ABSTRACT
Increasing awareness of health benefits of plant-based food/drinks has resulted in increased preference of consumers for functional beverages as compared to conventional sugar-laden drinks/beverages which only quench thirst but provide little or no nutritional or health benefits. However, most commercially-available functional drinks/beverages are often expensive and not affordable for most people especially low-income earners in developing countries. This study aims to develop low-cost functional yoghurt from milk-cocoa powder blends so as to provide alternative to expensive commercially-available functional drinks and enhance direct consumption of bioactive-rich cocoa powder. Cocoa powder was used to partially replace milk in yoghurt at levels of 10-50%. Standard procedures were used to evaluate triplicate samples of yoghurts for proximate and mineral composition, vitamins, radical-scavenging abilities and phenolic content. Sensory attributes of the yoghurts were evaluated using trained and untrained panels. pH, acidity and brix ranged from 4.29-4.34, 0.67-0.88% and 12-13.5%, respectively. Cocoa powder inclusion significantly increased crude protein (33.7-38.89% DW), fat (0.07-0.14%), ash (0.71-1.01%), fibre (0.1-0.21%), Ca, Mg, K, Zn, Fe, vitamins A, B1, B2, B3, C and E; while vitamin D reduced from 1.64 to 0.72-1.08 mg/L. Similarly phenolic and flavonoid contents, DPPH, FRAP and ABTS increased; values ranged from 0.03-0.16 mg/gGAE; 0.03-0.06 mg/gQUE, 40.12-72.72%, 0.43-1.17 mg/g and 22.49-26.09 mmol/ml, respectively. While the trained panel preferred 50:50 milk-cocoa yoghurt, untrained panel preferred 90:10 milk-cocoa yoghurt, indicating both panels’ preference for milk-cocoa yoghurts as compared to the plain yoghurt. Organoleptically acceptable, nutritionally-rich, low-cost functional yoghurt high in bioactive compounds can be developed from milk and cocoa powder. Its production requires no sophisticated machines since yoghurt is already being produced by both rural and urban dwellers/processors in Nigeria. This milk-cocoa yoghurt provides a cheaper alternative not only packed with bioactive compounds from cocoa powder but also high in protein and micro and macronutrients.
Key words: Antioxidant, cocoa, fermented milks, functional foods, Nigeria, radical-scavenging activity, trained panel, yoghurt.
Globally, the increasing demand for foods that provide much more than nutrition but also promote health and well-being has resulted in astronomical growth of functional foods and preventative/protective foods with associated health claims in the global market (Mollet and Lacroix, 2007). Diet has been reported to play significant roles in 5 of the ten leading causes of death worldwide and there are established evidences pointing to the protective effects of plant-based diets on degenerative diseases such as cardiovascular disease (CVD) and cancer. This is because bioactive compounds from legumes, cereals, grains, fruits and vegetables are effective in reducing lipid and cholesterol levels, increasing bone mineral density and antioxidant status and possess anticancer properties (Eskin and Tamir, 2006). Hence, new functional food products have been made through fortification or addition of desirable nutrients and bioactives that include vitamins, minerals, antioxidants, omega-3 fatty acids, plant extracts, prebiotics and probiotics, and fibre enrichments, many of which are derived from plants (Smith and Charter, 2010). Coincidentally, many plant products that possess important bioactive compounds with protective roles against oxidative stress that can lead to neuro-degenerative and cardiovascular diseases in humans are still grossly underutilized in areas where they are abundant. Most times, this may be due to possession of objectionable sensorial properties of the plants and their products.
Cocoa powder is one of such plant products that has not enjoyed wide consumer acceptance in Nigeria due to its bitter aftertaste and astringency. It has however, recently received global attention as a functional food product/ingredient in the food and confection industry for numerous applications (Borchers et al., 2000). Cocoa powder has been reported to confer health-promoting benefits including promotion of cardiovascular health, reduction of low density lipoprotein (LDL) cholesterol and oxidation of LDL to prevent atherosclerosis or plaque formation. It also elevates high density lipoprotein (HDL) cholesterol, suppresses decay-causing bacteria and plaque formation; acts as an anti-depressant, has euphoric and stimulant effects and general improvement in health and well-being of elderly men (Taubert et al., 2007; Corti et al., 2009). This is owing to its rich content of natural antioxidants, having been reported to exhibit greater antioxidant capacity than many other flavanol-rich foods and food extracts including red wine, blueberry, garlic, strawberry and green and black tea (Lee et al., 2003). Reported numbers of antioxidants in cocoa and its products (621) triple those in green tea, double those in red wine and are far higher than those in blueberries which are known to be a great source of antioxidants (Cooper et al., 2008; Miller et al., 2008). Nutritionally, cocoa powder is a rich source of protein (22%), contain useful amounts of vitamin A, riboflavin and nicotinic acid, several minerals including iron, calcium, copper, magnesium, phosphorus, potassium, sodium and zinc (Steinberg et al., 2003). Thus, it may serve the dual purpose of enriching food with vital nutrients and health-promoting bioactive compounds.
Although cocoa production is predominated by countries in the tropical region (West Africa being a major producer with its production alone accounting for approximately 70% of global production), consumption is mostly by countries in temperate regions of the world (Cadoni, 2013). Cocoa is abundant in Nigeria being the world's fourth largest producer after Ivory Coast, Indonesia and Ghana and the third largest exporter after Ivory Coast and Ghana. Unfortunately, most of the cocoa produced in Nigeria is exported to meet the increasing demand in the international market, while very little quantity is used locally for manufacturing of cocoa-based foods, drinks and confections (Cadoni, 2013; Verter and BeÄváÅ™ová, 2014; Adelodun, 2017). Despite cocoa powder’s nutritional and health benefits and abundance in Nigeria, its direct consumption is still very low. This poor acceptance and low consumption have been linked to its bitter aftertaste and astringency. However, for any functional food to benefit the target population group they must be consumed in consumable forms as part of the usual daily diet. Also, consumers expect such foods to have good organoleptic qualities and to be of similar qualities to the traditional foods (Kwak and Jukes, 2001). Hence, to encourage wider direct consumption of cocoa powder so as to harness its nutritional and health benefits, it may be necessary to use it as a functional ingredient in the development of food products which have the ability to mask the bitter aftertaste and astringency. Also, the food must be one that is commonly and frequently consumed.
Beverages are an important group of easily-digestible, commonly-consumed food taken majorly to quench thirst; although some provide nutritional benefits to the consumers. In particular, the functional beverages are used for the delivery of bioactive compounds that impact positively on the health of consumers. Among these is yoghurt, a lactic acid-fermented milk product made using the starters Streptococcus thermophilus and Lactobacillus delbrueckii spp. bulgaricus (FDA, 1998). It is an important, easily-digested fermented milk product popular for its probiotic effect and is one of the foods commonly used for the delivery of bioactives including vitamins, minerals, probiotics, fibre, etc. Yoghurt and other fermented milks are currently the most sought-after functional food products and their consumption worldwide has greatly increased over the past several decades with the most dramatic increase occurring during the 1980s and 1990s (Dannon, 2002). Nigeria is not an exception; yoghurt production and consumption have steadily increased even at the local level, making it an important dairy product that is enjoyed by all (Akinnubi, 1998).
Yoghurt is widely known for its probiotic effect on gastrointestinal tract resulting from activities of the fermenting or additional probiotic microbiota. However, the significant increase in research on bioactive compounds and development of new materials, processes, ingredients and products that can contribute to the development of functional foods (Sanguansari and Augustin, 2010), may have significantly imparted on development of new yoghurt variants in the market. As such, besides meeting the need for desirable sensory and aesthetic properties, fermented milks including yoghurt are supplemented with ingredients that are rich in bioactive compounds. These ingredients possess strong antioxidant capacity, which results mainly from high content of polyphenolic compounds and antioxidant vitamins (C, E, carotenoids). Examples include caffeine, guarana, green tea extract, Q10 coenzyme, ginseng, aloe vera, cranberry, dietary fibre, omega-3 fatty acids, phytosterols and phytostanols as well as prebiotic oligosaccharides (Cossu et al., 2009; Stankiewicz, 2009). Since cocoa powder has been reported as one of the plant products that exhibit the highest antioxidant capacity, incorporating it into yoghurt may enhance its direct consumption and also promote the health of yoghurt consumers in Nigeria. Also, supplementation of milk with cocoa powder for yoghurt production will bring about a new yoghurt variant packed with bioactive compounds with enhanced functionality. This beverage will be a cheaper, affordable functional beverage as compared to the expensive commercially available functional drinks.
Although the use of cocoa powder and chocolate in the supplementation of milk for production of yoghurt has been previously reported (Jayeola et al., 2010; Essia-Ngang et al., 2014), these previous studies only provided information on the physicochemical and sensory properties. Information is however lacking on the antioxidant capacity of yoghurt supplemented with cocoa powder from Nigeria. This is vital to give stronger impetus to the commercialization and consumption of this yoghurt variant. This study has investigated the effect of co-fermenting milk with cocoa powder on the nutritional composition, antioxidative capacity and consumer acceptability of yoghurt. This present study has provided a more objective sensory evaluation by comparing evaluations from both trained and untrained panels.
Cocoa powders (both natural and alkalized) were obtained from OLAM Nigeria Limited, Akure, Nigeria. Skimmed powder milk, sugar, and other ingredients were purchased locally from Akure main market, Ondo State, Nigeria, while yoghurt starter culture (Yoghumet - a product of Lyo-San Inc., 500, Aeroparc, C.P 598, Lachute, Quebec, Canada) containing Lactobacillus bulgaricus and Streptococcus thermophillus was purchased from a commercial dealer in Lagos, Nigeria. A preliminary study to ascertain consumers’ preference for the powders in the yoghurt was carried out and actual studies were thereafter done using the alkalized powder since preference for yoghurts made with the alkalized powder was higher, probably due to the astringent flavour of the natural powder which is usually reduced during alkalization process. Alkalization also reduces bitterness and improves solubility and these factors are important for beverage product applications (Miller et al., 2008).
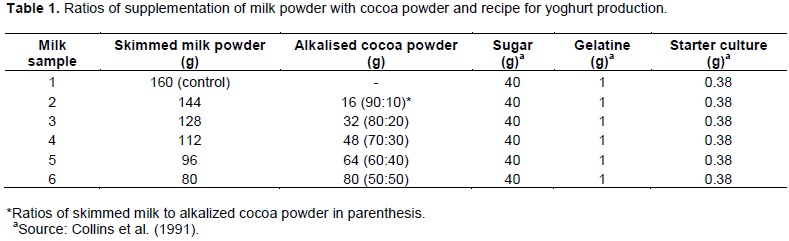
Supplementation of milk with cocoa powder and production of yoghurt
Skimmed milk was supplemented with alkalized cocoa powder at graded levels of 10-50%. Recipe for yogurt production was adopted from Collins et al. (1991) as shown in Table 1, while yoghurt was produced by the method of Bille et al. (2004). Briefly, distilled sterile water (1 L) was boiled, cooled to 40°C and used to mix the ingredients (milk, cocoa powder-milk mixes and sugar) in sterile containers. This was done aseptically to avoid contamination and using clean, sterile materials and utensils. Each milk mix was thereafter heated by raising the temperature to about 50°C for 10 min to dissolve uniformly and filtered by passing through a 75 micro sieve into clean, oven-dried 1 L beakers. The filtered milk mix was homogenized using a blender for 15 min during which 1 g of gelatin was added to each beaker. The beakers were covered with foil paper lid to avoid heat escape and pasteurized at 78 ±2°C (monitored using a digital thermometer) for 30 min with constant stirring to avoid coagulation or sticking to the base. After pasteurization, the samples were cooled to 43-45°C (and monitored to avoid temperatures below 43°C which is the optimal temperature for yoghurt starter culture) using a cooling bath at a set temperature of 40°C. The pasteurized samples were aseptically inoculated with 0.38 g freeze-dried yoghurt starter culture and stirred with a sterile stainless steel spoon. The beakers were incubated at a set temperature of 45°C. pH of the incubated samples was monitored until it reached 4.2-4.5 after which incubation was stopped. This process took between 5-7 h, depending on the ratio of cocoa powder to milk in each beaker. The yoghurts were aseptically filled into clean sterile plastic bottles (in a sterile environment to avoid cross and post-processing contamination) and labeled appropriately. The bottles were immediately placed in freezers of about 5–7 oC to terminate the fermentation process.
Determination of some physical properties and chemical composition of milk-cocoa powder yoghurt
pH
Triplicate determination of pH was done by the potentiometric method using Jenway pH meter (Model 3505, serial number 03132, Barloworld Scientific Ltd, Dunmow Essex UK). The meter was first calibrated with buffer solutions of pH 4 and 7 and the probe was thereafter placed and the values read digitally.
Titratable acidity
This was determined by titrating the samples against 0.1N NaOH.
Viscosity
Viscosity was measured using RION viscotester (Model VT04F009, serial number 84311991, manufactured by RION Co, Ltd, China) which measures in deca Pascal (dpa-s). The experiment was carried out according to manufacturer’s instruction. Results were read when the values on the digital readout became stable.
Refractive index
This was measured using a refractometer (serial number N-3000, ATAGO, Japan) whose prisms were cleaned with ethanol before being used. The refractometer temperature was maintained at 20°C during the measurement.
Proximate composition of the yoghurts
This was determined using standard AOAC (1990) methods. Crude protein content was determined by the micro Kjeldahl nitrogen method and a conversion factor of 6.25 was used to convert the nitrogen content to protein. Carbohydrate content was estimated by difference (100 − [moisture + total ash + crude fat + crude fiber + protein]).
Mineral element composition
This was determined by official methods described by AOAC (2002) using atomic absorption spectrophotometer for calcium, magnesium, iron, manganese, copper and zinc; while sodium and potassium were determined using the flame photometer.
Determination of vitamin contents
Vitamins A, D and E were determined using methods described by Pearson (1976), while vitamins B1, B2 and B3 were determined by Okwu and Josiah (2006) methods and vitamin C using methods described by Benderitter et al. (1998).
Determination of anti-oxidant potentials and free radical scavenging activity
DPPH free radical scavenging ability
DPPH free radical-scavenging ability of the yoghurt samples was measured using the method of Gyamfi et al. (1999). An aliquot of 2 mL of each sample was mixed with 2 ml of 0.1 mM methanolic DPPH solution and vortexed for 1 min and incubated in the dark at room temperature for 30 min. Absorbance was measured at 517 nm on a JENWAY UV–Visible spectrophotometer (JENWAY Inc.). Radical scavenging abilities of the beverages were calculated with reference to the control which contains all the reagents with exception of the test sample. The percentage DPPH inhibition was calculated with the following equation:
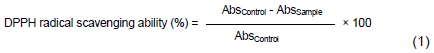
ABTS scavenging activity
The ABTS scavenging activity of the extract was determined according to the method described by Re et al. (1999). The ABTS was generated by reacting an ABTS aqueous solution with K2S208 (2.45 M/l final conc.) in the dark for 16 h and adjusting the absorbance at 734 nm to 0.700 with ethanol. 0.2 ml of the appropriate dilution of the extract was then added to 2.0ml of ABTS solution and the absorbance was read at 732 nm after 15 min. The TROLOX equivalent antioxidant capacity was subsequently calculated (264.32 g).
Total phenolic content (TPC)
TPC of the samples was determined using Singleton et al. (1999) methods with slight modification with gallic acid as standard. Yoghurt samples were diluted appropriately with water and 0.25 ml was oxidized with 2.5 ml of 10% Folin–Ciocalteau’s reagent (v/v). The reaction was allowed to proceed for 5 min at room temperature and neutralized using 2 ml of 7.5% sodium bicarbonate (Na2CO3). Thereafter, the mixture was incubated in the dark for 40 min at 37°C and the absorbance measured at 750 nm in a JENWAY UV–Visible spectrophotometer. Total phenolic content of the beverages was subsequently calculated from the standard curve of absorbance of gallic acid and reported as gallic acid equivalent per millilitre.
Flavonoid content
Flavonoid content was determined using the aluminum chloride colorimetric assay of Meda et al. (2005) with slight modifications. Briefly, 0.5 ml of the diluted sample was added to 0.5 ml methanol, 10% AlCl3 (50 μl), 1 M potassium acetate (50 μl) and water to a total volume of 2.5 ml and incubated for 40 min at room temperature. Absorbance was measured at 415 nm. Total flavonoid content was determined using standard plot with rutin (0-50 mg/L) and expressed as milligram rutin equivalent per millilitre of the beverage.
Ferric reducing antioxidant property (FRAP)
The ferric reducing antioxidant property of the beverages was determined using the method of Pulido et al. (2003). Sample quantity of 2.5 ml was mixed with equal volumes of 0.2 M phosphate buffer (pH 6.6) and 1% potassium ferricyanide [K3Fe(CN)6] and incubated for 20 min at 50°C. Thereafter, 2.5 ml of freshly prepared 10% trichloroacetic acid was added and centrifuged at 600 rpm for 10 min. The supernatant (5 ml) was mixed with equal volume of distilled water and 1 ml of 0.1% FeCl3. Absorbance was immediately read at 700 nm using a UV-visible spectrophotometer, with ascorbic acid used as standard and the ferric reducing power was determined as ascorbic acid equivalent per milliliter of the sample extract.
Sensory evaluation of yoghurt samples
Sensory evaluation of the yoghurts was done using two sets of 15 panelists each; the first set of untrained panelists was selected from among students and staff of the Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria who are familiar with yoghurt. The second set of trained panelists was made up of assessors of OLAM Nigeria Ltd trained by Nestle Nigeria Plc sensory team who are part of the sensory evaluation team of OLAM. They were trained through the assessment of different cocoa beverages, where they made use of their perception to touch, taste and aroma over a period of 2-4 weeks. The complex sensations that result from interaction of their taste are used to measure the food quality and their ability to taste. The training procedure used involved the following:
(i) Panelists must be objective, precise and reproduce judgment.
(ii) Panelists must refrain from smoking, chewing gum, eating or drinking for at least 30 minutes before testing.
(iii) In selection and screening, pure chemicals were used; e.g. for sweetness (pure sucrose), for saltiness (pure NaCl), for bitterness (quinine or caffeine) and for acidity (tartaric acid).
(iv)Panelists must have inherent sensitivity to the characteristic being tested and be able to replicate judgment.
The samples were strictly prepared using the same parameters and under the same conditions, except for differences in the blend ratios of milk-cocoa powder. Thereafter, they were labeled differently using 3-digit random numbers and presented to the panelists with a sachet of clean water for palate cleansing between tasting periods. The trained and untrained sessions were conducted in 2 different days in a well-lit room with two replicates per session. A 9-point hedonic scale where 9 represented extremely like and 1 extremely dislike was used to measure attributes including colour (appearance), aroma, mouth feel/texture, taste and overall acceptability of the yoghurts (Larmond, 1977).
Statistical analysis of data
Triplicate data obtained were subjected to Analysis of Variance (ANOVA) using SSPS version 17.0. Means were separated between the different levels of yoghurt supplemented with cocoa powder by Duncan’s Multiple Range Test.
Physicochemical properties of cocoa powder-supplemented yoghurt
Physical properties of the yoghurt as influenced by cocoa powder supplementation are presented in Table 2. pH ranged from 4.29-4.34 showing no significant difference (p ≤ 0.05). This may indicate that cocoa powder inclusion did not inhibit growth or fermentative ability (to ferment milk sugar, lactose to produce acid) of the fermenting microorganisms. Invariably, cocoa powder may also not hinder probiotic culture potential of yoghurt. This is very important from the standpoint of using yoghurt as a food carrier for probiotics since it is expected that viability of probiotics should be retained during and after processing of food in order to exert their health benefits within the consumer’s body. Furthermore, the values obtained in this study are in agreement with those reported by Jayeola et al. (2010). Total titratable acidity (TTA) ranged from 0.67-0.88%, plain yoghurt had the lowest value of 0.67%, while 90:10 milk-cocoa yoghurt had the highest (0.88%). Hence, these yoghurts may be classified as good quality yoghurts since values reported here compare favourably with pH 4.18-4.38 and TTA 0.5-0.87% recommended for good quality yoghurts (Lee and Lucey, 2003).
Viscosity reduced with cocoa powder addition; while plain yoghurt had the highest value (0.35 dpa-s), 60:40 and 50:50 milk-cocoa yoghurts were the lowest (0.28 dpa-s). Casein and whey proteins (alpha lactalbumin and beta lactoglobulins) influence hydrophilic properties and hydration of casein micelle, causing precipitation at pH below 4.6 during fermentation of milk and resulting in thickening and increased viscosity of the product (Lucey et al., 1998). However, reduction observed in this study may be due to dilution resulting from cocoa powder supplementation. A similar reduction was reported by Essia-Ngang et al. (2014). On the other hand, brix increased from 12% in plain milk yoghurt to a range of 12.5-13.5% as a result of addition of 10-40% cocoa powder, while 50% cocoa addition had no significant effect.
Proximate and mineral element composition of cocoa powder-supplemented yoghurt
Table 3 shows significantly reduced moisture content on cocoa powder addition from 87.15% in the plain yoghurt to 82.01% in 50:50 milk-cocoa yoghurt. This is expected since cocoa powder is a dry product with moisture content of 3-5% (Borchers et al. 2000) and may aid in concentration of other nutrients, give the milk-cocoa yoghurts added body and a storage advantage. Consequently, there was significant increase in crude protein (ranging from 33.7-38.89% DW), crude fat (0.07-0.14%), total ash (0.71-1.01%), crude fibre and carbohydrate contents as cocoa powder supplementation increased. Increase in crude protein may be attributed to inclusion of cocoa powder which has high protein content of 22.5 g. This may be advantageous in reducing protein energy malnutrition prevalent in developing countries like Nigeria. The consistent increase in crude fibre content due to cocoa powder addition may have health benefits for consumers of this yoghurt since plain yoghurt normally lacks crude fibre. Consumption of fibre-rich foods have been reported to impart positive benefits on health by increasing the volume of feacal bulk, thereby decreasing the time of intestinal transit, reducing cholesterol, glycemic levels and trapping mutagenic and carcinogenic agents (Beecher, 1999).
Results in Table 4 present the trend for mineral elements in the following descending order: K>P>Mg>Ca>Na>Zn>Fe>Cu. Potassium was the most abundant which may be due to inclusion of cocoa powder, being reported as most abundant mineral element in cocoa powder which is generally rich in minerals (Steinberg et al., 2003). Except for Cu, there was a consistent increase in mineral contents as cocoa powder addition increased. Specifically, nutritionally important mineral elements such as Ca, Mg, K, P and Fe were significantly increased. High calcium content in the samples may be particularly beneficial to young children in preventing rickets, and brittle and weak bones in adults by contributing significantly to strengthening of bones. Pointillart et al. (1986) reported that calcium from yoghurt may lead to greater bone mineralization in animals than calcium from non-fermented dairy products. Furthermore, the significant increase in iron (from 0.08 mg/l to a range of 1.47-3.42 mg/L) may make milk-cocoa yoghurts healthier and more nutritious than the conventional plain milk yoghurt. Similarly, there was consistent increase in most of the vitamins, although there were inconsistent trends in B vitamins (Table 4). Values ranged from 0.15-0.83mg/l (B1); 0.18-0.72 mg/L (B2); 0.09-0.83 mg/L (B3); 355.28-925.31 mg/L (A); 0.00-2.11 unit/ml (C); 0.72-1.64 mg/L (D); and 0.02-0.32 mg/L (E). Vitamin A was the most abundant while vitamin D decreased. This reduction in vitamin D may be due to dilution since milk is a rich source of vitamin D. On the other hand, increase in vitamins C and E is an indication of higher antioxidative potential of the yoghurt since these vitamins are antioxidant nutrients. Thus consumption of these yoghurts may further aid in safeguarding cells from damage by free radicals, among other benefits of antioxidants (Padayatti et al., 2003).
Antioxidative potential of milk-cocoa powder yoghurts
Results presented in Table 5 showed that total phenolic content (ranging from 0.03-0.16mg/gGAE), flavonoids (0.03-0.06 mgQUE/g), FRAP (0.43-1.17 mg/g), DPPH (40.12-72.72%) and ABTS (22.49-26.09 mmol.ml) increased significantly (p< 0.05) with increase in cocoa powder addition. Thus, yoghurt sample with 50% cocoa powder had the highest antioxidative and free radical-scavenging ability. Although yoghurt has been reported to possess large antioxidant capacity resulting from the presence of different bioactive peptides from milk proteins through proteolysis by LAB (Kudoh et al., 2001), significant increase observed here may be due to the rich antioxidant content of cocoa which has been reported to exhibit greater antioxidant capacity than many other flavanol-rich foods (Lee et al., 2003; Cooper et al., 2008; Steinberg et al., 2003). Since cocoa products have been previously associated with reduction of risk to cardiovascular diseases and blood glucose levels due to its antioxidant capacity (Cooper et al., 2008), consumption of this cocoa powder-supplemented yoghurt may be beneficial in reducing risk to cardiovascular diseases and blood glucose levels. Also, the increase in flavonoid content may be advantageous in improving brain blood flow and impacting cognitive behaviour, thereby offering potential for debilitating brain conditions including dementia and stroke. Flavonoids may also cause modulation and prevent oxidation and increase in LDL which could put a subject at a higher risk of coronary heart disease (Sorond et al., 2008; Osakebe et al., 2001).
Sensory attributes of yoghurt supplemented with cocoa powder
There were significant variations between the evaluations of the untrained (15) and trained (15) panels for the sensory attributes of the yoghurts (Table 6). While the untrained panel scored plain yoghurt highest (8.20) and 50:50 milk-cocoa yoghurt lowest (5.80) for colour/appearance, the trained panel scored yoghurts with 10 and 30% cocoa powder least (6.80) and 50:50 milk-cocoa yoghurt highest (8.20) (Table 6). The preference of the trained panel for colour/appearance of the milk-cocoa yoghurts as compared to plain yoghurt is understandable since they have been trained using cocoa-based products. This may explain why the trained panelists seemed to score yoghurts containing larger amounts of cocoa powder higher than those with less cocoa powder or none at all. This was the trend for all parameters evaluated, except for mouth feel, where the trained panelists scored 90:10 milk-cocoa yoghurt highest, while 80:20 milk-cocoa yoghurt was scored lowest. However, for overall acceptability, the trained panelists had the highest preference for the 50:50 milk-cocoa yoghurt. On the other hand, preference of the untrained panel for plain yoghurt in most parameters evaluated shows that the panelists were already used to plain yoghurt. With proper and adequate education, consumers’ acceptability for the new milk-supplemented-cocoa yoghurts would improve. This is corroborated by the highest preference of the untrained panelists for overall acceptability of 90:10 milk-cocoa yoghurt. This is an indication that cocoa powder inclusion improved the sensory attributes of yoghurt as shown by preference of both sets of panelists for the milk-cocoa yoghurts as compared to plain milk yoghurt.
Although demand for functional foods/beverages has been on the increase, their high cost has limited affordability especially for rural dwellers in developing countries like Nigeria. Since yoghurt is a popular beverage consumed by both rural and urban dwellers, inclusion of cocoa powder which is abundant in Nigeria may be a cheaper alternative. The present study has shown that supplementation of milk with cocoa powder significantly improved the nutritional composition, antioxidant potential and acceptability of yoghurt. This may serve as impetus to its commercial production; however, adequate enlightenment would encourage its consumption which will contribute to ensuring food security and improved health.
The authors have not declared any conflict of interests.
The authors are grateful to OLAM Nigeria Limited for raw cocoa powder and utilization of the chemical laboratory for preparation of the yoghurts. We also appreciate Mrs Olabiran and Mr Festus for their technical assistance and the sensory assessors for their patience and useful suggestions.
REFERENCES
|
Adelodun A (2017). Analysis, Food and Agribusiness. Available at:
View, accessed 14 Jan, 2019
|
|
|
|
Akinnubi S (1998). Yoghurt drink manufacturing: Business Guide. National Concord. May 22, pp. 22.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (1990). Official Methods of Analysis. 4th ed. Association of Analytical Chemist, Washington D.C.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (2002). Offical Methods of Analysis. 18th ed. Association of Official Analytical Chemists, Washington DC.
|
|
|
|
|
Beecher GR (1999). Phytonutrients role in metabolism: effects on resistance to degenerative processes. Nutrition Reviews 57:3-6.
Crossref
|
|
|
|
|
Benderitter M, Maupoil V, Vergely C, Dalloz F, Briot F, Rochette L (1998). Studies by electron paramagnetic resonance of the importance of iron in the hydroxyl scavenging properties of ascorbic acid in plasma: effects of iron chelators. Fundamental Clinical Pharmacology 12(5):510-516.
Crossref
|
|
|
|
|
Bille PG, Vovor MN, Goreseb J, Keye EL (2004). Evaluating the feasibility of adding value to goat milk by producing yoghurt using low cost technology method for rural Namibia. African Journal of Food Technology 5:139-144.
Crossref
|
|
|
|
|
Borchers AT, Keen CL, Hannum SM, Gershwin ME (2000). Cocoa and chocolate: composition, bioavailability, and health implications. Journal of Medicinal Foods 3:77-103.
Crossref
|
|
|
|
|
Cadoni P (2013). Analysis of incentives and disincentives for cocoa in Nigeria. Technical notes series, MAFAP, FAO, Rome.
|
|
|
|
|
Collins JL, Ebah CB, Mount JR, Demott BJ, Draughon FA (1991). Production and evaluation of milk-sweet potato mixtures fermented with yoghurt bacteria. Journal of Food Science 56(3):685-691.
Crossref
|
|
|
|
|
Cooper KA, Donovan JL, Waterhouse AL, Williamson G (2008). Cocoa and health. British Journal of Nutrition 99(1):1-11.
Crossref
|
|
|
|
|
Corti R, Flammer AJ, Hollenberg NK (2009). Cocoa and cardiovascular health. Contemporary Reviews in Cardio Medicine 119:1433-1441.
Crossref
|
|
|
|
|
Cossu M, Juliano C, Pisu R, Alamanni MC (2009). Effects of enrichment with polyphenolic extracts from Sardinian plants on physico-chemical, antioxidant and microbiological properties of yogurt. Italian Journal of Food Science 4(21):447-459.
|
|
|
|
|
Dannon (2002). The History of Yogurt, The Dannon Institute, Tarrytown, NY.
|
|
|
|
|
Eskin NAM, Tamir S (2006). Dictionary of Nutraceuticals and Functional Foods. Boca Raton, FL: Taylor & Francis Group/CRC Press.
Crossref
|
|
|
|
|
Essia-Ngang JJ, Kouebou CP, Sado Kamdem SL, Djoulde DR (2014). Impact of partial milk substitution with cocoa powder on the properties of milk fermented by Lactobacillus bulgaricus and Streptococcus thermophilus. African Journal of Microbiology Research 8(9):903-907.
Crossref
|
|
|
|
|
FDA (1998). FDA Code of Federal Regulation, Title 21, Sections 131.200:290-294. US Government Printing Office, Washington, DC.
|
|
|
|
|
Gyamfi MA, Yonamine M, Aaniya Y (1999). Free radical-scavenging action of medicinal herbs from Ghana: Thonningia Sanguine on experimentally induced liver injuries. General Pharmacology 32:661-667.
Crossref
|
|
|
|
|
Jayeola CO, Yahaya LE, Igbinadolor RO (2010). Cocoa powder supplementation in yoghurt production. Journal of Food Technoogy 8(3):82-85.
Crossref
|
|
|
|
|
Kudoh Y, Matsuda S, Igoshi K, Oki T (2001). Antioxidative peptide from milk fermented with Lactobacillus delbrueckii ssp. bulgaricus IFO 13953. Nippon Shokuhin Kagaku Kogaku Kaishi 48:44-50.
Crossref
|
|
|
|
|
Kwak NS, Jukes DJ (2001). Functional foods. Part 2: the impact on current regulatory terminology. Food Control 12:109-117.
Crossref
|
|
|
|
|
Larmond E (1977). Laboratory Method of Sensory Evaluation of Food. Food Research Institute Public, Ottawa, Canada. pp. 1637.
|
|
|
|
|
Lee KW, Kim YJ, Lee HJ, Lee CY (2003). Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. Journal of Agricultural and Food Chemistry 51:7292-7295.
Crossref
|
|
|
|
|
Lee WJ, Lucey JA (2003). Rheological properties, whey separation and microstructure in set- style yoghurt: effect of heating temperature and incubation temperature. Journal of Texture Studies 34:515-536.
Crossref
|
|
|
|
|
Lucey JA, Temehana M, Singh H, Munro PA (1998). Effect of interaction between whey protein and casein micelles on the formation and rheological properties of acid skim milk gels. Journal of Dairy Research 65:555-567.
Crossref
|
|
|
|
|
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey as well as their radical scavenging activity. Food Chemistry 91:571-577.
Crossref
|
|
|
|
|
Miller KB, Hurst WJ, Payne MJ, Stuar DA, Apgar J, Sweigart DS, Ou B (2008). Impact of alkalization on the antioxidant and flavonol content of commercial cocoa powders. Journal of Agricultural and Food Chemistry 56(18):8527-8533.
Crossref
|
|
|
|
|
Mollet B, Lacroix C (2007). Where biology and technology meet for better nutrition and health. Current Opinion in Biotechnology 18:154-155.
Crossref
|
|
|
|
|
Okwu DE, Josiah C (2006). Evaluation of the chemical composition of two Nigerian medicinal plants. African Journal of Biotechnology 5(4):357-351.
|
|
|
|
|
Osakebe N, Baba S, Yasuda A, Iwamoto T, Kamiyama M, Takizawa T, Itakura H, Konda K (2001). Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radical Research 34(34):93-99.
Crossref
|
|
|
|
|
Padayatti SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003). Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of the American Colloids and Nutrition 22(1):18-35.
Crossref
|
|
|
|
|
Pearson D (1976). Chemical Analysis of Food. 8th ed. New York, NY: Chemical Publishing Co Inc. pp. 258-376.
|
|
|
|
|
Pointillart A, Cayron B, Gueguen L (1986). Utilization du calcium et du phosphore et mineralization osseuse chez le porc consommant du yagourt. Sciences des Aliments 6:15-30.
|
|
|
|
|
Pulido R, Bravo L, Saura-Calixto F (2003). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry 48:3396-3402.
Crossref
|
|
|
|
|
Re R, Pellegrin N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radication decolourization assay. Free Radical Biology and Medicine 26:1231-1237.
Crossref
|
|
|
|
|
Sanguansari V, Augustin MA (2010). Microencapsulation in functional food product development. In. Smith J, Charter E (eds.), Functional Food Product Development. West Sussex, UK: Wiley-Blackwell pp. 3-23.
Crossref
|
|
|
|
|
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Methods in Enzymology 299:152-177.
Crossref
|
|
|
|
|
Smith J, Carter E (2010). Functional Food Product Development. West Sussex UK: Wiley-Blackwell.
Crossref
|
|
|
|
|
Sorond FA, Lipsitz LA, Hollenberg NK, Fisher ND (2008). Cerebral blood flow response to flavanol-rich cocoa in healthy elderly human. Neurological Disease Treatment 4:433-440.
Crossref
|
|
|
|
|
Stankiewicz J (2009). Jakość mlecznych napojów fermentowanych suplementowanych dodatkami pochodzenia roÅ›linnego [The quality of fermented milk drinks supplemented with plant extracts]. Zesz Nauk Akad Morsk Gdyn 61:39-44.
|
|
|
|
|
Steinberg FM, Bearden MM, Keen CL (2003). Cocoa and Chocolate Flavonoids: implications for cardiovascular health. Journal of American Dietetic Association 103:215-223.
Crossref
|
|
|
|
|
Taubert D, Roesen R, Schomig E (2007). Effect of cocoa and tea intake on blood pressure: a meta-analysis. Archives of Internal Medicine 167(7):626-634.
Crossref
|
|
|
|
|
Verter N, BeÄváÅ™ová V (2014). Analysis of some drivers of cocoa export in Nigeria in the era of Trade Liberalization. AGRIS on-line Papers in Economics and Informatics, Czech University of Life Sciences Prague, Faculty of Economics and Management 6(4):1-11.
|
|