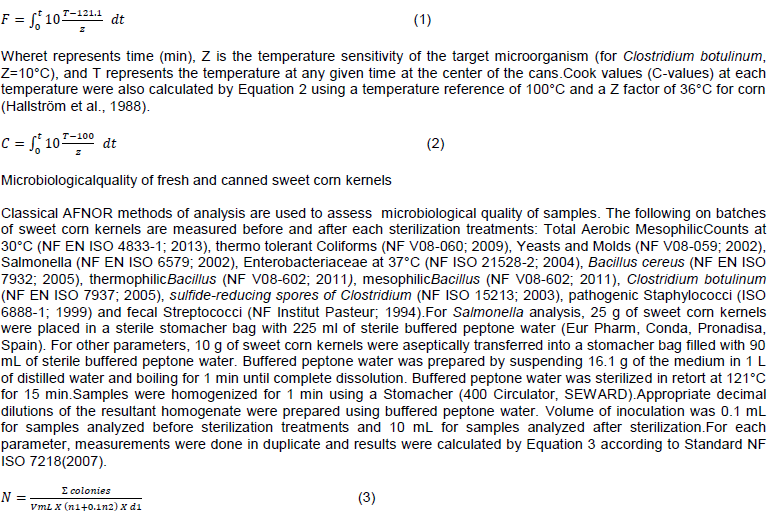
Hygiene level of sweet corn samples before canning
Table 1 shows the level of aerobic mesophilic total counts (AMC) expressed in Log10 CFU/g detected on sweet corn samples at different preliminary processing stage. The AMC was significantly highest after husking (5.4 log10 CFU/g) and lowest after blanching (1.8 log10 CFU/g). Cooling increasedthe AMC by 2 log10 CFU/g, while cutting and washing increased the AMC by 0.2 and 0.6 log10 CFU/g, respectively. There are no significant differences between cooling, cutting and washing operation while husking and blanching operations were statistically different. According to Pianetti et al. (2008), aerobic colony count does not relate to food poisoning and infections but is an indicator for food quality and shelf life. The aerobic bacterial count should be lower than 4 Log10 CFU/g for safe consumption (Khadka et al., 2017).
The exterior of vegetables is normally contaminated with bacteria and fungi. This fact could explain the AMC values found in fresh-husked sweet corn ears. Data are similar to those reported by Abadias et al. (2008) in fresh-cut vegetables (4.3 to 8.9 log10 CFU/g). Kumar et al. (2015) reported a level of AMC up to 8.4 log10 CFU/g in freshly shelled sweet corn kernels. The initial microbial load of raw material varies less or more in number according to its nature, its origin and the conditions for obtaining, transporting, and preparing (Andre et al., 2005). Blanching reducedAMC by 3.6 log10 CFU/g. Similar reduction in microbial load upon blanching (4 log10 CFU/g) has been reported for sweet corn kernels by Kumar et al.(2015). Blanching is a thermal process designed to inactivate the enzymes responsible for generating off-flavors and odors and to stabilize texture and nutritional quality and destroy microorganisms (Bahçeci et al., 2005). Furthermore, blanching is one of the stages of kernel technological production processes for consumer purposes (Szymanek et al., 2020). Many studies have demonstrated the positive effects of blanching on microbial quality of vegetables.
It is well established that fresh vegetables can be contaminated with pathogenic bacteria at any step from cultivation to consumption (Buyukunal et al., 2015). According to HACCP-TQM technical guidelines, raw foods containing < 4 log10 CFU/g; 4-6.7 log10 CFU/g; 6.7-7.7 log10 CFU/g and > 7.7 log10 CFU/g are rated as “good”, “average”, “poor” and “spoiled” respectively (Buyukunal et al., 2015). In our study, hygienic conditions of sweet corn samples were “average” after husking and cutting stages but “good” at blanching, cooling and washing steps. Therefore, to improve the hygiene level of sweet corn during canning, blanching was moved tothe last operation before can filling. Blanched sweet corn kernels were directly filled into cans followed by exhausting and seaming steps.
Effect of thermal sterilization on microbiological quality of canned sweet corn
Thermal treatment applied during processing of canned foods should destroy microorganisms which cause spoilage and foodborne illness (Mishra and Sinha, 2018). In this study, the impact of five combinations of heating temperature and holding time on the microbiological quality of canned sweet corn was evaluated (Table 2). No microorganisms related to food spoilage and public health concerns were detected in all canned sweet corn samples regardless of treatment. Indeed, C. botulinum and its related sulfide-reducer spores, Staphylococcus pathogens, mesophilic and thermophilic Bacillus, B. cereus and Salmonella were absent in canned sweet corn for all five sterilization treatments. Nevertheless, the AMC was 0.3, 0.5 and 1 Log10 CFU/g in canned sweet corn after treatments B, C and D, respectively. These were below the maximum limit of AMC (1.7 log10 CFU/g) allowed in canned vegetables (KEBS, 2016). The AMC acts as an indicator of food quality (Pianetti et al., 2008). Results indicated also the good hygiene level of sweet corn kernels before sterilization (all data were < 4 Log10 CFU/g).
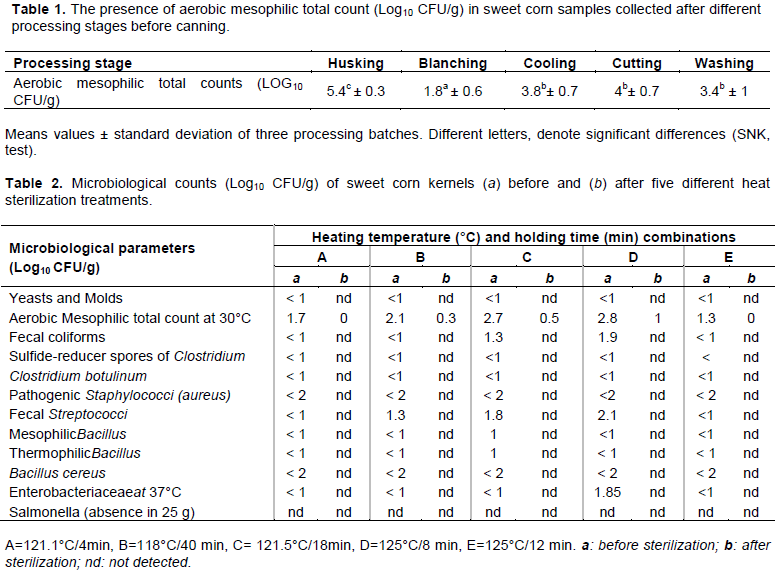
For heat thermal treatment validation, stability tests were performed on all canned sweet corn samples (Tables 3a and b). Results showed nomicro-leaks, bending, flocking and opening gas release for canned sweet corn samples incubated at 30 and 55°C. Macroscopic examination of color, texture and odor were also normal after incubation at 30 and 55°C. For treatment B, a pH difference > 0.5 between controls and samples was noticed and presence of thermophilic Bacillus in the samples incubated at room temperature and at 55°C. Canned sweet corn kernels sterilized with treatment B were not microbiologically stable and was not validated in our conditions of study.
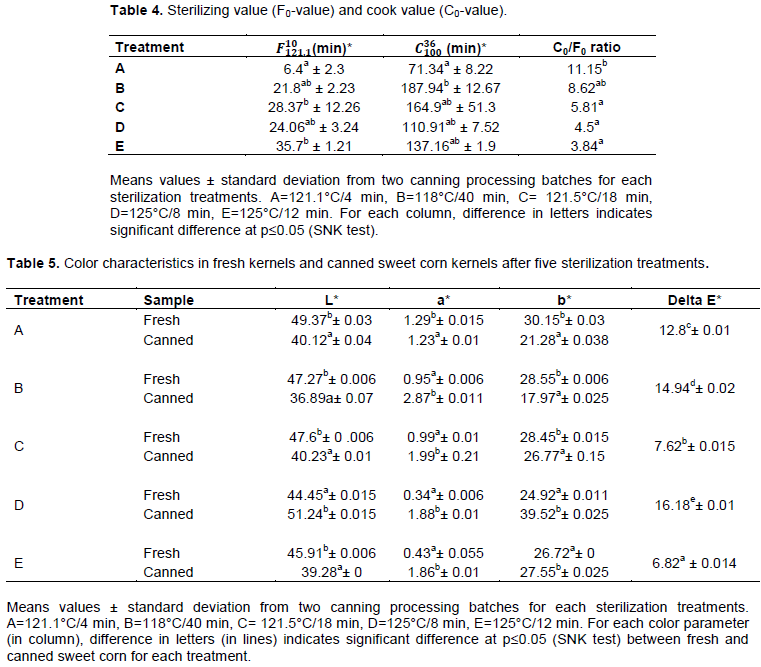
Sterilizing values (F-value) and cook values (C-value)
Sterilizing values (F-value) and cooking values (C-value) calculated from core temperatures recorded at the cold point of canned sweet corn kernels during thermal sterilization treatments are presented in Table 4. F-values for treatments C and Ewere significantly higher than treatment A. In thermal processing, pathogen survival depends on temperature and treatment time used to achieve the target lethality (Tola and Ramaswamy, 2015). According to Heinz and Hautzinger (2007), thermal processing of low-acid foods (pH > 4.6) such as sweet corn, conventionally uses sterilizing values equal to 2.58 min for destroying the spores of C.botulinum; but more severe conditions are still in need to control spoilage organisms because of mesophilic spore-forming bacteria (Clostridium sporogenes) and thermophilic bacteria (Bacillus stearothermophilus) which are more resistant than C. botulinum and could cause food spoilage (Stumbo, 1973)..Sterility can generally be accomplished when the number of viable spores in the population of mesophilic spore-forming bacteria is 10-4 after treatment time (Liato et al., 2016).While heat sterilization can kill microorganism, it also could have, in most cases, a negative impact on the overall quality of product (Mishra and Sinha, 2018).
The cook value (C-value) is a parameter for evaluating the impact of thermal processing on food. According to Sreenath et al. (2009), C-value is the measure of heat treatment with respect to nutrient degradation and textural changes that occur during processing. Thus, the cook value (C-value) should be minimized at any given F-value. Sensory parameters, texture and color of sterilized foods can be correlated with C-value/F-valueratio and can be used as an indicator to identify the process conditions that increase quality retention(Sreenath et al., 2009). In this study, treatments E, D, and C had statistically the lowest C0/F0 ratio while treatments A and B had the highest ratio. Therefore, processing canned sweet corn kernels at 125°C for 12 minwould result in better quality.
Effect on color
Color characteristics offresh and canned sweet corn kernels after five sterilization treatments are presented in Table 5. All thermal treatments had significant effect on color characteristics. Results showed that canned sweet corn kernels sterilized at 118°C for 40 min had the lowest L* parameter which led to the darkestkernels).It is well established that corn is rich in carotenoids, which are responsible to their yellow color (Gallon et al., 2013; O’Hare et al., 2015; Liato et al., 2016).According to Song et al.(2018), there was a good relationship between visual color L* value and dominant carotenoid content in sweet corn juice during thermal processing, suggesting that the lightness color value could be applied for monitoring the changes in carotenoid contents. Non- enzymatic browning at higher temperature could explain the darkness of color during heat treatment (Thakur et al., 2015). Furthermore, the combination of blanching and sterilization may contribute to the darkness color of kernels. Similar results were obtained by Liato et al. (2016) and Kachhadiya et al.(2018), where L* values decreased significantly after blanching and sterilization of sweet corn kernels. Treatment E had statistically the smallest total color change (Delta E* parameter) followed by treatment C while treatment D showed the largest total color change. According to Kachhadiya et al. (2018), the smallest total color change Delta E*, which can be assessed by human eye is 1.0, indicating noticeable change in color. A larger Delta E* denotes greater color change from the reference material (Mohammadi et al., 2008).
Effect on vitamin C
Table 6 presents the ascorbic acid content of fresh and canned sweet corn kernels sterilized at five different treatments. Ascorbic acid contents were statistically lower after treatments A, C, D and E. No significant difference was found between raw and processed kernels for treatment B.It is well established that vitamin C is unstable in foods and therefore processing and cooking caused significant losses depending on temperature, presence of oxygen, light, moisture content (Leskova et al., 2006). According to Jayathunge et al. (2015), vitamin C is very sensitive to light and oxygen and can be easily degraded by thermal treatment. The concentration of ascorbic acid was between 0.9-2.1 mg/100g in fresh kernels and between 0.53-0.77 mg/100g in canned kernels after sterilization treatment. These data were lower than those reported by Liato et al. (2016) for fresh sweet corn (3.34 mg/100g). On the other hand, ascorbic acid content of canned kernels in our study were higher than those reported by Liato et al. (2016) after treatment at 100°C for 22.27 min in electro activated brine solution (0.33 mg/100 g). Non vacuum-sealed canned sweet corn, heating and leaching into surrounding brine could explain losses in ascorbic acid noticed between fresh and canned sweet corn (Liato et al., 2016).
Shelf life study
Microbiological quality of canned sweet cornkept at room temperature was evaluated after five and 12 monthsof storage for each thermal treatment. Yeasts and Molds, sulfide-reducer spores of Clostridium, mesophilic and thermophilic Bacillus were absent in the canned sweet corn throughout the storage period regardless of treatment. The AMC after 12 months were up to 1 Log10 CFU/g for treatments A, D and E while they were less than 1 Log10 CFU/g for treatments B and C. The level of AMC in canned sweet corn kernels indicates their high hygiene level. In this study, pH values of canned sweet corn kernels were 7 to 7.2, which decreased after 12 months of storage, by 0.7, 1.1, 0.5, 0.3 and 0.1 pH units respectively for treatments A, B, C, D and E. Thus, treatment at 125°C exhibits the lowest variation in pH level. Kumar et al. (2015) had found pH value of 6.4 and 6.7 for fresh and processed sweet corn kernels.
The effects of combinations of heating temperature and holding time sterilization treatments on microbiological quality, color, vitamin C and shelf life were analyzed. Among the five sterilization regimes evaluated, treatment E (125°C for 12 min) will be recommended as processing sterilization parameters for canned sweet corn processing in this study. Indeed, canned sweet corn kernels sterilized at this condition were better in terms of microbiological stability and quality retention like color and vitamin C. The C-value/F-value ratio and total color change were also lowest at this temperature/time compared to other sterilization treatment. Nevertheless, canned sweet corn kernels were shelf stable after 12 months of storage at room temperature.
The authors have not declared any conflict of interests.
Support for this research was provided by USAID Cooperative Agreement number 685-A-00-10-00194-00 from the US Agency for International Development.
REFERENCES
|
Abadias M, Usall J, Anguera M, Solsona C, Vinas I (2008). Microbial quality of fresh minimally processed fruit and vegetables, and sprouts from retail establishments. International Journal Food Microbiology 123(1-2):121-129.
Crossref
|
|
|
|
Alan O, Kinaci G, Kinaci E, BudakBasciftci Z, Sonmez K, Evrenosoglu Y, Kutlu I (2014). Kernel Quality of Some Sweet Corn Varieties in Relation to Processing. Notulae BotanicaeHorti Agrobotanici 42(2):414-419.
Crossref
|
|
|
|
|
Andre S, Beckerich I, Bocquet D, Drouet P, Enfert E, Gasnier Y, Kowalski G, Lamy F, Laulan PY, Louvrier AL, Mercier D, Moinard V, Phelippeau M, Biton M, Cazier A, Descamps P, Zuber F (2005). Guide de Bonnes Pratiques pour l'établissement des traitementsthermiques des produitsappertisés. Avignon, CTCPA Documentation, 46p.
|
|
|
|
|
AOAC (Association of Official Analytical Chemists) (1990). Fruits and fruits products.In K. Helrich (Ed.), Official methods of analysis of the association of official analytical chemists. Arlington, USA 7:910-928.
|
|
|
|
|
Bahçeci K, Serpen A, Gökmen V, Acar J (2005). Study of lipoxygenase and peroxidase as indicator enzymes in green beans: change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. Journal of Food Engineering 66:187-192.
Crossref
|
|
|
|
|
Berry MR, Pflug IJ (2003). Canning: principles. In Caballero B, Trugo L, Finglas PM (eds), Encyclopedia of Food Science and Nutrition. San Diego, CA: Academic press, pp. 816-824.
Crossref
|
|
|
|
|
Buyukunal SK, Issa G, Aksu F, Vural A (2015). Microbiological quality of fresh vegetables and fruits collected from supermarkets in Istanbul, Turkey. Journal of Food and Nutrition Sciences 3(4):152-159.
Crossref
|
|
|
|
|
Ndiaye DN, Cissé M, DiopMbacké F, Diop A, Ndiaye S, Thompson T (2017). Physical and biochemical Characterization of Sweet Corn Ears of Four Varieties Grown in Senegal. European Scientific Journal 13(33):232-243.
Crossref
|
|
|
|
|
Erkmen O, Faruk Bozoglu T (2016). Spoilage of cannedfoods. In. Wiley J and Sons (eds), Food Microbiology: Principles into Practice. pp. 376-384.
Crossref
|
|
|
|
|
FAOSTATS (2019). Crops and Livestock's products; Senegal; Import quantity prepared or preserved Sweet corn.
|
|
|
|
|
Gallon CZ, Fuller SC, Fanning KJ, Smyth HE, Pun S, Martin IF, O'Hare TJ (2013). Increase in β-Ionone, a Carotenoid-Derived Volatile in Zeaxanthin-Bio fortified Sweet Corn. Journal of Agricultural and Food Chemistry 61(30):7181-7187.
Crossref
|
|
|
|
|
Hallström B, Skjöldebrand C, Trägårdh C (1988). Heat transfer & food products. New York: Elsevier Applied Science. 263p.
|
|
|
|
|
Heinz G, Hautzinger P (2007). Meat processing technology. RAP publication (FAO). Bangkok: Regional Office for Asia and the Pacific.
|
|
|
|
|
ISO 6888-1 (1999). Méthodehorizontale pour le dénombrement des Staphylocoques à coagulase positive. Partie 1: technique utilisant le milieu gélosé Baird Parker. Méthodeshorizontales de référence, tome 1, 8ème édition.
|
|
|
|
|
Jayathunge KGLR, Grant IR, Linton M, Patterson MF, Koidis A (2015). Impact of long-term storage at ambient temperatures on the total quality and stability of high pressure processed tomato juice. Innovative Food Science and Immerging Technologies 32:1-8.
Crossref
|
|
|
|
|
Kachhadiya S, Kumar N, Seth N (2018). Process kinetics on physico-chemical and peroxidase activity for different blanching methods of sweet corn. Journal of Food Science and Technology 55(12):4823-4832.
Crossref
|
|
|
|
|
Kenya Bureau of Standards (KEBS) (2016). Canned vegetables-Specification, DKS2685: 2016. Available at:
View
|
|
|
|
|
Khadka RB, Marasini M, Rawal R, Gautam DM, Acedo AL (2017). Effect of variety and post harvest handling practices on microbial population at different stages of the value chain of fresh tomato (Solanumlycopersicum) in West Teral of Nepal.BioMed Research international, pp. 1-6.
Crossref
|
|
|
|
|
Kumar S, Gautam S, Sharma A (2015). Hurdle technology including chlorination, blanching, packaging and irradiation to ensure safety and extend shelf life of shelled sweet corn kernels. Journal of Food Processing and Preservation 39:2340-2347.
Crossref
|
|
|
|
|
Leskova E, Kubikova J, Kovacikova E, Kosicka M, Porubska J, Holcikova K (2006). Vitamin losses: retention during heat treatment and continual changes expressed by mathematical models. Journal of Food composition and Analysis 19:252-276.
Crossref
|
|
|
|
|
Liato V, Labrie S, Benali M, Aïder M (2016). Study of the impact of a new hurdle technology composed of electro-activated solution and low heat treatment on the canned peas and corn quality and microbial safety. International Journal of Food Science and Technology 51:180-193.
Crossref
|
|
|
|
|
Mishra DK, Shina NK(2018). Principles of vegetable canning.InSiddiq M, Uebersax MA (eds.), Handbook of Vegetables and Vegetable Processing. UK: Blackwell Publishing Ltd. pp.365-380.
Crossref
|
|
|
|
|
Mohammadi A, Rafiee S, Emam-Djomeh Z, Keyhani A (2008). Kinetic Models for color changes in kiwifruit slices during hot air drying. World Journal of Agricultural Sciences 4(3):376-383.
|
|
|
|
|
More PG, Thakre SM, Khodke SU (2018). Qualityassessment of microwaveblanchedsweet corn kernels. International Journal of Agricultural Engineering 1:164-167.

Crossref
|
|
|
|
|
NF EN ISO 4833-1 (2013). Microbiologie de la chainealimentaire -Méthodehorizontale pour le dénombrement des microorganismes - Partie 1: Comptage des colonies à 30°C par la technique d'ensemencement en profondeur.
|
|
|
|
|
NF EN ISO 6579 (2002).Microbiologiealimentaire. Méthodehorizontale pour la recherche des Salmonella spp.
|
|
|
|
|
NF EN ISO 7218 (2007).Microbiologie des aliments. Exigencesgénérales et recommandations - Chapitre10.3 :Calcul et expression des résultatssur milieu solide.
|
|
|
|
|
NF EN ISO 7932 (2005a).Microbiologie des aliments. Méthodehorizontale pour le dénombrement de Bacillus cereus: méthode par comptage des colonies à 30°C.
|
|
|
|
|
NF EN ISO 7937 (2005b).Microbiologie des aliments - Méthodehorizontale pour le dénombrement de Clostridium perfringens - Technique par comptage des colonies.
|
|
|
|
|
NF Institut Pasteur (1994). Rechercheoudénombrement des Streptocoquesfécaux.Recueil de normesfrançaises 1994 - ENSP / Institut Pasteur (Lille).
|
|
|
|
|
NF ISO 15213 (2003). Microbiologie des aliments- Méthodehorizontale pour le dénombrement des bactériessulfito-réductrices se développant en conditions anaérobies.
|
|
|
|
|
NF ISO 21528 (2004). Microbiologie des aliments - Méthodeshorizontales pour la rechercheet le dénombrement des Enterobacteriaceae -partie 2: méthode par comptage des colonies.
|
|
|
|
|
NF V08-059 (2002).Microbiologie des aliments - Méthode de routine. Dénombrement des levuresetmoisissures par comptage des colonies à 25° C.
|
|
|
|
|
NF V08-060 (2009).Microbiologie des aliments. Dénombrement des coliformesthermotolérants par comptage des colonies obtenues à 44°C.
|
|
|
|
|
NF V08-401 (1997).Microbiologie des aliments - Contrôle de la stabilité des produitsappertisésetassimilés - Méthode de référence.
|
|
|
|
|
NF V08-602 (2011). Microbiologie des aliments - Dénombrement des spores dans les produitsalimentairesavanttraitementd'appertisation par comptage des colonies.
|
|
|
|
|
O'Hare TJ, Fanning KJ, Martin IF (2015). Zeaxanthinbio fortification of sweet-corn and factors affecting zeaxanthin accumulation and color change. Archives of Biochemistry and Biophysics 572:184-187.
Crossref
|
|
|
|
|
Pacurar GL, Apahidean M, Has V, Russu F, Apahidean AI, Boanta A (2019). Researches on some biological and ecological characteristics of sweet corn. Bulletin UASVM Horticulture 76(1):64-71
Crossref
|
|
|
|
|
Pankaj SK (2016). Thermal processing of food. In. RavishankarRai V, Whiley Blackwell (eds.), Advances in Food Biotechnology. UK: pp. 681-692.
Crossref
|
|
|
|
|
Pianetti A, Sabatini L, Citterio B, Pierfelici L, Ninfali P, Bruscolini F (2008). Changes in microbial populations in ready-to-eat vegetable salads during shelf life. Italian Journal of Food Science 20:245-254.

|
|
|
|
|
SCL (2019). Noslégumesfrais: le maïsdoux. Société de CultureLégumières. Available at
View
|
|
|
|
|
Siddiq M, Pascall MA (2011). Peas, Sweet corn, and Green beans. In Sinha NK (eds), Handbook of Vegetables and Vegetable Processing. UK: Blackwell Publishing Ltd, pp. 605-623.
Crossref
|
|
|
|
|
Simpson R, Abakarov A (2009). Optimal scheduling of canned food plants including simultaneous sterilization. Journal of Food Engineering 90:53-59.
Crossref
|
|
|
|
|
Song J, Meng L, Liu C, Li D, Zhang M (2018). Changes in color and carotenoids of sweet corn juice during high temperature heating. Cereal Chemistry 95(3):486-494.
Crossref
|
|
|
|
|
Sow A, Lagnane O (2011). Culture maraîchère: maïsdoux. Créneauxporteurs du secteurprimaire.Ministère de l'Economieet des Finances, Direction de l'Appui du secteur privé.18p.
|
|
|
|
|
Sreenath PG, Abhilash S, Ravishankar CN, Anandan R, Gopal TKS (2009). Heat penetration characteristics and quality changes of Indian mackerel (Rastrelligerkanagurta) canned in brine at different retort temperatures. Journal of Food Process Engineering 32:893-915.
Crossref
|
|
|
|
|
Stumbo CR (1973). Thermo bacteriology in food processing. New York: Academic Press. 336p.
|
|
|
|
|
Szymanek L, Dziwulska-Hunek A, Tanas W (2020). Influence of blanching time on moisture, sugars protein and processing recovery of sweet corn kernels. Processes 8:340-346.
Crossref
|
|
|
|
|
Thakur S, Kaur A, Singh N, Virdi AS (2015). Successive reduction dry milling of normal and waxy corn: grain, grit and flour properties. Journal of Food Science 80:1144-1155.
Crossref
|
|
|
|
|
Tola YB, Ramaswamy HS (2015). Microbiological design and validation of thermal and high pressure processing of acidified carrots and assessment of product quality. Journal of Food Processing and Preservation 39:2991-3004.
Crossref
|
|
|
|
|
Yu D, Bu F, Hou J, Kang Y, Yu Z (2016). A morel improved growth and suppressed Fusariuminfection in sweet corn. World Journal of Microbiology and Biotechnology 32(12):192.
Crossref
|
|