ABSTRACT
Milk is considered a nutritionally noble food and is therefore suitable for feeding children and adults. However, contamination of milk by mycotoxins may pose a health risk to the consumer. Aflatoxins, mycotoxins produced by fungi of the genus Aspergillus, can be found in several food products, including milk and its derivatives, which reinforces the importance of this type of study on the occurrence of the aflatoxin M1 (AFM1) in raw milk. We analyzed 45 samples of raw bovine milk from expansion tanks from 23 farms in different municipalities of the dairy belt of the state of Alagoas/Brazil. Samples were collected directly from the cooling tanks and transported under refrigeration for analysis. The method used for the extraction of AFM1 was that proposed by the Adolfo Lutz Institute. On the other hand, the detection of AFM1 occurred by high performance liquid chromatography (HPLC) by identifying retention times. The results of the analyses indicated that none of the collected samples presented contamination by aflatoxin M1, thus indicating that the milk commercialized in Alagoas shows a good quality against this toxic agent.
Key words: Aflatoxin M1, bovine milk, Alagoas.
Milk and its derivatives are important foods in the human diet since they are complete foods rich in calcium, protein and lipids, which serves as a basic nutrient for infant feeding (Castro et al., 2013). According to Vilela (2002), milk is one of the six most important products for Brazilian agriculture. Consequently, its contamination by mycotoxins poses a great risk to health in addition to causing significant economic losses mainly for the small producer.
Mycotoxins are secondary metabolites produced by fungi when there are favorable conditions for their development, such as moisture, temperature, oxygen presence, time for fungal growth, substrate constitution, genetic characteristics and competition among fungal lineages. More than 300 mycotoxins are known, and the human exposure to such substances result mainly from the consumption of food derived from plants contaminated by the ingestion of their metabolites present in products of animal origin, such as meat and eggs, or by exposure to air containing toxins (CAST, 2003; Bennett and Klich, 2003; Zain, 2011; Oliveira et al., 2014).
The main mycotoxin-producing fungi belong to the genera Aspergillus, Penicillium and Fusarium (Ribeiro, 2008; Queiroz et al., 2009; WHO, 2009; Codex Alimentarius, 2011). Among the main mycotoxins of interest in the food area are aflatoxins, patulin, ochratoxin, zearalenone, trichothecenes, fumonisins. Aflatoxins are the most important from a toxicological point of view (Jay, 2005; Codex Alimentarius, 2011). These substances are produced by three species of Aspergillus: Aspergillus flavus, Aspergillus parasiticus and, rarely, Aspergillus nomius, which contaminate plants and their products. A. flavus produces only the aflatoxin B, while the other two species produce aflatoxins B and G (Jay, 2005).
The toxic effects caused by mycotoxins to the human body range from acute problems to chronic diseases (Wild and Gong, 2010). Aflatoxins are severely toxic, immunosuppressive, mutagenic, teratogenic and carcinogenic chemicals. The main organ affected by toxicity and carcinogenicity is the liver (Aycicek et al
., 2005). When addressing mycotoxins, there is an aggravation, since their removal from food is very difficult. The most effective way of prevention is to control the growth of fungi in food (Erkekoglu et al., 2008). Therefore, in the preparation of formulas for infants, a strict control is necessary using quality raw materials (Mahdavi et al., 2010). When we speak of milk and milk products aflatoxin M
1 is the most significant, being a substance in the hydroxylated form of aflatoxin B
1, present in milk when the animals were fed with feeds contaminated with aflatoxin B
1 (
Flor-Flores, Lizarraga, López de Cerain and González-Peñas, 2015).
Aflatoxins B1, B2, G1 and G2 are named for fluorescence of blue (blue) and green (green) when exposed to ultraviolet light (Franco and Landgraf, 1996). These toxins are generally found together in many foods, cereals and rations - feed. various proportions (Galvano et al., 1996; Bakirci, 2001; Creppy, 2002). On the other hand, the aflatoxins M1 and M2 are hydroxylated metabolites of aflatoxins B1 and B2, respectively, produced by animals and generally excreted in the milk and urine of cattle and other mammalian species that consumed food or feed contaminated by these aflatoxins (Creppy, 2002). The designation "M" originates from "milk toxin" because it is a toxin excreted in milk. The aflatoxin M1 has a high genotoxic activity, although it has a lower carcinogenic potential compared to the aflatoxin B1 (Baggio, 2006).
Several countries around the world have laws controlling aflatoxins in food, and most indicate the maximum levels allowed for specific products. The European Union determines limits of 2-12 μg kg-1 for the aflatoxin B1 and 4-15 μg kg-1 for total aflatoxins (B1, B2, G1 and G2) in nuts, dried fruits, cereals and spices. For milk and dairy products, the established limit is 50 μg kg-1 of aflatoxin M1 (AFM1). However, in the case of baby foods, these limits should be 0.10 μg kg-1 for B1 and 25 μg kg-1 for M1. The United States, in its food safety regulation, includes a total limit of 2×104 μg kg-1 of aflatoxins (B1, B2, G1 and G2) in all feed except milk, which should be 5×10-4 μg kg-1 for aflatoxin M1 (FAO, 2004). In Brazil, the Ministry of Health, in the resolution of the Collegiate Board of Directors (RDC-07), of February 18, 2011, establishes the maximum limits of mycotoxins allowed for foods. In Brazil the limits for aflatoxin M1 are 0.5, 5.0 and 2.5 μg kg-1, respectively for fluid milk, milk powder and cheeses (Brazil, 2011). For the determination of AFM1, a classical method is generally that of high performance liquid chromatography (HPLC), HPLC being considered the reference method for this analysis (Andrade et al., 2013).
Brazil, in 2014, ranked fifth in the world ranking of milk production, behind the European Union, the United States, China and India (IBGE, 2014). The national milk production this year was 35.17 billion liters. The northeast region was responsible for the production of 3.88 billion liters, of which 305 million liters were produced in the state of Alagoas (EGL, 2016). The dairy belt of the state of Alagoas has approximately 3,000 producers, generating 25,000 direct jobs, which is relevant to the state economy (CPLA, 2016; EMBRAPA, 2016).
AFM1 contamination of milk is a result of contaminated and metabolized animal feed in the liver of this (Andrade et al., 2013) by ingesting preformed toxins through diets and foods contaminated with fungi (The AFM1 contamination of milk is the result of feed contaminated with fungi given to the animal, which is metabolized in in its liver (Andrade et al., 2013) by ingestion of preformed toxins). This contamination is mainly due to grains already contaminated by toxins or rations stored under inadequate conditions, as well as by ingestion of forage containing endophytic fungi (Zain, 2011). The rations in which the mycotoxin producing fungi are most prone their development are peanuts, maize and wheat, beans, rice, cocoa, barley, cottonseed, chestnut, wheat and others (Rosmaninho et al., 2006; Oliveira et al., 2014). Developed countries have already realized that reducing mycotoxin levels in food not only reduces the financial burden on public health care, but also brings advantages in international trade and can increase exports (Schwarzer, 2009). The European Union more strictly restricts the concentration of AFM1 in milk (0.05 ng/mL) (Commission Regulation, 2014).
Due to the great importance of milk for human consumption and the economy of a country, the objective of this work was to evaluate the presence of AFM1 in raw milk samples from 45 community expansion tanks, these tanks being represented by all producers in the region, collected from January to May 2013 in 23 municipalities in the state of Alagoas/Brazil.
Sample collection
Samples of milk were collected in triplicate of 45 community expansion tanks from 23 municipalities in Alagoas, the tanks had an approximate capacity of 2000L and a cooling temperature of 4°C, the municipalities and quantities of tanks collected were: 6 tanks in the municipalities of Batalha and Igreja Nova, 4 tanks in the municipality of Penedo and 3 tanks in the municipality of Senador Rui Palmeira, 2 tanks in the municipalities of Belo Monte, Cacimbinhas, Craíbas, Porto Real do Colégio, Girau do Ponciano, Mar Vermelho and Traipú, and 1 tank in the municipalities of Ibateguara, Jacaré dos Homens, Jaramataia, Junqueiro, Teotônio Vilela, Viçosa, Tanque D'Arca, São Brás, São Sebastião, Paulo Jacinto, Piaçabuçu and Quebrangulo. The collections were carried out from January to May 2013. About 500 mL of milk were collected and stored in pre-sterilized glass containers. The transport was carried out using isothermal boxes containing recyclable ice for the Bioprocess Laboratory, belonging to the Coordination of Chemistry of the Federal Institute of Alagoas (IFAL), Maceió campus. The samples were kept at 4°C in a refrigerator for analysis within 24 hours. The methodology used is described by the Adolfo Lutz Institute (2008).
Sample preparation
The extraction of AFM1 was performed according to methodology described by the Adolfo Lutz Institute (2008). For the extraction of AFM1 in bovine milk, 37.5 mL of the sample plus 300 mL of methanol A.P. and 12.5 g of celite (SiO2) were used, kept under stirring for 30 min. Subsequently, the filtration of the sample was performed on qualitative filter paper, transferring to a settling funnel to collect the phases after separation. After separation of the phases, the methanolic phase was collected. To the methanolic phase 50 mL of hexane and 112.5 mL of 4% NaCl (sodium chloride) m/v solution were added while stirring for 3 min. At the end of the stirring, followed by separation of the phases, the methanolic phase was collected again, discarding the hexane phase. An additional 50 mL of hexane was added, and the methanolic phase was collected by discarding the hexane phase. In the methanolic phase, 50 mL of CHCl3 (chloroform) was added, maintaining it by shaking for 3 min. Subsequently, the chloroform phase was collected, and this procedure was repeated once again. After the chloroform phase was collected, it was transferred to a settling flask, and 150 mL of 4% m/w NaCl solution was added thereto, while stirring, then adding about 5 g Na2SO4 (anhydrous sodium sulfate) for water removal. The chloroform phase was filtered, collected and transferred to a volumetric flask for rotoevaporation. After evaporating and forming a viscous extract, the extract was re-suspended in 2 mL of CHCl3 (chloroform), and transferred to an amber flask. Using nitrogen gas, the sample was evaporated. The resuspension of this dried extract was performed in 1mL of a 57% solution of deionized H2O, 17% acetonitrile and 26% methanol. After this step, the samples were homogenized by placing them on ultrasound equipmento for 15 minutes. Then, the filtration was performed on membranes of 0.45 μm Millex (Millipore, HV filter hydrophilic, MA, USA), and stored in amber bottles.
Chromatographic conditions
Previously, tests were performed on raw milk samples to optimize the separation and detection of aflatoxin M1 (AFM1) by High Performance Liquid Chromatography (HPLC) (Shimadzu®, Kyoto, Japan). They were used in a C18 reverse phase packed column (Shim-pack VP-ODS, 4.6 mm x 150 mm, 4.6 μm) mounted on an HPLC system coupled to an excitation fluorescence detector of 365 nm and emission of 460 nm. The mobile phase was composed of 55% deionized water acidified with 1% acetic acid, 35% methanol (Sigma-Aldrich) and 10% acetronitrile (Sigma-Aldrich) in isocratic mode with a flow of 0.8 mL min-1 and injection of 20 μL of material (analytical standard and sample) previously filtered with a 0.45 μm Millex membrane. Identification of the compound was made from the comparison of the retention time (RT) of the pure analytical standard (Sigma-Aldrich). For the construction of the AFM1 analytical curve, concentrations of 0.3, 0.35, 0.4, 0.5, 0.6, 1.0 and 5.0 μg L-1 were used, each point being the result of the average of three replicates. The quantification was performed with the interpolation of the areas of the chromatographic peaks of the samples through the linear function obtained from the linear regression of the calibration curve.
Aflatoxin M1 recovery test
For this test of recovery, the procedure of extraction of AFM1 was the same as applied to the samples. During preparation of the extraction of the milk for validation of the method, they were contaminated at three different levels, where to each three samples of milk containing 37.5 mL were added, respectively, 2.0, 4.0 and 8.0 μL of the standard to perform the recovery test; in addition, the test was also performed with a sample without contamination.
The performance of the analytical method by HPLC with reversed-phase C18 column was determined by the RF-20A diode array detector for the determination of the aflatoxin M1 by the recovery of these three levels of contamination with the respective concentrations: 0.0267, 0.0534 and 0.10680 μg/L and a sample without contamination.
Determination of aflatoxin M1 in the sample extract
20 μL aliquots of the purified sample extract were injected using the same chromatographic conditions as the preparation of the calibration curve. The aflatoxin M1 peak of the sample solution was identified by comparison with the retention time obtained by the injection of aflatoxin M1 standard solutions. The calculation of the mass of AFM1 was performed according to AOAC 17, adapted for use of area in place of sample peak, obtaining the concentration of AFM1 in μgL-1.
where: A = peak area of ​​the sample; A' = peak area of ​​the standard; C' = standard concentration (μg/mL); VI' = injected volume of the standard; VI = injected volume of the sample; V = final sample volume (μL); VL = volume of milk represented at the end of the extract (mL).
Statistical analysis
Statistical analysis was performed using the SAS program (Statistical Analyzes System, 2003).
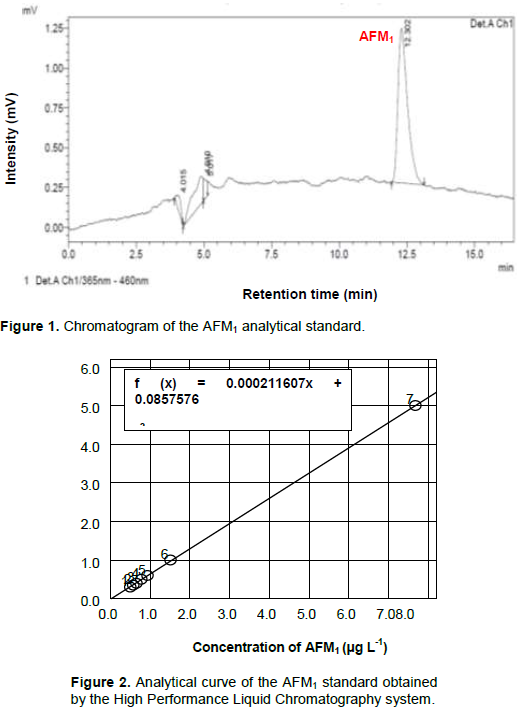
The chromatogram for the AFM1 analytical standard (Figure 1) shows that the retention time for this compound was 12.3 minutes. It presents an excellent chromatographic resolution with the chromatographic conditions applied. The linear regression from 0.3 to 5.0 μg L-1 of the calibration curve generated a linear function f (x) = 0.000211607x + 0.0857576, with coefficient (r2) of 0.9996 (Figure 2). The obtained R2 value for the AFM1 curve demonstrates the linearity of the obtained curve. Thus, the working range of the method, which corresponds to the concentration range used in the composition of the calibration curve, was linear and can be used for quantification. The limit of detection (LD) was 0.08 μg L-1, considering the minimum amount of AFM1 generating a measurable signal relative to the background noise as a ratio of 3:1. The limit of quantification (LQ) was 0.026 μg L-1.
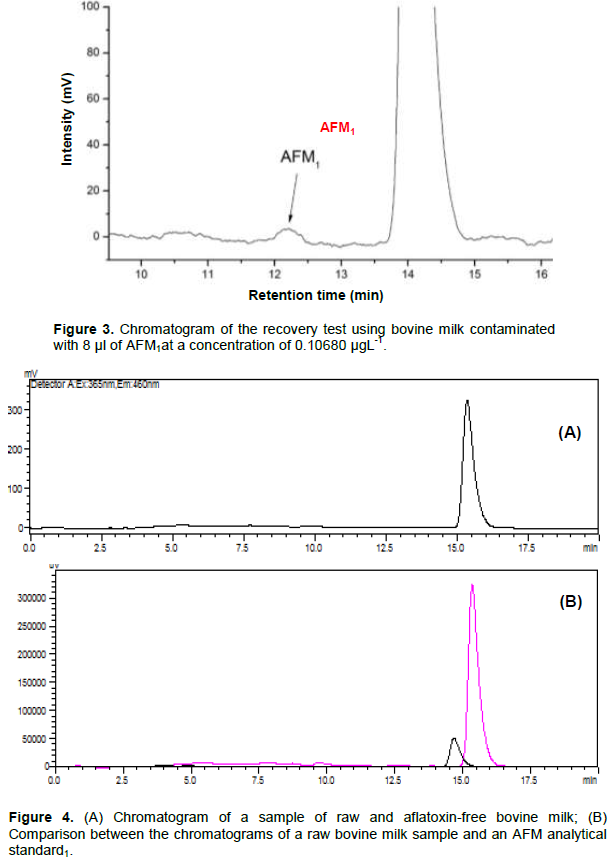
For the recovery test, as mentioned in the methodology, 3 milk samples were contaminated at different levels of contamination, and one was absent from intentional contamination. For this test, the same extraction treatment was applied to the milk samples collected in the expansion tanks, as well as the same chromatographic conditions. The contamination during this extraction procedure was made in order to validate the method and to prove that the extraction of the AFM1 is effective (Figure 3). The analysis of the chromatogram showed that AFM1 extraction method used is applicable and suitable for samples of raw bovine milk when contaminated with 8 μL of AFM1 at the concentration of 0.10680 μgL-1.
The results found in the evaluation of the 45 samples collected from the community expansion tanks were satisfactory, since none of them was within the limits of detection of the method used, contamination by AFM1, these results confirm research by other authors. Weigel (2007), for example, evaluated the condition of 128 samples of UHT milk and milk in natura in which the presence of the toxin was not detected. As found by Santos et al. (2014), 82 samples of pasteurized milk from Paraná State also obtained positively satisfactory results for the absence of AFM1. Figure 4A shows the chromatogram of one of the crude bovine milk samples, and Figure 4B shows a comparison between the
chromatograms of a raw milk sample and an analytical standard of AFM1. In this comparison, clearly the AFM1 shows different retention times than the majority compound present in the milk. It is possible for this method to be used without interference.
Allcroft and Galvo, quoted by Weigel (2007), hypothesize that the absence of contamination, as discovered by them, may have occurred due to a uniform distribution in milk, so that it may have been very easily mixed and diluted when incorporated into large quantities of milk, as in expansion tanks. This hypothesis can also be applied to this study, since the samples also come from expansion tanks of several producers in the region. Another hypothesis regarding the lack of contamination by the toxin in milk is related to the climatic conditions of the region on the date of collection, because the conditions of production and storage of the feed may not favor the production of mycotoxins. In addition, small producers of milk produce animal feed in their respective properties so that feed are produced in small quantities, preventing fungus proliferation in a timely manner, and thus avoiding the contamination of dairy cattle (Martins and Martins, 1986; Santos et al., 2014).
Liu et al. (2016) observed in their analytical results that four of the 17 samples were contaminated with very low concentrations of AFM1 and that all concentrations of AFM1 were below the regulatory limits established by the FDA of Taiwan and the USA (0.5 ng / mL) and the level in a sample slightly exceeded the regulatory limit established in the European Union (0.05 ng / mL). This study was consistent with a study where pAb-based cdELISA was used to analyze milk (Wang et al., 2011). Chadseesuwan et al. (2016) also observed in their research for aflatoxin M1 in fortified raw milk that the samples were within the acceptable range of detection.
Although the presence of aflatoxin M1 has not been verified in the samples collected in the dairy belt of the state of Alagoas/Brazil, control of animal feed is necessary to avoid possible human exposures to AFM1 through the consumption of milk. Regarding the method used for the identification of AFM1 in bovine milk, it showed a good sensitivity and precision for the quantification of aflatoxin within the Brazilian parameters, although there are also very sensitive methods for detection of aflatoxin M1 (Vdovenko et al., 2014; Kanungo et al., 2011).
The authors have not declared any conflict of interests.
REFERENCES
|
Adolfo Lutz Institute (2008). Métodos físico-químicos para análise de alimentos. 4. ed. Coordenadores Odair Zenebon, Neus Sadocco Pascuet e Paulo Tiglea. São Paulo. pp. 763-764.
|
|
|
|
Andrade PD, Silva JLG, Caldas ED (2013). Simultaneous analysis of aflatoxins B1, B2, G1, G2, M1 and ochratoxin A in breast milk by high-performance liquid chromatography/fluorescence after liquid–liquid extraction with low temperature purification (LLE–LTP). Journal of Chromatography A 1304: 61-68.
Crossref
|
|
|
|
|
Aycicek H, Aksoy A, Saygi S (2005). Determination of aflatoxin levels in some dairy and food products which consumed in Ankara, Turkey. Food Control 16:263-266.
Crossref
|
|
|
|
|
Baggio ECR (2006). Determinação de aflatoxina M1 emleitepasteurizadopelosmétodos de CCD e CLAE utilizandocoluna de imunoafinidade. 2006. 95 f. Dissertação (MestradoemTecnologia de Alimentos) – Programa de Pós-GraduaçãoemTecnologia de Alimentos, Universidade Federal do Paraná, Curitiba.
|
|
|
|
|
Bakirci I (2001). A study on the occurrence of an aflatoxin M1 in milk and milk products produced in Van province of Turkey. Food Control 12:47-51.
Crossref
|
|
|
|
|
Bennett JW, Klich M (2003). Mycotoxins. Clinical Microbiology Reviews 16(3):497-516.
Crossref
|
|
|
|
|
Brazil (2011). Ministério da Saúde. Resolução RDC nº 07, de 18 de fevereiro de 2011 da ANVISA. Regulamento Técnico sobre limites máximos tolerados (LMT) para micotoxinas em alimentos. Diário Oficial da União – D.O.U. de 09 de março de 2011.
|
|
|
|
|
CAST (2003). Council for Agricultural Science and Technology. Mycotoxins: Risks in Plant, Animal and Human Systems. Report n. 139, Ames, Iowa, USA, 2003.
|
|
|
|
|
Castro IM, Teixeira AS, Anjos MR, Santos SN (2013). Contaminante em Leite: Análise de Aflatoxina M1 porCromatografiaLíquida de Alta Eficiência com DetecçãoporFluorescência -CLAE/DFL2. Comunicado 199 Técnico, ISSN 0103 5231.
|
|
|
|
|
Chadseesuwan U, Sangdokmai A, Pimpitak U, Puthong S, Palaga T, Komolpis K (2016). Production of a monoclonal antibody against aflatoxin M1 and its application for detection of aflatoxin M1 in fortified milk. Journal of Food and Drug Analysis 24(4):780-787.
Crossref
|
|
|
|
|
Codex Alimentarius (2011). Proposed Draft Maximum Levels for Deoxynivalenol (DON) and Its Acetylated Derivates in Cereals and Cereal-based Products. Codex Committee on Contaminats in Food. European Union, Hague, Netherlands. CX/ CF 11/5/6.
View.
|
|
|
|
|
Commission Regulation (EC). (2014). No 519/2014 of 16 May 2014 amending regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Official Journal of European Union, L147, 29-43.
|
|
|
|
|
CPLA (2016). Cooperativa de Produção Leiteira de Alagoas Ltda. Perfil do Estado de Alagoas: Bacia Leiteira de Alagoas. Disponível em:
View. Acesso em: 02/03/2016.
|
|
|
|
|
Creppy EE (2002). Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicology Letters 127:19-28.
Crossref
|
|
|
|
|
Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) (2016). Indicadores: Leite e Derivados. Coordenadores: Glauco Rodrigues Carvalho e Alziro Vasconcelos Carneiro. Ano 7, n. 52, Juiz de Fora, 19 p.
|
|
|
|
|
Erkekoglu P, Sahin G, Baydar T (2008). A special focus on mycotoxin contamination in baby food: Their presence and regulation. FABAD Journal of Pharmaceutical Sciences 33:51-66.
|
|
|
|
|
Food and Agriculture Organization of the United Nations (FAO) (2004). Worldwide regulations for mycotoxins in food and feed in 2003, Paper 81, Roma 2004. Disponível em:
View. Acessoem: 20/04/2016.
|
|
|
|
|
Flor-Flores ME, Lizarraga E, López de Cerain A, González-Pe-as E, Presence of mycotoxins in animal milk: A review. Food Control 53:163-176.
Crossref
|
|
|
|
|
Franco BD, Landgraf M (1996). Microbiologia dos Alimentos. São Paulo: Atheneu.
|
|
|
|
|
Galvano F, Galofaro V, Galvano G (1996). Occurrence and stability of aflatoxin M1 in milk and milk products: a worldwide review. Journal of Food Protection 59(10):1079-1090.
Crossref
|
|
|
|
|
Instituto Brasileiro de Geografia e Estatística (IBGE) (2014). Instituto Brasileiro de Geografia e Estatística. Produção da Pecuária Municipal 42:36.
|
|
|
|
|
Jay JM (2005). Microbiologia dos alimentos. 6. ed. Porto Alegre: Artmed. pp. 634-645.
|
|
|
|
|
Kanungo L, Pal S, Bhand S (2011). Miniaturised hybrid immunoassay for high sensitivity analysis of aflatoxin M1 in milk. Biosensors and Bioelectronics 26(5):2601-2606.
Crossref
|
|
|
|
|
Liu BH, Chu KC, Yu FY (2016). Novel monoclonal antibody-based sensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 66 (2016) 1-7.
Crossref
|
|
|
|
|
Vdovenko MM, Lu CC, Yu FY, Sakharov IY (2014). Development of ultrasensitive direct chemiluminescent enzyme immunoassay for determination of aflatoxin M1 in milk. Food Chemistry 158:310-314.
Crossref
|
|
|
|
|
Mahdavi R, Nikiniaz L, Aleshosseini SR, Jabbah MV (2010). Determination of aflatoxin M1 in breast milk samples in Tabriz-Iran. Maternal and Child Health Journal 14:141-145.
Crossref
|
|
|
|
|
Martins JLS, Martins IS (1986). Aflatoxina M1 no leite tipo "B" comercializado no município de São Paulo, SP (Brasil). Revista de Saúdepública 20(4):303-308.
Crossref
|
|
|
|
|
Oliveira PM, Zannini E, Arendt EK (2014). Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: From crop farming to cereal products. Food Microbiology 37:78-95.
Crossref
|
|
|
|
|
Queiroz VAV, Santos JP, Tibda CS, Queiroz LR (2009). Boas práticas e sistema APPCC na fase de pós-colheita de milho. Sete Lagoas: Embrapa Milho e Sorgo. Circular Técnica 122:28.
|
|
|
|
|
Ribeiro MG (2008). Princípios terapêuticos na mastite em animais de produção e de companhia. In: ANDRADE S. F. (Ed.). Manual de terapêutica veterinária, 3. ed. São Paulo: Roca. pp. 759-771.
|
|
|
|
|
Rosmaninho JF, Oliveira CAF, Reis TA, Correa B (2006). Aflatoxina M1 e ácido ciclopiazônico em leites de consumo comercializados no Município de São Paulo, S.P., Brasil. Brazillian Journal of Food Technology III JIPCA 55-59.
|
|
|
|
|
Santos AL, Bando E, Machinscki Junior M. (2014). Ocorrência de aflatoxina M1 em leite bovino comercializado no estado do Paraná, Brasil. Semina: CiênciasAgrárias, Londrina 35(1):371-374.
Crossref
|
|
|
|
|
Schwarzer K (2009). Harmful effects of mycotoxins on animal, physiology. In: 17th Annual ASAIM SEA Feed Technology and Nutrition Workshop, Hue, Vietnam. P 132.
|
|
|
|
|
Vilela D (2002). Leite: suaimportânciaeconômica, social e nutricional. Minas de Leite, Juiz de Fora 3(2):17-18.
|
|
|
|
|
Wang JJ, Liu BH, Hsu YT, Yu FY (2011). Sensitive competitive direct enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 22:964-969.
Crossref
|
|
|
|
|
Weigel M (2007). Avaliação da contaminação por aflatoxina M1 em leite cru e UHT. 2007. Dissertação (Mestrado em Ciência e Tecnologia de Alimentos) – Universidade Federal do Rio Grande do Sul, Porto Alegre. Rio Grande do Sul. Disponível em:
View. Acessoem: 22/01/2016.
|
|
|
|
|
World Health Organization (WHO) (2009). Dampness and mould: WHO guidelines for indoor air quality. Denmark: Copenhagen, 230 p.
|
|
|
|
|
Wild CP, Gong YY (2010). Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis 31(1):71-82.
Crossref
|
|
|
|
|
Zain ME (2011). Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society 15(2):129-144.
Crossref
|
|