ABSTRACT
Plant growth promoting bacteria can be an alternative to increase plant production, and reduce production costs and environmental impacts. Ruminal bacteria have several abilities and some of them are related to plant growth promotion. The aim of this study is to evaluate the increase in maize and soybean plants and in soils promoted by the inoculation of three ruminal bacteria: Bacillus cereus, Lactobacillus acidophilus and Succinovibrio dextrinosolvens. The experiments were conducted in a complete randomized block design with five treatments and six replicates as follows: T1 = control; T2 = B. cereus, T3 = L. acidophilus, T4 = S. dextrinosolvens, T5 = B. cereus + L. acidophilus + S. dextrinosolvens. In vitro tests showed that bacteria were able to fix nitrogen, solubilize phosphorus, and synthesize indole acetic acid and amylase. S. dextrinosolvens increased the root dry matter of maize plants, L. acidophylus increased the phosphorus concentration in maize roots along with the mixture of the three bacteria and increased the shoot dry matter of soybean plants and also the phosphorus and nitrogen concentration in soybean plants. This is the first report showing that L. acidophilus and S. dextrinosolvens have great potential to be used as plant growth promoting agents.
Key words: Rhizobacteria, indoleacetic acid, nitrogen fixation, Zea mays, Glycine max.
Maize (Zea mays L.) is a crop that originates in Mexico. It is now cultivated in many parts of the world and is of great importance economically or socially (Dowswell, 2019). Brazil ranks third in world’s production, second in exports and fourth in consumption. For 2018/2019, the performance of the country will oscillate, reaching 361.4 million tonnes (CONAB, 2019). Soybean (Glycine max) probably originated in China, but spread to Europe, North and South America. In 1882, it was brought to Brazil, specifically Bahia State, and taken to the southern region of the country, where it was better adapted (Oliveira and Schneider, 2016). According to CONAB (2019), today, Brazil and the United States are ranked as the largest soybean producers in the world, followed by Argentina
and China. It is estimated that by the year 2020, Brazil will lead this ranking. The use of chemical inputs in combination with genetic improvement and type of management provide an increase in the yield of these grains (Duncan et al., 2018). There is concern about the excessive use of chemical fertilizers, as they cause soil pollution, soil eutrophication and emission of greenhouse gases (Pavinato et al., 2017).
The major challenge of agriculture is to increase or maintain the productivity of agricultural crops with lower production costs and environmental impact. Plant growth promoting rhizobacteria appear as an alternative, which are a group of microorganisms capable of stimulating plant growth through direct mechanisms (production of plant hormones, enzymes, hydrocyanic acid, phosphate solubilization and nitrogen fixation), and / or indirect mechanisms (biological control, space and nutrient petition, parasitism, resistance induction and cross protection) (Hungria et al., 2010). These rhizobacteria normally inhabit root surfaces, internal plant tissues and rhizosphere. Interestingly, some ruminal probiotic bacteria also have several plant growth promoting characteristics such as rhizospheric bacteria and could be tested for this purpose.
Bacillus cereus is among these bacteria, which is a cylindrical, gram-positive, spore forming, facultative anaerobic and mesophilic bacterium. Its spores facilitate adhesion on surfaces and resistance to high temperatures and sanitization processes, that is, the bacterium can remain in a state of "dormancy" until the environment becomes favorable. B. cereus is a producer of phospholipases and food degrading enzymes such as amylases, proteases and lectinase (Granum and Lindbäck, 2013). Bacillus cereus has a positive effect in modulating immunity and intestinal microbiota, which is very important for the exploration of new probiotics (Li et al., 2009).
Lactobacillus acidophilus is a probiotic organism that degrades several enzymes, widely used as a nutritional supplement; it is produced by the food industry, with the function of maintaining the balance of the intestinal microbiota (Flesch et al., 2014). It adheres to specific receptors on the intestinal membrane competing with pathogens, in addition to producing antimicrobial substances, called bactericides (Marco et al., 2006). In addition, L. acidophilus is used in food and pharmaceutical application to balance disturbed intestinal microbiota (Sinn et al., 2008).
Succinovibrio dextrinosolvens is an anaerobic, gram-negative amylolytic (degrading starch) bacterium, with optimum pH around 6.0 to 7.0. This bacterium is usually found in the bovine rumen; it helps with other microorganisms to make best use of nutrients (Stewart et al., 1997).
In agriculture, there are many products that have active ingredient species of the genus Bacillus such as Bacillus thuringiensis, Bacillus subitilis, Bacillus amyloliquefaciens, among others. Microorganisms promote many gains for plant nutrition and phosphorus solubilization, which is a consequence of the presence of this group of microorganism in the rhizosphere (Canbolat et al., 2006). However, there is no research in literature related to the application of ruminal probiotic bacteria as a growth promoter of maize and soybean plants. There are many benefits to use probiotic bacteria in food and pharmaceutical applications, but they are not used to promote plant growth. As we have demonstrated that these probiotic bacteria have abilities to produce IAA, siderophores, solubilize phosphorus, and fix nitrogen, this study aims to evaluate if B. cereus, L. acidophilus and S. dextrinosolvens would promote maize growth in greenhouse condition.
Experimental design and statistical analysis
Experiments were conducted in a complete randomized block design with five treatments and six replicates: T1 = control; T2 = B. cereus; T3 = L. acidophilus; T4 = S. dextrinosolvens; T5 = "MIX" (mixture of three microorganisms). Analyses were performed using AgroEstat software (Barbosa and Maldonado, 2010). Data were submitted to analysis of variance (ANOVA) with 5% significance level by the F test and means were compared by the Duncan test at 5% probability. The designs were the same for maize and soybean plants.
Bacterial isolates
Microorganisms used (B. cereus, L. acidophilose and S. dextrinosolvens) were provided by the Federal University of Viçosa - UFV, and belong to the collection of isolates from one of its laboratories. Bacteria were cultured in 60 mL Erlenmeyer flasks in nutrient broth culture medium at 28°C for 24 h, and suspensions were adjusted in bacterial concentration 108 colony forming unit (CFU) mL-1.
In vitro tests of isolates
Production of siderophores
Siderophore production in a liquid medium using the Chrome Azurol Solution (CAS) was performed as previously described (Louden et al., 2011). 5 mL of the PMS7-Ca medium was inoculated and incubated for 72 h. The suspension was then centrifuged at 4000 × g for 10 min and 1 mL of the filter-sterilized supernatant was incubated 1:1 with the CAS. The OD630 was then measured and a 10% difference between the sample and un-inoculated PMS7-Ca with CAS was considered as positive (Machuca and Milagres, 2003).
Starch agar
The following reagents were used for the preparation of the starch production medium: K2HPO4 0.3 g/L; MgSO4.7H2O 1.0 g/L; NaCl 0.5 g/L; NaNO3 1.0 g/L; Starch 10 g/L; pH = 6.9.
Cellulolytic activity
Cellulolytic activity was assayed by monitoring the oxidation of L- 3,4-dihydroxyphenylalanine (L-DOPA; Sigma) in the presence of hydrogen peroxide (28). A final volume of 1.0 ml of reaction mixture contained 4.0 mM hydrogen peroxide, 0.1 M potassium phosphate buffer (pH 7.0), and 1.0 mM L-DOPA. A concentrated crude enzyme preparation (100 to 200 ul) was used in the assay. The reaction was initiated by the addition of hydrogen peroxide, and the increase in the A470 was monitored for 5 min at 37°C. Reactions containing all reagents except the crude enzyme extract served as controls. One unit of enzyme was expressed as the amount of enzyme. The methodology of culture medium described by Ramachandra, Crawford and Pometto with no alterations was used (Ramachandra et al., 1987).
Production of indoleacetic acid
The bacteria evaluated were screened for IAA production (15). Briefly, the bacterial culture was inoculated in the respective medium (Jensen’s/nutrient broth) with tryptophan (1, 2, and 5 mg/ml) or without tryptophan incubated at 28 ± 2°C for 15 days. Cultures were centrifuged at 3000 rpm for 30 min. Two milliliters of the supernatant was mixed with 2 drops of orthophosphoric acid and 4 ml of Solawaski’s reagent (50 ml, 35% perchloric acid; 1 ml 0.5 FeCl3). Development of a pink colour indicates IAA production. O.D. was read at 530 nm using Spectronic 20D+. The level of IAA produced was estimated by a standard IAA graph.
P quantification in test tubes
For phosphate solubilization quantification, the modified methodology of Malavolta et al. (1997) was used. In a 120 ml Erlenmeyer flask containing 50 ml of Nahas medium (1994), 200 μl of inoculum from each isolate was added. Erlenmeyer flasks were incubated for 48 h at ± 28°C with stirring at 180 rpm, and after incubation, 5 ml of each sample was transferred to tubes and centrifuged at 9000 rpm for 15 min. Then, 1 ml of supernatant from each isolate, 4 ml of distilled water and 1 ml of ammonium molybdate-vanadate reagent formed by mixing equal volumes were added to a new tube for further reading (after 5 min) in spectrophotometer at 470 nm.
Nitrogen quantification in test tubes
The method of Kuss et al. (2007) was used in the nitrogen quantification analyses by isolates. In the determination of nitrogen in foodstuffs (1), a digestion mixture of 40 g of sodium sulfate and 1.6 g of copper sulfate per 100 ml of acid is recommended, with a digestion time of 6 h. For the micro determination of protein in 50% glycerol (51), bromine is used as an oxidizing agent, supplemented by 30% hydrogen peroxide.
Planting
Experiments were conducted in greenhouse belonging to the Laboratory of Agricultural Microbiology of UNESP-FCAV (coordinates - Latitude: 21° 14 '05 "S Longitude: 48° 17' 09" W). For studies with maize, seeds of variety 2B587PW Dow Agro-Science-transgenic were used; in experiments with soybean, seeds of variety 95R95IPRO Piornner were used. In both cases, seeds were pre-inoculated with B. cereus, L. acidophiluse and S. dextrinosolvens, deposited in pots (5 L), filled with red eutrophic latosol type soil, sieved and fertilized. Fertilization was performed according to soil chemical analysis and recommended for crops (Table 1).

Inoculations
Four inoculations were performed, the first through seeds, which were immersed in 125 ml Erlenmeyer flasks containing nutrient broth in bacterial concentration of 108 CFU ml -1 for 15 min in 120 rpm orbital shaking and then sown. The second, third and fourth inoculations were performed every week, seven days of sowing, adding 20 ml of each inoculum at the same concentration as above.
Evaluations in corn and soybean plants
Dry mass
Roots were collected from both cultures, washed in running water to remove excess soil and dried on absorbent paper. Shoots were separated from roots and both were dried in oven with forced air circulation at 65°C for approximately 72 h until reaching constant weight. The last step was the weighing of all the material, using analytical scale to determine the mass (g) of root dry matter (RDM) and shoot dry matter (SDM).
Nitrogen concentration in shoots and roots
In order to determine the nitrogen concentration (N), the plant material was ground in Willey mill (mesh 20) and submitted to N-leaf analysis using the method proposed by Bremmer and Mulvaney (1982) and modified by Bezerra and Barreto (2011).
Shoot and root phosphorus concentration
Phosphorus concentrations (P) were determined by nitroperchloric digestion, followed by the molybdo-vanadate colorimetric method according to methodology proposed by Haag et al. (1975) with modifications by Bezerra and Barreto (2011).
Soil assessments
Simple soil samples were collected from the maize and soybean rhizosphere; they were collected at random points from pots and then divided into two parts: the first was kept in plastic bags at 4°C until the moment of use for total bacteria counting and the second air was dried and stored at room temperature (28°C) to determine the amount of soluble phosphorus, total nitrogen and carbon of the bacterial biomass (Nahas and Assis, 1992).
Total nitrogen content was determined by Berigari (1975). For nitrogen determination, the plant material was ground in Willey-type mill (20 mesh) and submitted to leaf nitrogen analysis according to Bremmer and Mulvaney (1982) and modified by Bezerra and Barreto (2011); 0.1 g of plant sample was weighed, placed in a digester and digested using 7 ml of sulfuric acid. The material was digested, and then, 10 ml of distilled water was added. Distillation was performed by using the Kjeldahl method with the aid of 25 ml of NaOH (50%). The distilled material was recollected in 10 ml of boric acid as an indicator solution, resulting in 20 ml of distilled material. Ammonium titration was performed using 0.05 N H2SO4 as the standard.
Soluble phosphorus
Soluble phosphorus was measured according to Watanabe and Olsen (1965), where 0.6 g of dry soil was sampled and transferred to Erlenmeyer flasks containing 12 ml of extractor sodium bicarbonate solution and Whatman filter paper. For determination, 2.0 ml of sodium bicarbonate was pipetted, and 0.2 ml of sulfur solution (5 M) and 0.8 ml of B reagent were filtered. Then, the material was incubated at 45°C for 20 min. Next, a reading was taken using a spectrophotometer at 820 nm.
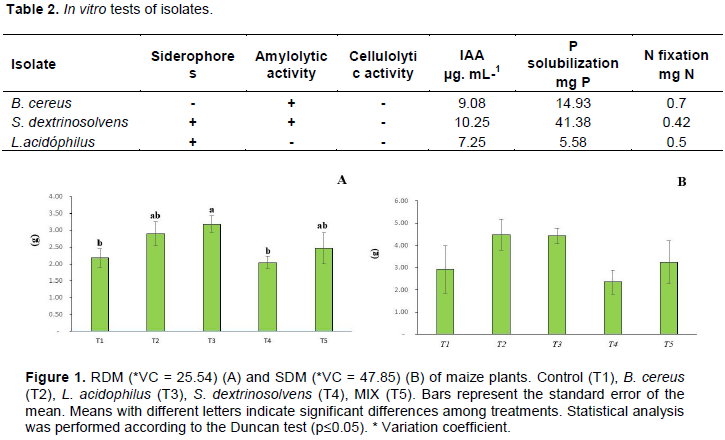
The results of in vitro laboratory analyses to evaluate the ability of isolates to produce siderophores, amylolytic and cellulolytic activity, and the quantitative values ​​of IAA production, nitrogen fixation and phosphorus solubilization in tubes are shown in Table 2. For root dry matter (RDM) (Figure 1A), L. acidophilus (T3) bacterium promoted a 3.2 g increase (p> 0.05) compared to control treatment. For shoot dry matter (SDM) (Figure 1B), treatments did not differ from each other, although numerically, B. cereus isolate was almost twice as large as control (without application of bacteria).
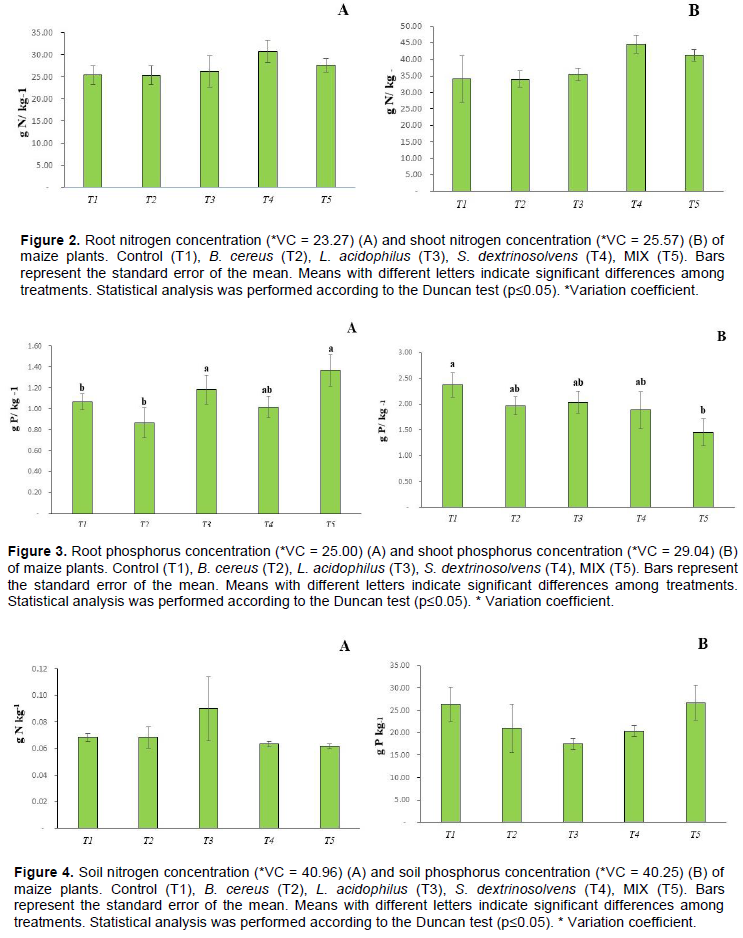
The root nitrogen content ranged from 25.36 to 30.73 g N / kg-1, and there was no significant difference (p <0.05) among treatments (Figure 2A). The shoot nitrogen content ranged from 34.5 to 43.5 g N / kg-1 and there was no significant difference among treatments (Figure 2B).
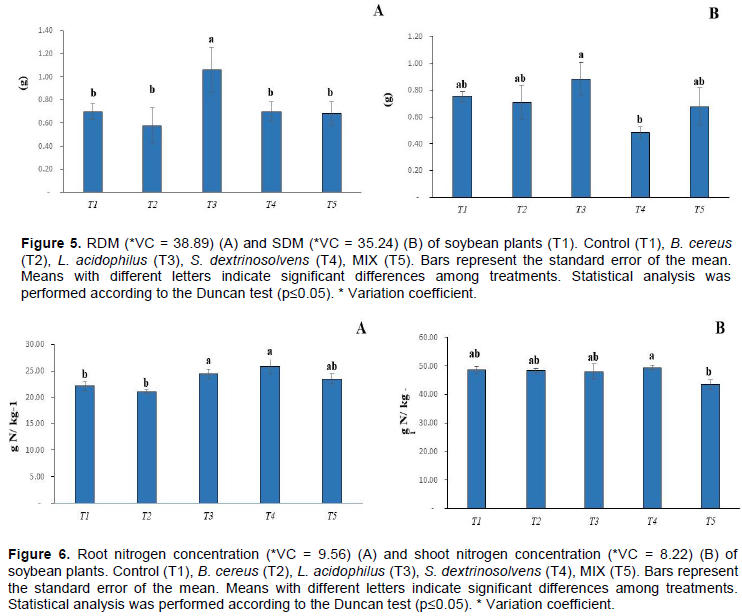
The bacterial MIX promoted an increase in the phosphorus concentration of 1.4 g of P / kg of plant compared to control, followed by L. acidophilus bacterium (Figure 3A). Interestingly, the bacterial MIX decreased shoot P concentration of 1.7 g of P per kg of maize plant compared to control of 2.3 g of P per kg of plant (Figure 3B). Nitrogen concentrations ranged from 0.06 to 0.9 g N per kg of dry soil while phosphorus concentrations ranged from 5 to 35 g P / kg -1 of dry soil. However, there was no significant difference among treatments (Figure 4). L. acidophilus showed the highest root dry matter compared to control treatment and the other treatments (Figure 5A), while, there was no significant difference (p <0.05) in the shoot dry matter (Figure 5B).
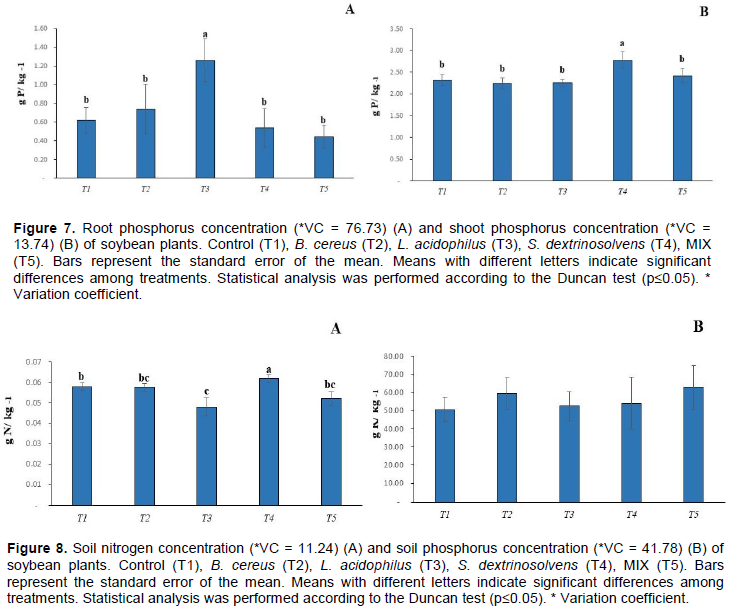
L. acidophilus and S. dextrinosolvens bacteria promoted an increase in the nitrogen concentration in soybean roots (p > 0.05), 23 and 25 g of N kg-1, respectively, compared to the control treatment, 22 g of N kg-1 plant (Figure 6). L. acidophilus bacterium (T3) promoted the highest root phosphorus concentration, 1.1 g of P kg-1, while S. dextrinosolvens promoted the highest shoot phosphorus concentration, 2.8 g of P kg-1, compared to control treatments (p> 0.05). However, no significant difference (p <0.05) in relation to root and shoot concentrations for the other treatments was observed (Figure 7A-B). In relation to soil nitrogen, S. dextrinosolvens bacterium (T3) promoted the highest concentration, 0.065 g of N kg-1, compared to control treatment (p> 0.05). On the other hand, L. acidophilus decreased the soil nitrogen concentration, 0.05 g of N kg-1, compared to control treatment (Figure 8A). In relation to soil phosphorus concentrations, no significant difference between control and the other treatments was observed (Figure 8B).
Ruminal probiotic bacteria presented important characteristics related to plant growth promotion such as synthesis of siderophores, indole acetic acid, nitrogen fixation and phosphorus solubilization. Therefore, these bacteria were evaluated in maize and soybean plants to verify the potential of each for their plant growth promoting effect. Probiotic bacteria are bacteria whose inadequate amounts promote any benefit to the host (Martin and Langella, 2019). These benefits may be the consequence of nutrient supply and / or the reduction of pathogens that impair host development (Kleerebezem et al., 2019).
Plant growth promoting bacteria are generally isolated from the rhizosphere or from within plant tissues and have plant growth promoting abilities (Calvo et al., 2019).
In the same way that probiotic bacteria promote host development, plant growth promoting bacteria also provide nutrients and reduce the harmful effects of plant pathogens. In a way, it was found that the mode of action of probiotic bacteria and plant growth promoting bacteria is very similar. As a consequence of these similarities, B. cereus, L. acidophilus and S. dextrinosolvens were inoculated in maize and soybean plants and some plant growth parameters were evaluated in comparison with control treatment.
Interestingly, L. acidophilus bacterium increased root dry matter and the phosphorus concentration in the roots of maize plants. L. acidophilus also increased root dry matter and the nitrogen and phosphorus concentration in the roots of soybean plants. Probably, these effects promoted by L. acidophilus were due to its ability to synthesize phytohormones that in certain amounts can stimulate or inhibit the root development of plants and as a consequence, increase the concentration of certain nutrients in the plant (Barnawal et al., 2019).
The increase of phosphorus concentration in maize roots and phosphorus and nitrogen concentration in soybean roots is a very interesting aspect promoted by the plant / microorganisms interaction, in which the nutritional efficiency of plants is increased. Nutrients such as phosphorus and nitrogen are essential for plant growth and development (Stewart et al., 2019; Klamer et al., 2019) and when the association with a microorganism allows their absorption more efficiently, this microorganism has great potential to be used in a more sustainable agricultural production system (Syed and Tollamadugu, 2019), allowing reductions in production costs and environmental impact (Baron et al., 2018).
S. dextrinosolvens increased the nitrogen concentration in roots and soil as well as phosphorus concentration. These results are very interesting from the point of view of plant nutrition and show that ruminal probiotic bacteria have potential to be used as plant growth promoting bacteria. There is a positive correlation between plant nutritional status, microbioma and productivity (Pii et al., 2016). In this sense, the action of bacteria such as S. dextrinosolvens and L. acidophilus can be very positive for plant production. The bacteria / plant interaction depends on several factors such as plant species, soil type, climatic conditions and characteristics that are intrinsic to microorganisms used (Bulgarelli et al., 2013). L. acidophilus and S. dextrinosolvens bacteria showed a certain affinity with the plant species tested, promoting increases in plant and nutritional development and soil fertility. In this sense, more studies are needed to verify the best conditions of use of these bacteria such as dose, mode of application and plant species in order to optimize the increases promoted by the microorganisms.
This is the first report on the use of ruminal probiotic bacteria as plant growth promoting bacteria. It shows great potential for their use, since L. acidophilus increased dry matter in soybean and corn plants and S. dextrinosolvens promoted increases in the nutritional status of soybean and soil plants. In the future these ruminal probiotic bacteria could be used in agricultural production as inoculates, allowing significant reduction of mineral fertilizer levels and contributing to more sustainable production.
The authors have not declared any conflict of interests.
REFERENCES
|
Barbosa J, Maldonado JW (2010). AgroEstat: sistema para análises estatísticas de ensaios agronômi.
|
|
|
|
Barnawal D, Singh, R, Singh RP (2019). Role of plant growth promoting rhizobacteria in drought tolerance: Regulating growth hormones and osmolytes. PGPR Amelioration in Sustainable Agriculture 107-128.
Crossref
|
|
|
|
|
Baron NC, Costa NTA, Mochi DA, Rigobelo EC (2018). First report of Aspergillus sydowii and Aspergillus brasiliensis as phosphorus solubilizers in maize. Annals of microbiology 68(12):863-870.
Crossref
|
|
|
|
|
Berigari M (1975). Determination of total protein in plant tissues from nitrogen analysis by a modified Kjeldahl digestion and Nesslerization method. Argonne National Laboratory Radiological and Environmental Research Division Annual Report, January-December 1974. ANL75-3 (Pt3).
|
|
|
|
|
Bezerra NE, Barreto L (2011). Análises químicas e bioquímicas em plantas. Recife: UFRPE.
|
|
|
|
|
Bremmer J, Mulvaney C (1982). Salicylic Acid Thisolfate Modification of Kjeldahl Method to Include Nitrate and Nitrite. Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties 621-622.
|
|
|
|
|
Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaat EVL, Schulze-Lefert P (2013). Structure and functions of the bacterial microbiota of plants. Annual Review of Plant Biology 64:807-838.
Crossref
|
|
|
|
|
Calvo P, Zebelo S, McNear D, Kloepper J, Fadamiro H (2019). Plant growth-promoting rhizobacteria induce changes in Arabidopsis thaliana gene expression of nitrate and ammonium uptake genes. Journal of Plant Interactions 14(1):224-231.
Crossref
|
|
|
|
|
Canbolat MY, Bilen S, Cakmakç R, Åžahin F, Aydın A (2006). Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biology and Fertility of Soils 42(4):350-357.
Crossref
|
|
|
|
|
CONAB (2019). CNDEA. Indicadores da Agropecuária. CONAB/MAA 10(05).
|
|
|
|
|
Dowswell C (2019). Maize in the third world: CRC Press.
Crossref
|
|
|
|
|
Duncan EG, O'Sullivan CA, Roper MM, Biggs JS, Peoples MB (2018). Influence of co-application of nitrogen with phosphorus, potassium and sulphur on the apparent efficiency of nitrogen fertiliser use, grain yield and protein content of wheat. Field Crops Research 226:56-65.
Crossref
|
|
|
|
|
Flesch AGT, Poziomyck AK, Damin DDC (2014). The therapeutic use of symbiotics. ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo) 27(3):206-209.
Crossref
|
|
|
|
|
Granum PE, Lindbäck T (2013). "Bacillus cereus." Food microbiology. American Society of Microbiology, pp. 491-502.
Crossref
|
|
|
|
|
Haag H, Sarruge J, de Oliveira, G, Dechen A (1975). Nutrição mineral do cajueiro (Anacardium occidentale L.): I-deficiência dos macronutrientes-nota prévia. Anais da Escola Superior de Agricultura Luiz de Queiroz 32:185-190.
Crossref
|
|
|
|
|
Hungria M, Campo RJ, Souza EM, Pedrosa FO (2010). Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant and soil 331(1-2):413-425.
Crossref
|
|
|
|
|
Klamer F, Vogel F, Li X, Bremer H, Neumann G, Neuhäuser B, Hochholdinger F, Ludewig U (2019). Estimating the importance of maize root hairs in low phosphorus conditions and under drought. Annals of Botany 124(6):961-968.
Crossref
|
|
|
|
|
Kleerebezem M, Binda S, Bron PA, Gross G, Hill C, van Hylckama Vlieg JE, Lebeer S, Satokari R, Ouwehand AC (2019). Understanding mode of action can drive the translational pipeline towards more reliable health benefits for probiotics. Current Opinion in Biotechnology 56:55-60.
Crossref
|
|
|
|
|
Kuss AV, Kuss VV, Lovato T, Flôres ML (2007). Nitrogen fixation and in vitro production of indolacetic acid by endophytic diazotrophic bacteria. Pesquisa Agropecuária Brasileira 42(10):1459-1465.
Crossref
|
|
|
|
|
Li S, Zhao X, Wang J (2009). Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poultry Science 88(3):519-525.
Crossref
|
|
|
|
|
Louden BC, Haarmann D, Lynne AM (2011). Use of blue agar CAS assay for siderophore detection. Journal of Microbiology and Biology Education 12(1):51.
Crossref
|
|
|
|
|
Machuca A, Milagres A (2003). Use of CASâ€agar plate modified to study the effect of different variables on the siderophore production by Aspergillus. Letters in Applied Microbiology 36(3):177-181.
Crossref
|
|
|
|
|
Malavolta E, Vitti GC, de-Oliveira SA (1997). Avaliação do estado nutricional das plantas. Princípios e aplicações. https://repositorio.usp.br/single.php?_id=001070906&locale=en_US
|
|
|
|
|
Marco ML, Pavan S, Kleerebezem M (2006). Towards understanding molecular modes of probiotic action. Current Opinion in Biotechnology 17(2):204-210.
Crossref
|
|
|
|
|
Martin R, Langella P (2019). Emerging health concepts in the probiotics field: streamlining the definitions. Frontiers in Microbiology 10:1047.
Crossref
|
|
|
|
|
Nahas E, Assis LC (1992). Efeito da concentraçäo de fosfato na solubilizaçäo de fluorapatita por Aspergilllus niger. Revista de Microbiologia 23(1):37-42.
|
|
|
|
|
Oliveira GDL, Schneider M (2016). The politics of flexing soybeans: China, Brazil and global agroindustrial restructuring. The Journal of Peasant Studies 43(1):167-194.
Crossref
|
|
|
|
|
Pavinato PS, Rodrigues M, Soltangheisi A, Sartor LR, Withers PJA (2017). Effects of cover crops and phosphorus sources on maize yield, phosphorus uptake, and phosphorus use efficiency. Agronomy Journal 109(3):1039-1047.
Crossref
|
|
|
|
|
Pii Y, Borruso L, Brusetti L, Crecchio C, Cesco S, Mimmo T (2016). The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiology and Biochemistry 99:39-48.
Crossref
|
|
|
|
|
Ramachandra M, Crawford DL, Pometto AL (1987). Extracellular enzyme activities during lignocellulose degradation by Streptomyces spp.: a comparative study of wild-type and genetically manipulated strains. Applied and Environmental Microbiology 53(12):2754-2760.
Crossref
|
|
|
|
|
Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, Kim YH, Kim JJ, Rhee JC, Rhee PL (2008). Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Digestive Diseases and Sciences 53(10):2714-2718.
Crossref
|
|
|
|
|
Stewart C, Flint H, Bryant M (1997). The rumen bacteria. The Rumen Microbial Ecosystem, pp. 10-72.
Crossref
|
|
|
|
|
Stewart SD, Young MB, Harding JS, Horton TW (2019). Invasive nitrogen-fixing plant amplifies terrestrial-aquatic nutrient flow and alters ecosystem function. Ecosystems 22(3):587-601.
Crossref
|
|
|
|
|
Syed S, Tollamadugu NP (2019). Role of Plant Growth-Promoting Microorganisms as a Tool for Environmental Sustainability. Recent Developments in Applied Microbiology and Biochemistry, pp. 209-222.
Crossref
|
|
|
|
|
Watanabe F, Olsen S (1965). Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Science Society of America Journal 29(6):677-678.
Crossref
|
|