Listeria monocytogenes is a foodborne pathogen that is widely dispersed in the environment; it is found in soil, water and plant material, and can grow at refrigeration temperature and at unfavourable conditions of pH (up to pH 4.7) and salt (up to 10%). It can persist in the harsh conditions of the food processing environment from which it can contaminate food. Listeriosis, infection with L. monocytogenes, can be mild but the ability of the pathogen to cross the epithelial barrier of the intestinal tract, the blood brain barrier and the feto-placental barrier can also result in more severe illness including bacteremia and meningitis or spontaneous miscarriage. Although relatively rare, infection with L. monocytogenes can have a mortality rate of up to 30%, resulting in a serious hazard, particularly for the high risk groups of the elderly and immunocompromised individuals. As consumer demand for less processed, less preserved, longer shelf-life ready-to-eat food increases, the threat of L. monocytogenes to public health and the food industry continues to rise. In addition to being a public health threat, L. monocytogenes is a major economic burden on industry in terms of costs of analysis and potential product recalls. Awareness of its ubiquitous nature and understanding of its physiology and survival are important aspects of its control in the food processing environment with the aim of reducing the public health concern. Appropriate methodologies are required for its detection and isolation. Characterisation of strains by pulsed field gel electrophoresis (PFGE) and other genotypic methods can facilitate identification of putative contamination routes. Whole genome sequencing (WGS) of outbreak strains is becoming a part of outbreak investigation. Such WGS will lead to a greater understanding of the physiology of the organism as well as contribute to understanding epidemiology and pathogenicity. However, despite the advances, the best mechanism of public health protection is still prevention. Awareness of its presence, and control by conventional hygiene methods or by novel biocontrol methods such as bacteriocins and bacteriophage will help prevent cross-contamination of food from the environment and therefore reduce the public health burden.
In 2005, the Food and Agriculture Organisation and the World Health Organisation (FAO/WHO) held a joint regional conference on food safety for Africa. As a part of this, they identified the agency responsible for food safety in each country and highlighted issues like lack of international standards in food safety legislation, and the need for regional cooperation and collaboration as being important. It was also noted that there is a large degree of underreporting of foodborne illnesses (FAO/WHO, 2005).
In Africa, in general, there is little awareness or regulation relating to L. monocytogenes. For example, a recent amendment to the South African Foodstuffs, Cosmetics and Disinfectants Act (1972), referring to microbiological standards has nothing on Listeria spp. The Dairy Standard Agency (DSA) has guidelines in its Codes of Practice relating to L. monocytogenes in raw milk for final consumption, pasteurised milk, UHT milk, cream and salted butter (DSA, 2012). In these products, the guidelines recommend the absence (in 25 g) of L. monocytogenes in raw milk for consumption and in other products.
In general, companies that export, use the relevant regulation in the country they export to. One South African voluntary standard (South African National Standard [SANS] 885:2011) that specifically refers to the prevalence of L. monocytogenes in processed meat products, allows a maximum of 100 cfu/g at the end of shelf-life.
In Europe, Regulation (EC) No 2073/2005 (EC 2005) sets the microbiological criteria for L. monocytogenes in foods that must be complied with. This regulation primarily covers RTE food products, and requires that L. monocytogenes must be absent from foods (10 x 25 g) intended for infants and for special medical purposes, and allows different criteria depending on the ability of the food product to support growth of L. monocytogenes. For RTE foods unable to support the growth of L. monocytogenes, the levels should be <100 cfu/g throughout the shelf-life of the product (5 x 25 g). On the other hand, for RTE foods that are able to support the growth of the bacterium, L. monocytogenes must not be present in 5 x 25 g samples at the time of leaving the production plant; however, if the producer can show, to the satisfaction of the competent authority, that the product will not exceed the limit of 100 cfu/g throughout its shelf-life, the level should be <100 cfu/g throughout the shelf life of the product (5 x 25 g).
In Canada (http://www.hc-sc.gc.ca/fn-an/legislation/ pol/policy_listeria_monocytogenes_2011-eng.php) and Australia/New-Zealand (http://www.foodstandards.gov.au/code/microbiollimits/Pages/Criteria-for-Listeria-monocytogenes-in-ready-to-eat-foods.aspx), the regulations are in line with European regulations, allowing a differentiation between foods that can and cannot support growth.
However, in the USA there is ‘zero tolerance’ of L. monocytogenes (absence in 5 x 25 g of food is required at all times, and in the processing environment), where any occurrence is considered an offence (http://www.fsis.usda.gov/wps/portal/fsis/topics/regulatory-compliance/listeria).
Further discussion on regulations in different jurisdictions is reviewed in a special issue of Food Control published in 2011 (Anonymous, 2011).
Occurrence of L. monocytogenes in foods and food processing environments
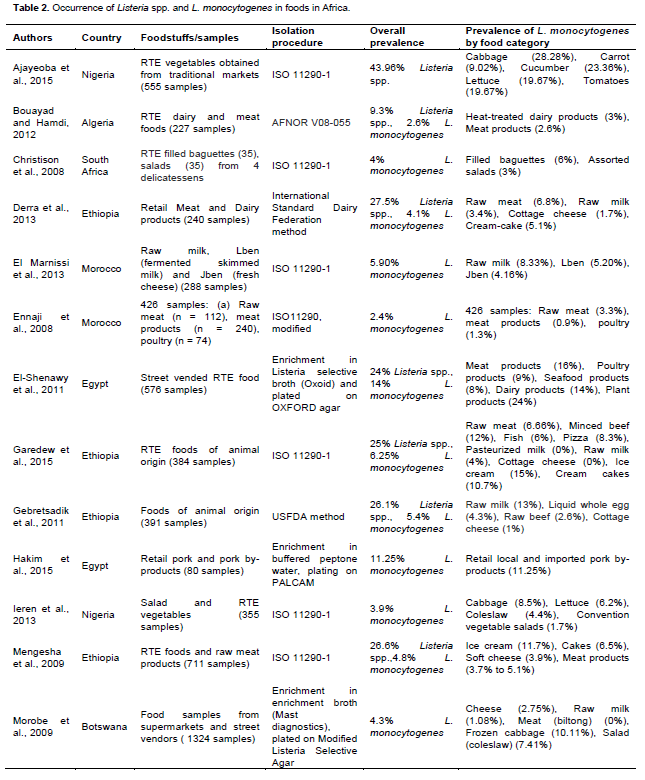
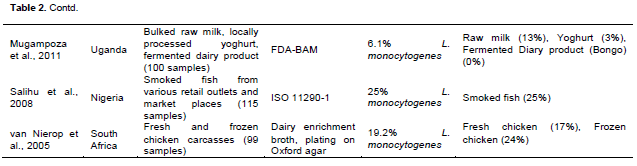
Because L. monocytogenes is ubiquitous in the environment and frequently present in the processing environment, it can contaminate food. A number of studies have shown the occurrence of L. monocytogenes in foods from Africa and other countries (Table 2). The occurrence of L. monocytogenes in some of these due to surveys was relatively high. These high values could be several factors for example, water quality or inadequate hygiene management in companies. Occurrence in/on food is a particular problem if the food can support the growth of the organism.
L. monocytogenes is frequently present in raw foods of both plant and animal origin (including fish), and it can be found in cooked foods due to post-processing contamination. Thus, it has been isolated from foods such as raw and unpasteurized milk, cheese, ice cream, raw vegetables, fermented meats and cooked sausages, raw and cooked poultry, raw meats, and raw and smoked seafood. In addition, its ubiquitous presence also leads to the potential for contamination of the food processing environment, where occurrence and persistence of
L. monocytogenes is frequent (
Nakari et al., 2014;
Vongkamjan et al., 2013; Jami et al., 2014).
A number of surveys of
L. monocytogenes in foods (especially RTE foods) and processing environments within food processing facilities have been performed in recent years. Table 2 shows the frequency of its presence in surveys conducted in Africa. Such surveys give valuable information for particular cases, but tend to be focused on a single analysis time at a few facilities. Surveys conducted over time at several processing facilities provide greater information on the ecology and persistence of
L. monocytogenes. For instance, in the particular case of Ireland, a study of the occurrence and persistence of
L. monocytogenes in foods and food processing environments of 48 food businesses involving regular sampling and characterization of isolates by serotyping and pulsed field gel electrophoresis (PFGE) has been recently published (
Leong et al., 2014). A European-wide survey on occurrence in different dairy and meat processing facilities over a 12-month period has also been reported (
Muhterem-Uyar et al., 2015). Additionally, varying occurrence of
L. monocytogenes has been reported in smoked fish products and processing facilities (Tocmo et al., 2014; Jami et al., 2014), dairy processing facilities (Pritchard et al. 1995) and ready-to-eat food producing facilities (Kovacevic et al., 2012).
Occurrence of L. monocytogenes at retail level
Contamination of RTE foods by L. monocytogenes can occur at various stages of the processing and distribution chain, including at retail level, although studies of occurrence at retail level do not necessarily imply that contamination occurred in the retail environment. Cross-contamination with L. monocytogenes at retail has been identified as the main source of L. monocytogenes in RTE deli products (Sauders et al., 2009; Tompkin, 2002; Vorst et al., 2006). Data from some surveys have indicated that RTE deli products handled at retail level have a significantly higher L. monocytogenes prevalence than products pre-packed by the manufacturer and not handled at retail (Gombas et al., 2003). For instance, Gombas et al. (2003) analysed 31,705 samples from retail markets in the USA and found an overall L. monocytogenes prevalence of 1.82%, with the prevalence ranging from 0.17 to 4.7% among the product categories tested. Interestingly, these authors observed significantly (p < 0.001) higher prevalence for in-store packaged samples than for manufacturer-packaged samples of luncheon meats, deli salads and seafood salads.
Gombas et al. (2003) analysed 31,705 samples from retail markets in the USA and found an overall L. monocytogenes prevalence of 1.82%, with the prevalence ranging from 0.17 to 4.7% among the product categories tested. Interestingly, these authors observed significantly (p < 0.001) higher prevalence for in-store packaged samples than for manufacturer-packaged samples of luncheon meats, deli salads and seafood salads.
It is important to note that recently conducted risk assessments for L. monocytogenes in deli meats indicated that the majority of listeriosis cases and deaths associated with deli meats are probably due to contamination of products at retail (Endrikat et al., 2010; Pradhan et al., 2010). Endrikat et al. (2010) estimated that 83% of human listeriosis cases and deaths attributable to deli meats are due to retail-sliced products, and Pradhan et al. (2010) performed a risk assessment using product-specific growth kinetic parameters that indicated that 63 to 84% of human listeriosis deaths linked to deli ham and turkey can be attributed to contamination at retail. Occurrence and cross-contamination at retail level do not attract much research, but are obviously an important source of listeriosis.
The persistence of
L. monocytogenes in the food-processing environment is well-documented but poorly understood (Carpentier and Cerf 2011; Lomonaco et al., 2009). This is partly due to the loosely defined term “persistence”. Generally, strains of
L. monocytogenes that have been repeatedly isolated from the same environment over a long period of time for example, over six months, are regarded as being persistent. Persistence of
L. monocytogenes isolates has been shown, often for many years, at larger scale cheese production facilities (Lomonaco et al., 2009), smaller artisan facilities (Fox et al., 2011), in the salmon industry (Tocmo et al., 2014), in meat processing plants (Gomez et al., 2015) and in poultry production plants (Lawrence and Gilmour, 1995; Ojeniyi et al., 2000). Nevertheless, although it is probable that these strains are surviving and persisting in the food-processing environment, it is also possible that consistent contamination from outside sources, for example, from raw materials, act as a continuous source of particular
L. monocytogenes strains (
Carpentier and Cerf, 2011).
The survival of
L. monocytogenes in food processing conditions which would be inhospitable to most bacteria can be due to several factors including: (1) ability to grow at a wide range of temperatures, especially refrigeration temperatures (
Schmid et al., 2009), (2) resistance to acid stress, (3) resistance to desiccation (
Takahashi et al., 2011), (4) resistance to sanitation agents and (5) biofilm formation (
Gandhi and Chikindas, 2007;
Galvão et al., 2012). This ability to survive where other bacteria cannot, allows
L. monocytogenes to grow with little competition from other bacteria.
Persistent strains do not appear to have any particular resistance genes to help them survive and persist in the environment, but
L. monocytogenes strains in general are hardy and resistance to various stresses is commonly seen (
Carpentier and Cerf, 2011). These characteristics allow
L. monocytogenes to survive and possibly even thrive in environments which would be considered unfavourable for general bacterial growth.
A major step to discourage bacterial growth in food processing is storage at refrigeration temperatures of 4°C. Although the majority of food pathogens cannot grow at this temperature,
L. monocytogenes can. Therefore, refrigerated storage essentially selects for
L. monocytogenes growth. Cold shock proteins have been shown to be essential for
L. monocytogenes’ ability to survive at low temperature as well as its ability to survive osmotic stress (
Schmid et al., 2009). An alternative sigma factor σ
B, encoded by
sigB, plays a vital role in
L. monocytogenes stress response. The
sigB gene has been shown to be vital in the survival of
L. monocytogenes in prolonged cold storage (
Moorhead and Dykes, 2004).
Harbourage sites are also a very important factor in the persistence of
L. monocytogenes. When used correctly, cleaning and sanitising procedures should be adequate to remove
L. monocytogenes from the environment (Cruz and Fletcher, 2012). However, a harbourage site could be an area where sanitation agents do not properly reach so
L. monocytogenes is not properly removed. When used correctly and in a high enough dosage,
L. monocytogenes does not seem to have increased resistance to disinfectants as compared to other bacteria (
Kastbjerg and Gram, 2012;
Lourenço et al., 2009). However, a harbourage site may be an area where the disinfection product reaches but at a lower concentration and it may not be properly dried so that a sub lethal amount of the product remains in the site. This may allow
L. monocytogenes strains sufficient time to develop a resistance to the product so that a community of
L. monocytogenes which is resistant to the cleaning product develops. This strain could then be spread out from the harbourage site to contaminate other areas of the facility (
Carpentier and Cerf, 2011).
Methods of detection
L. monocytogenes contamination usually occurs in very low numbers both in foods and in the processing environment so it is vital that any analysis performed includes one or more enrichment steps which inhibit other microflora, and allow both the increase of L. monocytogenes in sufficient numbers to allow detection and the recovery of injured/stressed cells. Three methods of analysis are most commonly used: the International Standard (ISO-11290) method which uses a two-step enrichment in Fraser broth, the United States Department of Agriculture (USDA) method which uses a two-step enrichment in University of Vermont media (UVM) and the One-broth Listeria method which has been approved for use by the Association Française de Normalisation (AFNOR) and takes considerably less incubation time and yields results in 2 days as opposed to the 4-5 days needed for the other two methods (
Gómez et al., 2013;
Zhang et al., 2007)
. All these methods involve plating on Listeria selective agar (traditional or chromogenic agars) and require confirmation of isolates as L. monocytogenes by biochemical or molecular tests.
The use of real-time PCR (RTi-PCR), in combination with traditional culture, to detect the presence or absence of Listeria has also been explored in recent years (Dalmasso et al., 2014; Rossmanith et al., 2010). By amplifying Listeria specific genes through PCR and quantifying them by the detection of a fluorescent probe attached to the DNA fragments, even low numbers of the bacteria can be detected within a few hours (after enrichment) as opposed to the several days it takes to complete traditional plating techniques. For best use, RTi-PCR should be combined with the traditional methods so that isolates can be obtained from the traditional method for strain typing. PCR is not suitable
for direct detection of L. monocytogenes in food as it lacks the required sensitivity, may be subject to inhibition by food ingredients and can detect the presence of DNA from live as well as dead cells
There is a wide range of different test methods for Listeria spp. and L. monocytogenes that have been reviewed by Välimaa et al. (2015). These include antibody-based tests, enzyme linked immunosorbent assay (ELISA), immune-capture methods, molecular methods targeting different genes and biosensor methods. Commercial kits are available for many of these methods, but it is not within the scope of this review to give detail of all these methods.
Characterisation of isolates
In order to identify the source or route of contamination, it is necessary to identify the strain type of
L. monocytogenes contaminating the food or the processing environment rather than just give a positive/negative result. Differentiation of
L. monocytogenes strains by serotyping is one of the oldest methods of typing and is based on the somatic (O) and flagellar (H) antigen differences between strains. As more exacting typing techniques have since been developed, serotyping of strains now offers little in terms of strain identification but can be helpful in the characterisation of strains (
Morobe et al., 2012). Thirteen serotypes are currently recognized which can be broadly split in 4 different serogroups. Doumith et al. (2004) have developed a widely used multiplex PCR which can be used to divide
L. monocytogenes strains into their serogroup (
Doumith et al., 2004). However, to further differentiate strains into their serotype, testing with antisera needs to be performed, which can be prohibitively expensive. Some reactions in antisera testing can be variable, for instance, currently serotypes 4b and 4e cannot be separated by this method. The vast majority of listeriosis outbreaks, approximately 90%, are caused by 1/2b and 4b serotypes, both of which are commonly found in food and food processing facilities. In general, serotype 1/2a has been isolated most frequently from food and the food processing environment (
Leong et al., 2014;
Shen et al., 2013). Although, it is thought that some serotypes may be generally more virulent than others, currently all
L. monocytogenes strains must be treated as virulent. Therefore, the identification of certain serotypes in a food or a processing facility does not mean that they will or will not cause disease.
The gold standard for
L. monocytogenes sub-typing remains pulsed field gel electrophoresis (PFGE), although other methods do offer advantages. PFGE is quite expensive, takes several days and requires trained staff to perform. However, it offers better discriminatory power than most other methods and can be compared between labs if performed according to international standard practices (
PulseNetUSA, 2009). Briefly, PFGE involves the lysis of cells to release the genomic DNA, the immobilisation of the DNA by trapping it in an agarose plug, the restriction digest of the DNA by specific enzymes and the migration of the DNA by gel electrophoresis over a long period of time, generally 21 h. The restriction by a specific restriction digest enzyme gives a distinct pattern of bands, a PFGE pulsotype, which can be used to identify a strain. Generally, two separate restriction digests are performed in two separate PFGE runs which gives a much better differentiation than the use of a single enzyme (
Borucki et al., 2004). The resulting PFGE pulsotypes can then be analysed by specialised software in order to accurately compare PFGE pulsotypes and the percentage similarity between strain patterns observed can be calculated. In this way, the same strain found in more than one area of a processing facility or over a period of time can be identified and the likely route/source of contamination may be identified (
Strydom et al., 2013).
Sub-typing of isolates, using methods such as pulsed field gel electrophoresis (PFGE), allows analysis of the molecular diversity of
L. monocytogenes strains present in processing facilities. Strains recurring in the processing environment over time (persistent strains) can be identified (
Stessl et al., 2014). Persistent strains in the environment represent an increased risk of contamination of food products. Control of these persistent strains, in particular, is an important part of a food processing facility food safety programme. After characterising the molecular diversity of isolates in the environment in question, putative routes of transmission and/or sources of entry into the environment can be identified. Muhterem-Uyar et al. (2015) identified three potential contamination scenarios that can increase the risk of food contamination, hot-spot contamination (where a specific area is contaminated), widespread contamination (where contamination is spread throughout the facility) and sporadic contamination (where non-persistent contami-nation occurs on an irregular basis). Visualisation of the contamination on a facility map can help identify the putative contamination routes (
Dalmasso and Jordan, 2013). Thus, control strategies can be adjusted/targeted to remove the source of contamination and interrupt the route of transfer to the food. Analysis of such results can not only identify persistent strains, but can also identify an area which may be colonised by a particular strain, leading to possible recontamination events. It can also be used to prevent the spread of strains throughout the facility.
Multilocus sequence typing (MLST) is also commonly used in strain typing, by sequencing a specific set of alleles of housekeeping genes and analysing the variations in the sequences, which allows identification of strain differences. Although less discriminatory than PFGE, the evolutionary distance between strains can be measured, by inspecting the number of alterations in the sequences, which cannot be performed by PFGE (
Haase et al., 2014).
PCR to detect different genes present in
L. monocytogenes strains is also commonly used for strain characterization. The presence/absence of different genes can be a good indication of whether or not a strain is virulent or whether it possesses genes which may help it to persist in a food processing facility. Several genes, such as the stress survival islet SSI-1 and the Tn
6188 transposon, which confers resistance to certain quaternary ammonium compounds, have been identified which appear to confer advantages to strains which may help them to survive in the seemingly inhospitable environment of a processing facility (
Müller et al., 2013;
Ryan et al., 2010). Similarly, several genes which contribute to virulence have been identified, for example listeriolysin S (LLS) and
actA, and the use of PCR to detect these genes can help to evaluate strains ability to cause disease (
Cotter et al., 2008;
Jacquet et al., 2002).
Other options for characterization of
L. monocytogenes isolates include Multiple-Locus Variable Tandem Repeat Analysis (MLVA), ribotyping, phenotypic or biochemical arrays and Fourier Transform infrared spectroscopy (
Stessl et al., 2014).
In recent years, the price of whole genome sequencing (WGS) has lowered significantly allowing the use of WGS in more routine applications. As opposed to PFGE or MLST, WGS examines the entire sequence of a genome, rather than just part of it, and so gives a much higher strain differentiation (
Gilmour et al., 2010). Individual genes can also be examined through the use of WGS. For example, in the Quargel cheese outbreak in Austria in 2009/2010, WGS was used to identify 2 distinct 1/2a
L. monocytogenes strains (QOC1 and QOC2) which overlapped to form the outbreak (
Rychli et al., 2014). Through WGS, specific genes which contribute to invasion and survival were also identified including the presence of a
vip homologue in QOC2 which encodes a surface protein, likely responsible for the higher invasion efficiency of QOC2 in comparison with QOC1. As costs continue to fall, WGS is increasingly being used in outbreak investigations as it offers a much more comprehensive overview of a strain and gives a significantly higher confidence in strain identification.
Certain foods are categorized in a higher risk category for contamination with L. monocytogenes. These are ready-to-eat (RTE) foods (including soft cheese, RTE meats and smoked fish), since the heat step of cooking, which would kill any L. monocytogenes present, is missing in these foods. Thus, if the food product is able to support the growth of L. monocytogenes, bacterial numbers can reach high levels, even at refrigeration temperatures, posing a health risk for consumers.
Determining the ability of RTE foods to support the growth of
L. monocytogenes is important, especially in those jurisdictions where there is no “zero tolerance” policy for
L. monocytogenes (e.g. Europe, Canada and Australia). The ability of
L. monocytogenes to grow in food products may be estimated based on specifications of the physico-chemical characteristics of the product, consultation of the available scientific literature, or predictive mathematical modelling. There are many tools that support predictive modelling of
L. monocytogenes in food. These include for example, general pathogen models such as Combase (www.combase.eu) and Pathogen Modelling Programme (PMP; http://pmp.errc.ars.usda.gov/PMPOnline.aspx), and more specific
L. monocytogenes models such as those at http://safesmokedfish.food.gov.uk/ or http://fssp.food.dtu.dk/. Such predictive models are useful, but for many reasons, including the possibility of overestimation/underestimation of growth in food products, in most cases growth assessment will involve laboratory-based studies, so-called challenge tests. From a public health perspective, overestimation of growth is a ‘fail-safe’ scenario, although such overestimation can be inaccurate from a food producer’s perspective. For example, in 40% of cases Combase predicted growth in cheese when no growth was seen in growth experiments (Schvartzman et al. 2011). It was further shown that the growth characteristics of
L. monocytogenes were different in liquid and solid matrices (
Schvartzman et al., 2010).
A challenge test can be defined as a laboratory-based study that measures the growth of
L. monocytogenes in artificially contaminated food stored under foreseeable abuse conditions of transportation, storage at retail and at consumer level. Performing challenge tests to assess growth of
L. monocytogenes on foods is not simple, since different RTE foods may require different laboratory approaches. However, in order to harmonize the laboratory methodology, some agencies have published guidelines in the last decade for the execution of challenge tests. The Food Standards Agency of New Zealand has recently published guidelines for undertaking challenge studies (
FSANZ, 2014), although this document is not specifically related to
L. monocytogenes. On the other hand, Canada also has guidelines which specifically relate to
L. monocytogenes (
Health Canada, 2012). In Europe, in order to facilitate the task of performing challenge studies, the European Union Community Reference Laboratory for
L. monocytogenes (EURL
Lm) prepared a Technical Guidance document in 2008 (
EC, 2008). This guidance document, which was aimed at describing the microbiological procedures for determining growth of
L. monocytogenes using challenge tests in the frame of the application of Regulation (EC) No. 2073/2005, has been recently updated (
EC, 2014). The European Guidance document of 2014, recently reviewed by Alvarez-Ordóñez et al. (2015), helps the Food Business Operator to decide whether a challenge test would be required for their food product, and describes the laboratory methodology that must be followed when carrying out a challenge test. This guidance document differentiates two types of challenge tests: the ones that determine growth potential of an inoculated strain or strains and those that calculate the growth rate of the strain(s). Growth potential is defined as the difference between the log
10 cfu/g at the end of the shelf-life and the log
10 cfu/g at the beginning of the test. When this difference is greater than 0.5 log
10 cfu/g, the food is classified into RTE foods that are able to support the growth of
L. monocytogenes. Alternatively, when the difference is less than 0.5 log
10 cfu/g, the food is classified into RTE foods that are unable to support the growth of
L. monocytogenes. The growth rate is on the other hand calculated from the growth curve as the slope of the straight line resulting from plotting the log
10 of cell numbers against time in the exponential phase of growth. The growth rate is an important parameter of the growth curve which depends on the inoculated strain(s), the intrinsic properties of the food (e.g. pH, NaCl content, aw, associated microflora, antimicrobial constituents), and extrinsic properties (e.g. temperature, gas atmosphere, moisture). Once the growth rate is known for a given food at a given temperature, it is possible to estimate the concentration of
L. monocytogenes at a given day of the shelf-life if the initial concentration is known. It is also possible to extrapolate the growth rate at a given temperature to predict growth rates at other temperatures in the same food.
Control of L. monocytogenes
As L. monocytogenes is an ubiquitous organism, its complete elimination is an unrealistic aim. Control is a more practical approach. Such control can be achieved by attention to detail in hygiene strategies, monitoring occurrence of the organism or using novel control methods such as bacteriocins and bacteriophage.
Novel methods of control
In recent years, in addition to novel technologies such as high pressure processing and pulsed electric field, novel methods for control of pathogens (and spoilage organisms) has focused on the use of natural anti microbial agents such as bacteriocins and bacteriophage.
Bacteriocins
Bacteriocins are ribosomally-synthesised peptides that are pore-forming agents, which act by disrupting the integrity of the target cell membrane. They have the potential to inhibit other bacteria, including pathogens, in many cases resulting in cell death. Therefore, they have potential as a mechanism to control L. monocytogenes. The spectrum of activity can be broad, where a wide variety of unrelated species are inactivated, or narrow, where only closely related species are inactivated. To date, insufficient data has been generated to obtain a complete picture of the potential use for many bacteriocins. The current regulatory situation dictates against the use of bacteriocins as biocontrol agents as in many cases, there is currently insufficient supporting data to assure the regulatory authorities of their efficacy and safety (Cotter et al., 2013).
Bacteriophage
Bacteriophages are viruses that infect and can kill bacteria and are logical candidates for biocontrol of
L. monocytogenes in food. They exhibit a high degree of specificity towards their target host bacterium, and as a result, are safe for use in food processing, considering they will have no detrimental effect on the microflora of the eventual consumer, nor will they have an effect on any other desired bacteria in the food. They also have other desirable attributes, including a relative stability during storage, and the ability to self-perpetuate. Of particular importance in terms of suitability for biocontrol of
L. monocytogenes is finding a virulent bacteriophage phage that is strictly lytic, rather than a lysogenic phage which can be genetically unstable. Lytic phages are genetically stable, will always kill infected cells, and cannot therefore integrate its genome into that of the bacterial chromosome. It is also of critical importance that the full genome sequence of such phage is known, and that any phage applied to food does not encode any virulence factors or toxins which may be harmful (
Hagens and Loessner, 2010).
The consensus among microbiologists is that bacteriophages do not have any known adverse effects on humans, animals or the environment. For this reason, many scientists and food safety experts predict that bacteriophages could become a useful tool in the reduction of pathogens in the food chain. However, there are concerns that limited safety data testing has been undertaken, although bacteriophages have been widely used for treatment of human diseases in the former Soviet Union (
Chanishvili, 2012).
The renewed interest in the use of bacteriophage as biocontrol agents has resulted in the development of several commercial products designed for this purpose, such as LMP-102 phage preparation (now more commonly known as ListShieldTM) and ListexTM. Although products have been approved for use in some countries, their use is not permitted in others. Biocontrol of L. monocytogenes with bacteriophage was reviewed by Strydom and Witthuhn (2015).
National monitoring programmes
Monitoring the food processing environment for the presence of
L. monocytogenes can be an effective mechanism in its control (Dalmasso and Jordan, 2013). Indeed, EU regulations require that food processing environments are sampled, although they do not state the number of samples to be taken, or the frequency of sampling (
EC, 2005).
In South Africa, over the last decade, most of the major retailers have developed their own food safety standards and audit protocols in order to protect their brands, and ultimately the consumer. These standards are all based on national legal requirements, for example, regulation R692 governing microbiological standards for foodstuffs and related matters (Foodstuffs, Cosmetics and Disinfectants Act, 1972) and prerequisite programmes as defined by the voluntary national standards of the South African Bureau of Standards. These regulations apply to a wide range of foodstuffs and beverages and while the absence of specified genera and species of various pathogenic bacteria are required in the products mentioned in these regulations, Listeria was not mentioned. Similarly, in regulation 1555 relating to milk and dairy products, all pathogens are required to be absent from raw milk intended for further processing or consumption, with no specific mention of Listeria. In an attempt to rationalise the number of audits and create a national approach to a food safety management system, the Consumer Goods Council of South Africa (CGCSA) formed the Food Safety Initiative (FSI) to promote a single audit standard. As all major retailers are members of a similar international organisation known as the Global Food Safety Initiative (GFSI), the decision was taken to adopt the GFSI Global Markets Capacity Programme as the single audit standard. The GFSI Global Markets Programme was launched in 2008 by the Global Food Safety Initiative to help small or less developed companies achieve certification to GFSI recognised food safety schemes and market access. It also helps to build food safety capacity through a structured, step-by-step approach.
From a dairy perspective, the DSA is a non-profit making company that aims to promote the compliance of milk and other dairy products, on a national basis, with product composition, food safety and metrology standards. This is done by regular and systematic monitoring of dairy products on farms and on retail shelves. In the DSA Codes of Practice (Milk South Africa, 2015), guidelines recommend the absence (in 25 g of product) of L. monocytogenes in raw milk for human consumption, pasteurised milk, UHT milk, cream and salted butter.
In Austria, a National Monitoring Programme has been established on a voluntary basis in the cheese industry. This is aimed at early detection of L. monocytogenes followed by targeted intervention strategies. There are four levels of investigation; Level 1 deals with the routine monitoring of samples, Level 2 is an intervention phase if positive results are detected, Level 3 is an intensive sanitation phase and Level 4 is a verification phase to confirm successful control.
In the Republic of Ireland, a research project on Listeria monitoring in food processing environments commenced in March 2013. Sixty seven food businesses, categorised into several industry sectors, such as dairy, meat, fish and vegetables, were involved in the project. Every two months, each business submits six environmental swab samples and two food samples for analysis by the ISO 11290 method. Businesses are informed on presumptive results immediately so that corrective actions can be taken, if necessary. Confirmatory PCR, serotyping and PFGE are performed on all isolates obtained. PFGE allows the identification of persistent strains and businesses are offered advice especially if particular contamination issues (such as persistence) are identified. Through this programme, a pattern of contamination in Irish food processing facilities can be seen, and a general L. monocytogenes contamination level of 4.6% was found in the first year of the programme with a similar positive percentage found in food and environmental samples (Leong et al., 2014). A similar programme of monitoring has recently been established in Northern Ireland.
Control of L. monocytogenes in the processing environment
It is relatively difficult to maintain a completely L. monocytogenes-free processing environment as many varying factors can have an effect on the occurrence of L. monocytogenes in the processing facility. These can include, for example, contaminated incoming raw materials, staff members acting as L. monocytogenes carriers, inefficient cleaning strategies and sampling programmes in place, the facility design to prevent contamination, the location of the facility near a farm, etc. Another major factor in the occurrence of L. monocytogenes is the awareness of the processing facility management and staff. The operation of a processing facility requires constant vigilance against bacterial contamination through various methods, and lack of awareness in this area can lead to more significant problems in end products which can result in product recalls, damage to company reputation, lawsuits, illnesses or even death. Thus, sampling and analysis are key factors in successful control. If occurrence is detected it can be eliminated through targeted intervention measures that help to prevent product contamination.
Although, final product testing is important in L. monocytogenes control programmes, it does not give information on the source and routes of product contamination. On the other hand, environmental testing is a more effective way to monitor hygiene and prevent contamination events (Tompkin, 2002). Tracing the source of L. monocytogenes is critical in the control of the organism in a localised environment, although the ubiquitous nature of L. monocytogenes makes it difficult to positively identify the source of contamination in some occasions. The potentially long incubation time for L. monocytogenes to cause disease can also make it difficult to trace the disease to a specific food and source of contamination (Goulet et al., 2013). It is therefore important to remove as many sources of contamination as possible from the food processing environment to reduce the possibility of food contamination.
Of utmost importance when sampling a processing environment for L. monocytogenes is actively looking for it, as opposed to selecting for negative results in order to adhere to regulations. Sampling directly after disinfection or cleaning or sanitation, for example, should be discouraged, unless the sampling is being used to evaluate the efficacy of the cleaning procedures. Proper sampling of a processing environment should include several areas in which contamination is most likely to occur, including both food contact and non-food contact surfaces. One of the most common areas to be contaminated are floor drains as any contamination throughout the facility is likely to be washed through the drain where L. monocytogenes can persist in a harbourage site (Carpentier and Cerf, 2011). Sampling should be done with a sponge-type swab, allowing sufficient surface area to be sampled. Adequate sampling will allow problems of contamination to be pre-empted and addressed in a timely manner. L. monocytogenes contamination of food products is a much more serious problem which requires significantly more intervention than contamination at the processing stage.
The following guidelines may help in tackling problems with L. monocytogenes.
Understanding the nature of L. monocytogenes contamination and attaching importance to it
Most food processing environments are contaminated to some extent. Adequate sampling for L. monocytogenes will help identify issues, which should be addressed immediately.
Regulations should be taken seriously and a food processing environment monitoring plan developed as a core activity of good hygiene practices (GHP).
Choosing the right sampling sites and methodology
The processing environment should be sampled with a view to finding the organism. The most informative sampling sites can vary depending on the food commodity produced. The difference in information that will be gained from sampling of food contact materials versus non-food contact materials should be considered. Sampling is the most critical procedural step and, if done inappropriately is of little benefit. Swabs that have enough contact surface to sample the 900 cm2 mentioned in many guidelines should be used. Sampling sites from manufacturing or handling steps that are applied on most of the products produced should be chosen (e.g. conveyor belts before packaging, slicer blades, etc.).
Choosing the right sampling frequency
Recommendations on sampling frequency can only be expressed in general terms. If a food processing environment (FPE) is being sampled for the first time, a broad sampling approach is used. If the contamination status is already known, a restricted number of sampling sites should be tested frequently rather than a lot of sampling sites only once. Sampling frequency can be reduced if negative results are shown, but should be increased again if positive results are detected or if there are changes to the processing environment or manufac-turing process. Sampling frequency should be dynamic.
Establishing critical control areas
Prioritisation of counter-measures, clearly defining critical control areas (CCA) where FPE contamination is not acceptable under any circumstances should be facilitated. It makes a difference whether a L. monocytogenes positive drain is located in a general processing area or if it is located where food is handled prior to packing. Critical control areas should be clearly marked (e.g. by marks on floors, in construction maps) and hygiene barriers should prevent CCAs from being visited or trespassed by unqualified personnel. Hygiene barriers, such as footbaths and change of personal protective clothing should reduce the risk of cross-contamination with L. monocytogenes. The high hygiene standard that should exist in CCAs can only be monitored by taking an appropriate number of FPE samples.
Trace the route of transmission of isolates most importantly in CCAs
To combat contamination, it is vital to keep all isolates at a safe and appropriate place (e.g. a contract laboratory). Use molecular typing to identify the putative routes of transmission of a pathogen in the facility, if possible. To reduce the costs, start with combating contamination in a CCA where the risk for contamination of the food commodity is the highest.
Be particularly aware at times of construction
During building work, hygiene measures are usually difficult to maintain at a food processing facility. On the one hand, craftsmen of various occupations with no training in hygiene need to have access to the FPE. Recommending the use of hygiene protection (overshoes and overcoats) to craftsmen is frequently in vain because it limits their maneuverability. Building material, often stored outdoors before use, needs to be carried around. Insects and rodents can get access to the FPE. On the other hand, the food business operator (FBO) frequently needs to produce food in processing rooms adjacent to the construction area. Be aware of the increased risk of cross contamination during such construction periods, and construct physical barriers between food production and construction. The FBO should try to prevent access of craftsmen to production areas as much as possible. Careful and intensified sanitation programmes in the processing areas during the construction phase, and sanitisation of the entire FPE after completion of the construction phase should be observed. The success of this process should be verified by subsequent sampling of the FPE.
Critical review of the floor sanitation procedures applied in cases of widespread contamination
If FPE monitoring demonstrates a widespread contamination of a genetically indistinguishable L. monocytogenes strain, sanitation procedure (specify the type of sanitizer to be used and use it appropriately. Make sure all areas are covered. Allow all the surfaces to dry off before food), and the workflow system should be re-considered. Drain water sampling should be used to control the efficiency of sanitation.
Structuring your data and using a processing facility map (roughly drawn) to document your progress and efforts
Safe food production is possible even if there is contamination of a FPE. However, the following criteria must be met:
1. The extent of contamination must be known (implies intensified sampling)
2. Contamination must be never detected in the food commodity produced
3. FPE contamination must be infrequent (reported only irregularly)
4. Contamination must be detectable only in compartments where the risk for cross-contamination is low
5. The food produced must not support growth of L. monocytogenes on its surface.
Documentation is critical in any FBO communication process, either within an operation or with regulators or specialists from the outside. Documentation of ingredients and raw materials used as well as any contamination patterns is essential. A map of the facility (roughly drawn) can help with this.
To demonstrate that the FBO has met these requirements, is necessary to organize the data into a structured decision making process. The advice of experts that help to facilitate the decision making process should be sought.