ABSTRACT
This study aimed at substituting animal proteins by Mucuna flour and protein concentrate in beef sausage. Mucuna pruriens var pruriens grains were first transformed into flour (500 µm) from which the protein concentrate was obtained. The extraction process was done in an aqueous medium at pH 4.5. Following the characterization of the flour and protein concentrate, 5 sausages samples (3 units per sample) with varying rates of incorporation of flour and protein concentrates (25, 50, 75 and 100%) as well as a reference made with 100% beef were produced. Quality of emulsion sausages was evaluated in terms of physicochemical and functional characteristics. The results obtained showed that protein content of Mucuna flour and protein concentrate was significantly different (p < 0.05), with values of 29.92 ± 0.51 and 59.74 ± 0.32%, respectively. For the functional properties, water retention capacity (333.32 g/100 g/100 g), emulsifying capacity (60.44%), jellification capacity (12 g/100 g) and foaming capacity (52.38%) were higher in the flour sample; whereas the oil retention capacity (290.33 g/100 g DMC) was higher in the protein concentrate. Incorporation rate had a significant influence (p<0.05) on the physicochemical and functional properties of the sausage samples produced, since the technological yield was observed to vary from 73.10±0.71 (S25) to 99.49±0.05% (S100), hardness from 3.43 ± 0.35 (S100) to 3.94 ± 0.05 N (S25) and the water retention capacity varied from 33.34±0.79 (S25) to 51.93±0.045% (S100). These parameters were observed to increase with an increase in the rate of substitution.
Key words: Mucuna flour, Mucuna protein concentrate, beef sausage, functional characterizations.
Meat and meat products are nutritionally rich, providing a wide range of nutrients, such as proteins, fats, minerals and vitamins (Cosgrove et al., 2005). They have long been considered as a highly desirable and nutritious food, and have become a mass consumer product throughout the world with the highest consumption rates being recorded in industrialized western countries.A significant percentage of the recommended dietary requirements for proteins, vitamins-B, magnesium, iron and zinc are contributed by red meat and poultry (Pearson and Brooks, 1978). With the growing need for products with less fat or calorie content, it becomes necessary to develop meat products that are pertinent to consumer demand. Hence, basic formulations tend to evolve to account not only food safety concerns but also for changing economic conditions, raw material availability, consumer trends, and adaptation from one region to another (James and Lamkey, 1998). Several studies have demonstrated possible use of food hydrocolloids such as carrageenan, cellulose gum, konjac flour, guar gum and xanthan gum as fat replacements or the use of poultry meat as replacement for red meat in reduced-fat meat products (Troutt et al., 1992; Chin et al., 1998; Andrès et al., 2006; Bhattacharyya et al., 2007). Konjac flour, a glucomannan polysaccharide gum, has been used as a fat replacer in low-fat prerigor fresh pork sausages (Osburn and Keeton, 1994), low-fat bolognas (Chin et al., 1998), reduced-fat pork sausages (Akesowan, 2002a) and Thai traditional minced and preserved pork products (Akesowan, 2002b). In the same trend, soy proteins, one of non-meat proteins, are widely used as meat binders because of their several functionalities such as water-holding, binding and emulsifying properties (Arrese et al., 1991). Ahn et al. (1999) showed that addition of soy proteins resulted in better binding and texturization of sausages. Upon incorporation into comminuted meat, soy proteins improve physical and chemical properties of processed meat products such as frankfurters and ground meat patties (Alvarez et al., 1990). However, beany flavour of soy proteins also limits their expanded applications in foods (Ho et al., 1997). But concerns about high-cholesterol, allergens, animal welfare, the food industries impact on the environment, and high food costs have, however, resulted in an increased interest in using legume protein as meat replacers in foods (Hughes et al., 2011). Recent studies on Mucuna pruriens var. pruriens, another legume promoted by smallholder farmers in Africa, showed rich protein content (23 to 35%) (Mugendi et al., 2010) but remain a minor food crop due to the presence of antinutrients (Ngatchic et al., 2013). The effects of these anti-nutritional factors such as antitrypsin factors, tannins, anticoagulants, phytates and 3,4-dihydroxy-L-phenylalanine (L-Dopa) (Ravindran and Ravindran, 1988; Rosenthal et al., 1989), on the body are known as the causes of poor protein digestibility, reduce food intake, nutrients availability and can provoke deleterious effects on many organs (Esenwah and Ikenebomey, 2008). Some vegetables proteins, either soy or pea, were used as fillers or extenders to enhance the texture, stability of emulsion, replace the fat and to lower the cost (Edanane and Bernard, 2014). Since Mwasaru et al. (1999), Rangel et al. (2004) and Ngatchic et al. (2013) showed that techniques employed to obtain protein concentrates or isolates are effective in the elimination of the antinutrients in Mucuna seed, flour and protein concentrate can be incorporated in food preparations.
Therefore, the purpose of this study was to determine the effect of Mucuna flour and protein concentrate on physicochemical and textural properties of beef sausages.
Beef semi-membranous muscle (top round), beef bump fats and casings (sheep gut of about 20 mm diameter) were obtained from local processors (Ngaoundere, Cameroon). Beef muscles were trimmed of all visible extra-muscular fat and connective tissue before storage at 4°C for 72 h. Sheep guts were separated immediately after slaughtering, emptied, rinsed and stored in brine at 4°C until sausages were produced. Seed of M. pruriens were purchased from local markets in Ngaoundere (Cameroon) and manually separated from infested seeds impurities.
Preparation of Mucuna bean flours
The flours were produced from seeds legumes according to the method of Kaptso (2008). The seeds were soaked at ambient temperature (25°C) for overnight in tap water with bean to water ratio of 1 to 10 (w/v). After soaking, seeds were dried for 24 h at 50°C and dehulled manually. The dehulled seeds were ground to flour using a hammer mill and sieved with the 500 µm mesh sieve and stored in polyethylene bags at 4°C until analysis.
Preparation of protein concentrate
The Mucuna protein concentrate was prepared according to the process described by Wolf (1970) with minor modifications. Defatted Mucuna flour were dispersed in de-ionized water (1:10, w/v) at room temperature and the pH of the dispersion adjusted to 4.5 by addition of 1N HCl, stirred using a magnetic stirrer for 1 h. The slurry was then centrifuged (10,000 g, 30 min, 4°C) in a CR22G centrifuge (Hitachi Koki Co., Hitachinake, Japan). The precipitate was washed with de-ionized water, re-dissolved in de-ionized water, neutralized to pH 7.0 with 1 N NaOH at room temperature, and then freeze-dried.
Beef sausage processing
The emulsion sausage was prepared from a comminuted mixture of meat, fat, salt, condiments, spices mixtures and Mucunaflouraprotein concentrate at a level of 0, 25, 75 and 100% of substitution of meat batter. The beef lean meat (2 × 2 × 2 cm3 cut) cured with nitrite salts (NaCl:NaNO2 = 99.4:0.6) and beef bump fats (2 × 2 × 2 cm3 cut) were ground in a grinder (Manurhing type cutter: C-301, France) for 10 min, and then ice cubes were added and further comminuted for 5 min. As the mix absorbed the moisture received from molten ice, the other ingredients like salt, spices, condiment, and starches were added and chopping was further continued for 5 min and the end temperature in range of 14 to 16°C. Entire mix was filled in the stuffing machine and casing (sheep gut of about 20 mm diameter) was used for filling sausage. The finished sausage was cooked in sausage cooker for 20 min at 110°C temperature. Cooked sausages were exposing to chilled water and packed in nylon-polyethylene bags. The finished sausages were stored at 4°C for future study.
Proximate analysis of Mucuna flour, concentrate and sausages
All sausages, Mucuna flour and proteins concentrates were analysed for moisture, fat and ash contents according to the methods of AOAC (2000), numbers 950.46, 960.39 and 920.153, respectively. The protein content of samples was determined by the micro-Kjeldhal method (2000) through the use of the protein-nitrogen coefficient of 5.30 (Sze-Tao and Sathe, 2000). Carbohydrates were determined according to the method of Fisher and Stein (1961). The contents were expressed on a dry weight basis for Mucuna flour and protein concentrates and on fresh weight basis for sausages. Each analysis was done in triplicate, and data were reported as means ± standard deviation.
Bulk density
The bulk density was determined according to the method described by Adeleke and Odedeji (2010).
Functional properties of Mucuna flour and protein concentrate
Emulsifying activity (EA)
EA was determined according to the method described by Naczk et al. (1985) with some modifications. Samples (3.5 g) were homogenized at a speed of 10,000 rpm for 1 min at room temperature (about 25°C) in 25 ml de-ionized water. The sample solution was mixed with 25 ml of soybean oil followed by homogenization at a speed of 10,000 rpm for 1 min. Finally, the emulsion was centrifuged at 1300 g for 5 min. All analysis was performed in triplicate. Emulsifying activity was determined by:
Foaming capacity (FC) and foam stability (FS)
FC and FS were based on the method described by Sze-Tao and Sathe (2000) with minor modifications. 3 g samples were dispersed in 100 ml of de-ionized water. The solutions were stirred at a speed of 10,000 rpm for 2 min. The blend was immediately transferred into a 250 ml graduated cylinder. The volume was recorded before and after stirring. FC was expressed as the volume (%) increased due to stirring. For the determination of FS, foam volume changes in the graduated cylinder were recorded at 30 min of storage. All analysis was performed in triplicate. Foam capacity and foam stability were then calculated according to the following formulae:
Fat absorption capacity (FAC)
FAC was determined using the method described by Phillips et al. (1988) with minor modifications. 1 g of sample was weighed into 15 ml pre-weighed centrifuge tube and thoroughly mixed with 5 ml soybean oil. The emulsion was incubated at room temperature (about 25°C) for 30 min and then centrifuged at 5000 g for 30 min at 25°C. Then the supernatant was carefully removed, and the tube was reweighed. All analysis was performed in triplicate. FAC (gram of oil per gram of sample) was determined by:
where F0 is the weight of the dry sample (g), F1 is the weight of the tube plus the dry sample (g), and F2 is the weight of the tube plus the sediment (g).
Water absorption capacity (WAC)
WAC was determined using the method described by Rodriguez-Ambriz et al. (2005) with minor modifications. 1 g of sample was weighed into 15 ml pre-weighed centrifuge tube. Then, 10 ml of distilled water was added in small increments to the tube under continuous stirring with a glass rod. After being held at room temperature (about 25°C) for 30 min, the tube was centrifuged at 2000 g for 20 min. In the end, the amount of added distilled water resulting in the supernatant liquid in the test tube was recorded. All analysis was performed in triplicate. WAC expressed as grams of water per gram of sample, was calculated by:
where W0 is the weight of the dry sample (g), W1 is the weight of the tube plus the dry sample (g), and W2 is the weight of the tube plus the sediment (g).
Least gelation concentration (LGC)
LGC was estimated according to the method described by Deshpande et al. (1982). Samples of starch, 2 to 18% (w/v), were prepared in test tubes with 5 ml distilled water. The starch suspensions were mixed with a Vari-whirl mixer for 5 min. The test tubes were heated for 30 min at 80°C in a water bath, followed by rapid cooling under running cold tap water. The test tubes were further cooled at 4°C for 2 h. LGC was determined as that concentration when the sample from the inverted test tube did not fall down or slip.
Properties of sausages
pH determination
Raw and cooked sausages (10 g) were homogenised with 90 ml of distilled water and the pH was determined with a pH-meter (Eutech Cybernetics, Cyberscan 1000, Singapore) (AOAC, 1980).
Water holding capacity (WHC)
The Tsai and Ockerman (1981) press technique was used with some modification to measure the WHC of the raw sausages. A sample (0.5 g) was placed between 2 sheets of filter paper (Whatman no. 1, stored over saturated KCL) which was placed between two Plexiglas sheets and pressed for 30 min under 1 kg load. The area of pressed meat and a spread juice was measured and the water holding capacity was calculated as follows:
WHC = 100 – % Free Water
Cooking yield
After formulating sausages and placing them in casings of known weight, the sausages were weighed and then place in the sausage cooker. At the end of the cooking period, they were left to cool and then weighed again.
2-Thiobarbituric acid (TBA) values
The degree of lipid oxidation of the raw and cooked beef sausages was determined by the TBA cold extraction method, described by Wite et al. (1970). The results are expressed as mg malonaldehyde/kg of sausages.
Texture measurement
The texture, based on a compression test, was measured using texturometer (Brookfield Texturometer LFRA 4500 TA 115). The resistance to a given deformation (compression), characteristic of the hardness were measured and expressed in Newton (N). During the compression test, samples were cut in squares of 30 mm and a thickness of 10 mm, then placed on the stage of a texturometer and an aluminium cylinder probe with a 25 mm diameter, at a speed of 1.0 mm/s with a trigger of 5 g was used to compress the product to 60% of its original thickness. Two parameters were recorded: peak load and final load. The equal values of peak load and final load for a sample means it texture homogeneity.
Statistical analysis
The effect of each treatment was analyzed from the different preparations. Data were subjected to analysis of variance and the differences among means were obtained using Duncan’s multiple range test (significance p<0.05) using Statgraphics plus 5.0 software.
Physico-chemical characterization of Mucuna flour and protein concentrate
The proximate composition and bulk density of Mucuna flour and protein concentrate is shown in Table 1. Mucuna flour was used as starting material for the preparation of Mucuna protein concentrate. Protein content of Mucuna flour and protein concentrate was significantly different (p < 0.05), with values of 29.92 ± 0.51 and 59.74 ± 0.32%, respectively.
The protein content increased by about 50% from flour to protein concentrate. The same trend was observed for lipid and crude fibres while its density, ash and carbohydrate contents decreased significantly (p < 0.05) from 0.67 to 0.43, 3.20 to 2.16 and 20.66 to 16.85%, respectively (Table 1). The variation observed between the two samples may be attributed to the extraction method used.
In order to evaluate if a protein is applicable and suitable in certain food systems and food products, it is important to characterize the functionalities of the protein (Kinsella, 1982; Vaclavik and Christian, 2003). Some functional properties of flour and proteins concentrates of M. pruriens var. pruriens are presented in Table 2. LGC, used as the index of gelation, indicated that Mucuna flour exhibited high gelation ability than Mucuna protein concentrate, 12 and 20%, respectively. The difference observed here can be linked to the difference in carbohydrate content (20.66± 1.9 and 16.85± 3.30%, respectively) of the two samples. According to Lawal and Adebowale (2005), gel strength depends on strength of intra-granular binding forces within swollen starch granules. In this respect it can be believed that intra-granular bonding forces are higher in Mucuna flour. This observation corroborated the results of water absorption capacity.
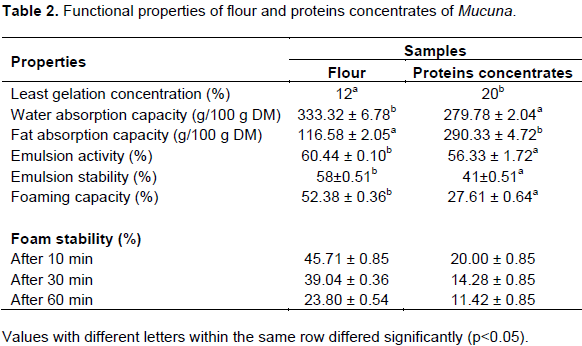
WAC of both samples were significantly different (p < 0.05), with flour having the highest WAC (333.32 ± 6.78 g/100 g). This was consistent with other studies (Prinyawiwatkul et al., 1997). Protein water absorption capacity of proteins is a function of several parameters, including size, shape, steric factors, conformational characteristics, hydrophilic-hydrophobic balance of amino acids in the protein molecules as well as lipids, carbohydrates and tannins associated with proteins. Carbohydrates contain hydrophilic parts, such as polar or charged side chains, which can enhance WAC (Jitngarmkusol et al., 2008). Flour water absorption capacity was enhanced, as the flour carbohydrate content (20.66 ± 1.95%) was significantly higher (p < 0.05) than that of protein concentrates (16.85 ± 3.30%). WAC value of Mucuna flour and protein concentrates were not significantly different than 3.551 g/g of commercial soy protein isolate reported by Zhu et al. (2010).
FAC of both samples were significantly different (p < 0.05), with protein concentrate having more than twice FAC (290.33 ± 4.72 g/100 g) than flour (116.58 ± 2.05 g/100 g). The differences in fat absorption capacity between the samples might be due to the presence of more non-polar amino acids in protein concentrates than in flour, and also due to the partial denaturation of proteins with exposure of hydrophobic amino acid groups during the protein concentrate production process. The presence of several non-polar side chains may bind the hydrocarbon chains of fats, thereby resulting in higher absorption of oil (Sathe et al., 1982). El Nasri and El Tinay (2007) reported that surface area and hydrophobicity improve fat absorption capacity and also high protein content shows high FAC. Campell et al. (1992) reported that FAC increased as protein content increased in sunflower and soy protein products. FAC values of Mucuna proteins concentrates was higher than 2.434 g/g of commercial soy protein isolate reported by Zhu et al. (2010). The ability of protein to bind fat is very important for applications as meat replacement and extenders, principally because it enhances flavour retention, and reputedly improves mouth feel (Ogunwolu et al., 2009). Results obtained indicated that Mucuna protein concentrates have good oil absorption capacity. High FAC of Mucuna protein concentrate renders it a good ingredient in cold meat industry, particularly for sausages, where the protein usually bridges the fat and
water in order to obtain good products. EA of Mucuna flour and protein concentrate, was significantly different (p < 0.05) with values of 60.44 ± 0.10 and 56.33 ± 1.72%, respectively. The results were in agreement with Chove et al. (2001), who stated that the emulsifying capacity of proteins tends to decrease as protein concentration is increased and this was also consistent with the results reported on winged bean protein concentrate (Sathe et al., 1982), sunflower protein isolate (Lin et al., 1974) and cashew nut protein concentrate and isolate (Ogunwolu et al., 2009). The same trend was observed for ES.
FC of flour was significantly higher than that of protein concentrate, with values of 52.38 ± 0.36 and 27.61 ± 0.64%, respectively. FS of flour was significantly higher than that of protein concentrates, and this stability decrease with time (Table 2). The results suggested that the flour had a more flexible protein structure in aqueous solutions and interacted strongly at the air-water interface to form more stable foams when compared with the protein concentrates.
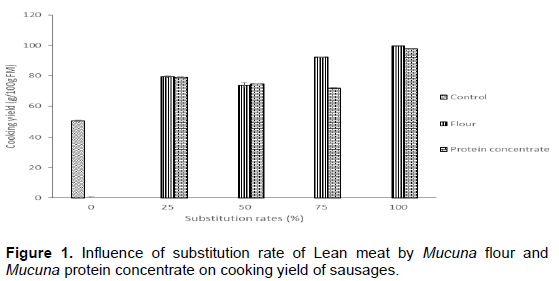
Cooking yield of sausages with Mucuna flour and protein concentrate at different rates as replacement for beef meat is shown in Figure 1. Cooking yield values exhibited a significant (p<0.05) increasing trend with incorporation of Mucuna flour and protein concentrate as compared to control. Indeed, Chin et al. (1998) reported that textural modifying ingredients, such as non-meat proteins or gums would be added in low-fat meat products to retain added water not to loss during cooking. At the same incorporation rate of Mucuna flour and protein concentrate, no significant differences (p>0.05) were observed on cooking yield of sausages except at 75 % incorporation rate. This was probably link the difference of WAC and FAC of Mucuna flour and protein concentrate. The beneficial effect of Mucuna flour and protein concentrate on reducing purge loss was similar to that reported by Chin et al. (2000) who found that low-fat bolognas containing 1% soy protein isolate had reduced purge loss after processing and during refrigerated storage.
The pH affects many functional properties such as color, flavor and texture of food, although the pH of food is important for microbial growth. The variations of pH of raw and cooked sausages with different substitutions rates of Mucuna flour and protein concentrate are shown in Table 3. Incorporation of Mucuna flour influence significantly pH of raw and cook sausages (p<0.05), the same trend was observed for sausages incorporated with Mucuna protein concentrate. The decrease of pH with the different substitution could be mainly due to the pH value of Mucuna flour and protein concentrate.
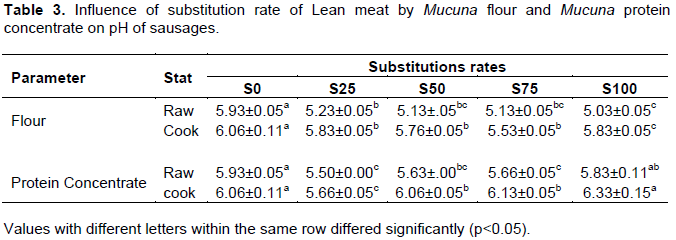
There is a general increase in pH of sausages after cooking compared to the raw. The increase of pH in sausages after cooking may be due to either the hydrolysis of protein during cooking with the release of peptide and amino acids leading to the increase of pH of the medium or the loss of short chains organic acids produced during maturation of meat released during cooking. The increase in pH of cooked samples compared to the raw samples has been reported by Manish et al. (2007) using sodium alginate as a fat replacer in pork patties.
WRC is important for the formation of gels and emulsions. WHC of the control (25.49±0.85 g/100 g FM) was significantly lower (P<0.05) than that of the substituted samples (Figure 2). These results clearly show that the substituted samples, which consisted of 25 to 100% substitution of Mucuna flour and protein concentrates of lean meat in sausages had a higher ability to hold water compared to the control. Despite the fact that protein is well-known for its ability to hold oil and water and form a stable emulsion due to the lipophilic and hydrophilic groups in the same polymer chain. Physical and chemical properties, such as size, shape, amino acid composition and sequence, net charges, hydrophobicity to hydrophilicity ratio, structures, molecular flexibility and the ability to interact with other components as starches also affect WHC (Egbert and Payne, 2009; Brewer, 2012).
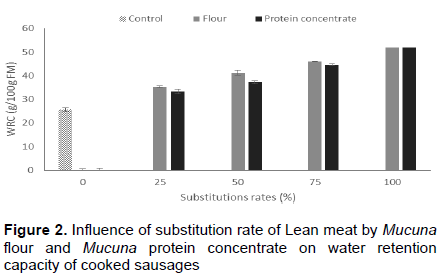
The imitation sausage incorporated with Mucuna flour and proteins concentrates keeps more water. Protein and carbohydrate brought by Mucuna flour and protein concentrates might have increased hydration, solubility, emulsification and gelation, which are the most important characteristics needed to qualify the stability and acceptability of end products. This observation is confirmed by the increase of WRC with substitution rates of Mucuna flour and protein concentrate in sausages.
The hardness of different sausages is presented in Figure 3. There were a general drop (p<0.05) of hardness of sausages with the in corporation of Mucuna flour and protein concentrate.
The high moisture content and high water binding capacity and even oil absorption capacity of Mucuna flour and protein concentrate might be a contributing factor to the lower hardness in sausages. Similar results have been reported by Arun et al. (2008) using soy bean paste in goat meat nuggets and Khalil (2000) using modified corn starch paste in beef patties as fat replacers. Also, the protein content is responsible for the hardness, as rheological parameters are strongly influenced by protein concentration in processed muscle foods such as sausage (Colmenero et al., 1995).
TBA values of sausages are important property, which relates to the quality and shelf life of the product (Figure 4).
TBA values of emulsion sausages incorporated with levels of (25, 50, 75 and 100%) were found to be higher (p<0.05). At the same rate of incorporation, sausages containing Mucuna protein concentrate are more oxidised (p<0.05) than they homologues containing Mucuna flour. The presence of minerals such as iron (pro-oxidant) and the type of lipids in Mucuna flour and protein concentrate can explain the increase in lipids oxidation of sausage (Zanardi et al., 2004).
Results from this study indicate that the composition of Mucuna flour and protein concentrates were different. Also, functional properties and bulk density of Mucuna protein concentrate were different from that of Mucuna flour. Finally, Mucuna flour and protein concentrate exhibited satisfactory functional properties as required in meat products, that is, high fat and water absorption capacity, good emulsion activity and stability. What has been confirmed by increasing of cooking yield with incorporation of Mucuna flour and protein concentrate as compared to control. There were also a general drop (p<0.05) of hardness of sausages with the incorporation of Mucuna flour and protein concentrate.
The authors have not declared any conflict of interest.
REFERENCES
|
Adeleke RO, Odedeji JO (2010). Functional Properties of Wheat and Sweet Potato Flour Blends. Pak. J. Nutr. 9(6):535-538.
Crossref
|
|
|
|
Ahn H, Hsieh F, Clarke AD, Huff HE (1999). Extrusion for producing low-fat pork and its use in sausage as affected by soy protein isolate. J. Food Sci. 6:267-271.
Crossref
|
|
|
|
Akesowan A (2002a). Reduced fat, added konjac gel pork sausage as affected by chopping times. J. Int. Soc. Southeast Asian Agric. Sci. 7:17-30.
|
|
|
|
Akesowan A (2002b). Effect of salt and added water (ice) contents on the yield, physical and sensory properties of low-fat Moo Yo containing a konjac gel. Thai J. Agric. Sci. 35:63-73.
|
|
|
|
Alvarez VB, Smith DM, Morgan RG, Booren AM (1990). Restructuring of mechanically deboned chicken and nonmeat binders in a twinscrew extruder. J. Food Sci. 55:942-946.
Crossref
|
|
|
|
Andrès S, Zaritzky N, Califano A (2006). The effect of whey protein concentrates and hydrocolloids on the texture and colour characteristics of chicken sausages. J. Food Sci. Technol. 41:954-961.
Crossref
|
|
|
|
AOAC (1980). Official Methods of Analysis of the Association of Official Analytical Chemists (éd. 13th) Washington, DC.
|
|
|
|
AOAC (2000). Official Methods of Analysis of AOAC (éd. 17th). (A. international, Éd.) Gaithersburg, USA.
|
|
|
|
Arrese EL, Sorgentini DA, Wagner JR, 80 5 Anon MC (1991). Electrophoretic, solubility and functional properties of commercial soy protein isolates. J. Agric. Food. Chem. 39:1029-1032.
Crossref
|
|
|
|
Arun K, Das ASR, Anjaneyulu H, Pragati (2008). Effect of full-fat soy past and textured soy granules on quality and shelf life of goat meat nuggets in frozen storage. Meat. Sci. 80(3):607-614.
Crossref
|
|
|
|
Bhattacharyya D, Sinhamahapatra M, Biswas S (2007). Preparation of sausage from spent duck-an acceptability study. J. Food Sci. Technol. 42:24-29.
Crossref
|
|
|
|
Brewer MS (2012). Reducing the fat content in ground beef without sacrificing quality: a review. Meat Sci. 91:385-395.
Crossref
|
|
|
|
Campbell NF, Shih FF, Marshall WE (1992). Enzymic phosphorylation of soy protein isolate for improved functional properties. J. Agric. Food Chem. 40: 403-406.
Crossref
|
|
|
|
Chin KB, Keeton JT, Longnecker MT, Lamkey JW (1998). Low-fat bologna in a model system with varying types and levels of konjac blends. J. Food Sci. 63: 808-813.
Crossref
|
|
|
|
Chin KB, Keeton JT, Miller RK, Longnecker MT, Lamkey JW (2000). Evaluation of konjac blends and soy protein isolate as fat replacements in low-fat bolognas. J. Food Sci. 65: 756-763.
Crossref
|
|
|
|
Chove BE, Grandison AS, Lewis MJ (2001). Emulsifying properties of soy protein isolate fractions obtained by isoelectric precipitation. J. Sci. Food Agr. 81: 759–763.
Crossref
|
|
|
|
Colmenero FJ, Barreto G, Mota N, Carballo J (1995). Influence of protein and fat content and cooking temperature on texture and sensory evaluation of bologna sausage. LWT – Food. Sci Technol. 28: 481-487.
|
|
|
|
Cosgrove M, Flynn A, Kiely M (2005). Consumption of red meat, white meat and processed meat in Irish adult in relation to dietary. Brit. J. Nutr. 93:933-942.
Crossref
|
|
|
|
Deshpande SS, Sath SK, Cornforth D, Salunk OK. (1982). Effects of dehulling on functional properties of dry bean (Phaseolus vulgaris L.) flours. Cereal Chem. 59: 396-401.
|
|
|
|
Edanane G, Bernard R (2014). University of Illinois .National Soybean Research Laboratory. Consulté le Juillet 2016, sur NSRL. 2014. Edanane and Garden soy.
View
|
|
|
|
Egbert WR, Payne CT (2009). Plants proteins. As ingredients in meat products: Properties, functionality and applications. (R. Tarte, Éd.) New York: Springer Science Business Media, LLC.
|
|
|
|
El Nasri NA, El Tinay AH (2007). Functional properties of fenugreek (Trigonella foenum-graecum) protein concentrate. Food Chem. 103:582-589.
Crossref
|
|
|
|
Esenwah CN, Ikenebomey MJ (2008). Processing effect on the nutritional and antinutritional content of African locust bean (Parkia Bliglobosa benth.) Bean. Pak. J. Nutr. 7(2): 214-217.
Crossref
|
|
|
|
Fisher EH, Stein EA (1961). DNS colorimetric Determination of Available Carbohydrates in foods. NS colorimetric Determination of Available Carbohydrates in foods. Biochem. Prep. 8:30-37.
|
|
|
|
Ho KG, Wilson LA, Sebranek JG (1997). Dried soy tofu powder effects on frankfurters and pork sausages patties. J. Food Sci. 62:434-437.
Crossref
|
|
|
|
Hughes GJ, Ryan DJ, Mukherjea R, Schastee CS (2011). Protein Digestibility-Corrected Amino Acid Scores (PDCAAS) for Soy Protein Isolates and Concentrate: Criteria for Evaluation. J. Agric. Food Chem 59:12707-12712.
Crossref
|
|
|
|
James W, Lamkey (1998). Non-Meat Ingredients for Meat Processing. Reciprocal Meat Conference Proceedings. 51: 48-52.
|
|
|
|
Jitngarmkusol S, Hongsuwankul J, Tananuwong K (2008). Chemical compositions, functional properties, and microstructure of defatted macadamia flours. Food Chem. 110: 23-30.
Crossref
|
|
|
|
Kalil AH (2000). Quality characteristics of low-fat beef patties formulated with modified starch and water. Food Chem. 68(1): 61-68.
Crossref
|
|
|
|
Kaptso KG (2008). Potentiel technologique des farines de niébé (vigna unguiculata) et de voandzou (Vigna subterranea) pour la préparation du koki (gâteau de pate cuite à la vapeur). Thèse de Doctorat, Ngaoundéré (Université de Ngaoundéré, Cameroun). 200p.
|
|
|
|
Kinsella J (1982). Relationships between structure and functional properties of food proteins. Food proteins. (F. P. J.J., Éd.) England: Applied science publishers LTD.
|
|
|
|
Lawal OS, Adebowale KO (2005). Physicochemical characteristics and thermal properties of chemically modified jack bean (Canavalia ensiformis) starch. Carbohydr. Polym. 60(3):331-341.
Crossref
|
|
|
|
Lin M, Humbert E, Sosulski F (1974). Certain functional properties of sunflower meal products. J. Food Sci. 39:368-370.
Crossref
|
|
|
|
Manish K, Sharma BD, Kumar RR (2007). Evaluation of sodium alignate as fat replacer on processing and shelf-life of low-fat. Asian-Aust. J. Anim. Sci. 20(4):588-597.
|
|
|
|
Mugendi JB, Njagi EN, Kuria EN, Mwasaru MA, Mureithi JG, Apostolides Z (2010). Effects of processing technique on the nutritional composition and antinutrient content of Mucuna bean (Mucuna pruriens L.). Afr. J. Food Sci. 4(4):156-166.
|
|
|
|
Mwasaru MA, Muhammad K, Bakar J, Che Man YB (1999). Effects of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeon pea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. Physicochemical properties. Food Chem. 67:435-443 .
Crossref
|
|
|
|
Naczk M, Diosady LL, Rubin LJ (1985). Functional properties of canola meals produced by a two phase's solvent extraction system. J. Food Sci. 50:1685-1692.
Crossref
|
|
|
|
Ngatchic TJ, Metsagang, Njintang YN, Oben JE, Mbofung CM (2013). Protein quality and antigrowth effect of protein isolate of Mucuna (Mucuna pruriens) and Canavalia (Canavalia ensiformis) seeds. SAJB 5(1):183-191.
|
|
|
|
Ogunwolu SO, Henshaw FO, Mock HP, Santros A, Awonorin SO (2009). Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) Nut. Food Chem. 115:852-858.
Crossref
|
|
|
|
Osburn WN, Keeton JT (1994). Konjac flour gel as fat substitute in lowfat prerigor fresh pork sausage. J. Food Sci. 59:484-489.
Crossref
|
|
|
|
Pearson AM, Brooks RF (1978). The contribution of meat to the diet of man. Proc. Relip. Meat Conf. 31:64p.
|
|
|
|
Phillips RD, Chinnan MS. Branch AL, Miller J, Mc Watter KH (1988). Effects of pre-treatment on functional and nutritional properties of cowpea meal. J. Food Sci. 3:805-809.
Crossref
|
|
|
|
Prinyawiwatkul W, Beuchat LR, McWatters KH, Phillips RD (1997). Functional properties of cowpea (Vigna unguiculata) flour as affected by soaking, boiling, and fungal fermentation. J. Agric. Food Chem. 45:480-486.
Crossref
|
|
|
|
Rangel A, Saraiva K, Schwengber P, Narciso MS, Domont GB, Ferreira ST, Pedrosa C (2004). Biological evaluations of a protein isolate from cowpea (Vigna unguiculata) seeds. Food Chem. 87:491-499.
Crossref
|
|
|
|
Ravindran V, Ravindran G (1988). Nutritional and anti-nutritional characteristics of Mucuna (Mucuna utilis) beans seeds. J. Sci. Food Agric. 46:71-79.
Crossref
|
|
|
|
Rodriguez-Ambriz S, Martinez-Ayala A, Millan F, Davila-Ortiz G (2005). Composition and functional properties of Lupinus campestris protein isolates. Plant Foods Hum. Nutr. 60:99-107.
Crossref
|
|
|
|
Rosenthal GA, Berge MA, Bleiler JA (1989). Novel mechanism for detoxification of L canaline. Biochem Syst. Ecol. 17:203-206.
Crossref
|
|
|
|
Sathe S, Deshpande S, Salunkhe D (1982). Functional properties of winged bean (Psophocarpus tetragonolobus L. DC) proteins. J. Food Sci. 47:503-509.
Crossref
|
|
|
|
Sze-Tao K, Sathe S (2000). Functional properties and in vitro digestibility of almond (Prunus dulcis L.) protein isolate. Food Chem. 69:153-160.
Crossref
|
|
|
|
Troutt E, Hunt MC, Johnson DE, Claus JR, Kastner DH (1992). Characteristics of low-fat ground beef containing texturemodifying ingredients. J. Food Sci. 57(57):19-24.
Crossref
|
|
|
|
Tsai TC, Ockerman HL (1981). Water-binding measurement of meat. J. Food Sci. 46:697-701, 707.
Crossref
|
|
|
|
Vaclavik V, Christian E (2003). Essentials Of Food Science (éd. 2nd). (K. Academic, Éd.) New York: Plenum Publishers.
Crossref
|
|
|
|
Wite VC, krause GF, Bailey ME (1970). A new extraction method for determining 2-thiobarbituric acid value of pork and beef during storage. J Food Sci. 35: 582-585.
Crossref
|
|
|
|
Zanardi E, Ghidini S, Battaglia A, Chizzoli R (2004). Lipolysis and lipid oxidation in fermented sausages depending on different processing conditionsand different antioxidants. Meat Sci. 66:415-423.
Crossref
|
|
|
|
Zhu KX, Sun XH, Chen ZC, Peng W, Qian HF, Zhou HM (2010). Comparison of functional properties and secondary structures of defatted wheat germ proteins separated by reverse micelles and alkaline extraction and isoelectric precipitation. Food Chem. 123:1163-1169.
Crossref
|