Full Length Research Paper
ABSTRACT
Klebsiella pneumoniae is increasingly being isolated from the stool of Nigerian patients. K. pneumoniae was isolated from stool samples submitted to two clinical laboratories in Nigeria from patients presenting with various symptoms of gastrointestinal tract infections, including diarrhoea. The authors characterized the virulence and antimicrobial resistance genes of these K. pneumoniae strains. Sixteen pure cultures of heavy growth of K. pneumoniae isolated from two facilities in Nigeria were subjected to susceptibility testing using a panel of antibiotic, with agar dilution method. Paired-end Illumina whole genome sequencing was completed using a NextSeq instrument. Virulence genes including astA, senB, and gad were found in 5 isolates. Multiple plasmid replicons were present; IncF and Col were common plasmids while others were IncR and IncY. Four different STs were found; ST914, ST1962, ST494, and novel ST. These isolates carried various important resistance genes to cephalosporins, fluoroquinolones, aminoglycosides, and so on, including blaCTX-M-15 in one of the isolates. Diarrhoeagenic K. pneumoniae is present, which is caused by plasmid-mediated virulence genes such as astA, senB, and gad. Fluoroquinolone and third generation cephalosporin resistance were discovered.
Key words: Klebsiella, diarrhoea, virulence genes, antibiotics resistance, genomics, Nigeria.
INTRODUCTION
The common diarrhoea-inducing pathogens include, rotavirus, norovirus, diarrhoeagenic Escherichia coli, Salmonella spp., Shigella spp., and Yersinia species (Operario and Houpt 2011; Sjoling et al., 2015). Klebsiella pneumoniae can also cause diarrhoea, but most studies focus on extra intestinal infections. Although, K. pneumoniae occurs as a commensal in the intestine, it may induce diarrhoea through the production of toxin (Guarino et al., 1989; Panigrahi et al., 1991). Some of these diarrhoeagenic strains encode thermostable toxins similar to enterotoxigenic or enteroaggregative toxins of E. coli. Enteroaggregative heat stable toxin 1 (EAST-1) is encoded by astA gene on a 60-MDa pAA plasmid (Telli et al., 2010). astA produces a toxin that stimulates the production of high levels of cyclic guanosine monophosphate (cGMP) in cells such that sodium/ chloride co-transport is inhibited and absorption of water and electrolytes from the intestine at villus tips is reduced, resulting in diarrhoea (Telli et al., 2010). Similarly, senB a plasmid-mediated gene has been described to produce the TieB enterotoxin in enteroinvasive and uropathogenic E. coli strains (Nataro et al., 1995; Touchon et al., 2009). K. pneumoniae isolation from stool is increasing in Nigerian hospitals, and they are usually ignored as native intestinal flora even when isolated as a pure culture from cases of diarrhoea in children and adults. The authors therefore investigated the presence of K. pneumoniae and characterized their virulence and antimicrobial resistance genes in Nigeria.
METHODS
Bacterial isolates
Sixteen pure cultures of heavy growth of K. pneumoniae isolated from stool of different patients from two clinical laboratory facilities in Nigeria (diagnosed with various diseases) were identified using cultural morphology, Gram reaction (Bartholomew and Mittwer, 1952), standard biochemical tests; indole, citrate, motility, methyl red, voges proskauer and sugars (Ewing, 1986; Barrows and Feltham, 1993), and API 20E strips (BioMérieux, Basingstoke, UK). Ethical approval was obtained for the study from Federal Capital Territory, Health Research Ethics Committee with approval number FHREC/2018/01/95/14-08-18, including informed written consent from the participants.
Antibiotic susceptibility testing
Susceptibility of all isolates to a panel of antibiotics classes in common clinical use in these hospitals were determined by the agar dilution method on Mueller–Hinton agar according to the recommendations of CLSI breakpoints (Wayne, 2018). All runs included the control organism E. coli (ATCC 25922).
Whole genome sequencing and bioinformatics
DNA was extracted using a QIAamp1 DNA Mini Kit (QIAGEN, Crawley, UK) according to the manufacturer's instructions. Paired-end Illumina whole genome sequencing was completed using a NextSeq instrument at the Quadram Institute Bioscience. Bioinformatics used an in-house pipeline hosted on an IRIDA instance; sequences were assembled with shovill and annotated with prokka and core snps identified with snippy. Furthermore, assemblies were used to search for plasmid content using the ‘PlasmidFinder’ tool hosted at the Centre for Genomic Epidemiology(https://cge.cbs.dtu.dk/services/PlasmidFinder), and for isolates likely to carry significant resistance genes in plasmids, ‘plasmidSPAdes’ was used to assemble likely plasmid contigs from the trimmed reads.
Comparative genomics
Strains belonging to sequence types implicated in globally disseminated disease were compared against completed genomes were available to determine relationships between sources of strains, Nigerian strains and others in global circulation. Reads were also mapped against reference strain HS11286, SNPs identified and phylogenetic relationships determined to determine whether clones in Nigeria are divergent or highly similar from those seen globally.
RESULTS AND DISCUSSION
In the 16 isolates of K. pneumoniae, 5 encoded virulence genes namely, astA, senB, and gad. All of these 5 isolates had the gad gene while only 2 had all the 3 genes (Figure 1). astA encodes enteroaggregative E. coli heat-stable enterotoxin (EAST 1), senB encodes TieB enterotoxin, while gad is a glutamate decarboxylase protein. The contribution to pathogenicity of gad has been debatable; this enzyme has been reported to be essential in the survival of enteric pathogens in the acidic conditions of the mammalian stomach (Lin et al., 1996). Two isoforms of glutamate decarboxylase (GAD) encoded by gadA and gadB are the most effective E. coli acid resistance system (De Biase et al., 1999). The major role of this system might be to facilitate the colonization of the intestines by commensal strains of E. coli. This is a housekeeping gene in E. coli, but not part of the K. pneumoniae core genome and here it was 31.3%.
EAST 1 has been identified as a plasmid-mediated enterotoxin of low molecular weight and associated with enteroaggregative E. coli. It shares about 50% protein identity with heat-stable enterotoxin (STa), and the gene has also been found in many enterotoxigenic E. coli strains (Contreras et al., 2011) and other members of Enterobacteriaceae such as Salmonella (Paiva de Sousa et al., 2001). The authors demonstrated the presence of the gene in different strains of K. pneumoniae (ST914 and ST1962) from two different geographic regions of Nigeria, and these patients presented with diarrhoea episodes. There are reports debating whether astA is sufficient to cause diarrhoea without other virulence factors in E. coli, but Soto et al. (2009) and Mirzarazi et al. (2015) reported that E. coli acquired this toxin to become a diarrhoea-causing agent, which may be the situation in these K. pneumoniae strains.
The two K. pneumoniae that encoded astA also encoded senB, a plasmid-mediated gene associated with enterotoxicity of enteroinvasive E. coli (EIEC) that codes the TieB protein. It has also been described to have some role in uropathogenic E. coli. Multiple plasmid replicons were present in all strains; however IncF and Col plasmids play a major role in the dissemination of these genes (Figure 1).
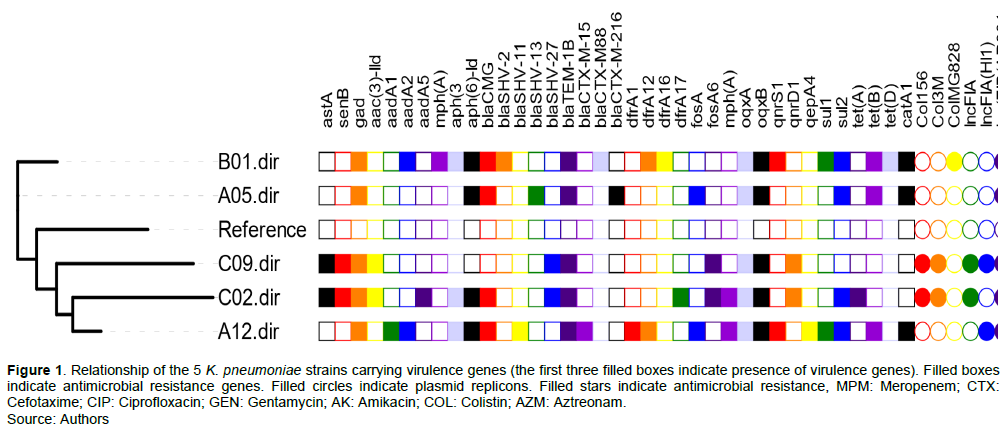
Susceptibility testing identified all 5 isolates with these virulence genes were sensitive to meropenem (MIC values of ≤ 0.03 µg/ml) while isolates with both astA and senB had low level resistance to ciprofloxacin and cefotaxime (MIC value of 8 µg/ml each), whereas other strains had high level resistance to ciprofloxacin (MIC value of >64 µg/ml). Resistance to colistin varied with MICs between 1 and >64 µg/ml. The isolates carried a range of important resistance genes to cephalosporins, fluoroquinolones, aminoglycosides, with the presence of various PMQR genes, aph(3'')-Ib, aac(3)-IId, variants of blaCTX-M including blaCTX-M-15 found in one of the isolates.
CONCLUSION
There is presence of diarrhoeagenic K. pneumoniae linked to carriage of plasmid mediated virulence genes such as astA, senB, and gad in diversity strains in Nigeria. This may represent a greater burden of diarrhoea caused by K. pneumoniae in future.
FUNDING
DOO was the recipient of an overseas scholarship from the Royal Society Newton International Fellowship (NF110504) and their Follow-on funding which supported this work, including support from Quadram Institute of Bioscience.
CONFLICT OF INTERESTS
The authors have not declared any conflicts of interests.
ACKNOWLEDGEMENTS
The authors thank the staff within the laboratory of the study site hospitals for their technical support during this study.
REFERENCES
|
Barrow GI, Feltham RKA (1993). Characters of Gram-negative bacteria. In: Cowan and Steel Manual for Identification of Medical Bacteria. 3rd edition, Cambridge University Press pp. 94-149. |
|
|
Contreras CA, Ochoa TJ, Ruiz J, Lacher DW, Rivera FP, Saenz Y, Chea-Woo E, Zavaleta N, Gil AI, Lanata CF, Huicho L (2011). Phylogenetic relationships of Shiga toxin-producing Escherichia coli isolated from Peruvian children. Journal of Medical Microbiology 60(Pt 5):639-646. |
|
|
De Biase D, Tramonti A, Bossa F, Visca P (1999). The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Molecular Microbiology 32(6):1198-1211. |
|
|
Ewing WH (1986). Edwards and Ewings Identification of Enterobacteriaceae. 4th edition. Elsevier Science Publishing, New York. |
|
|
Guarino A, Guandalini S, Alessio M, Gentile F, Tarallo L, Capano G, Migliavacca M, Rubino A (1989). Characteristics and mechanism of action of a heat stable enterotoxin produced by Klebsiella pneumoniae from infants with secretory diarrhoea. Pediatrics Research 25(5):514-518. |
|
|
Lin J, Smith MP, Chapin KC, Baik HS, Bennett GN, Foster JW (1996). Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Applied and Environmental Microbiology 62(9):3094-3100. |
|
|
Mirzarazi M, Rezatofighi SE, Pourmahdi M, Mohajeri MR (2015). Occurrence of genes encoding enterotoxins in uropathogenic Escherichia coli isolates. Brazilian Journal of Microbiology 46(1):155-159. |
|
|
Nataro JP, Seriwatana J, Fasano A, Maneval DR, Guers LD, Noriega F, Dubovsky F, Levine MM, Morris Jr JG (1995). Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infection and Immunity 63(12):4721-4728. |
|
|
Operario DJ, Houpt E (2011). Defining the causes of diarrhoea: novel approaches. Current Opinion in Infectious Diseases 24(5):464-471. |
|
|
Paiva de Sousa C, Dubreuil JD (2001). Distribution and expression of the astA gene (EAST1 toxin) in Escherichia coli and Salmonella. International Journal of Medical Microbiology 291(1):15-20. |
|
|
Panigrahi D, Roy P, Chakrabarthi AR (1991). Enterotoxigenic Klebsiella pneumoniae in acute childhood diarrhoea. Indian Journal of Medical Research 93:293-296. |
|
|
Sjoling A, Sadeghipoorjahromi L, Novak D, Tobias J (2015). Detection of major diarrhoeagenic bacterial pathogens by multiplex PCR panels. Microbiological Research 172:34-40. |
|
|
Soto SM, Guiral E, Bosch J, Vila J (2009). Prevalence of the set-1B and astA genes encoding enterotoxins in uropathogenic Escherichia coli clinical isolates. Microbial Pathogenesis 47(6):305-307. |
|
|
Telli M, Guiral E, Martínez JA, Almela M, Bosch J, Vila J, Soto SM (2010). Prevalence of enterotoxins among Escherichia coli isolates causing bacteraemia. FEMS Microbiology Letters 306(2):117-121. |
|
|
Touchon M, Hoede C, Tenaillon O, Barbe V, Baeriswyl S, Bidet P, Bingen E, Bonacorsi S, Bouchier C, Bouvet O, Calteau A (2009). Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genetics 5(1):e1000344. |
|
|
Wayne PA (2018). Clinical Laboratory Standard Institute: Performance Standards for Antimicrobial Susceptibility Testing: Informational Supplement, M100. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0