ABSTRACT
Dermatophytes cause superficial fungal infections that pose public health problem to man and animals. Long term treatment with antifungal agents is required to control these infections. Various parts of Azadirachta indica are claimed to have significant medicinal value in treatment of infections especially ringworm. To determine the antifungal activity of the oil and fractions against dermatophytes isolated from clinical cases. Seeds of A. indica were collected, dried, grinded and extracted with hexane using soxhlet and cold maceration. Physicochemical analysis of the oil was carried out as described by Association of Official Analytical Chemists methods (AOAC, 1990). Fractionation of the oil was subjected using column chromatography and Infra-Red (I.R) analysis using spectrophotometer. Swab samples were collected from pupils in Kudan, Kaduna State with suspected cases of Tinea corporis (ringworm). The causative fungal organisms were isolated and identified by routine mycological and biochemical procedures. The antifungal activities of the extracts were evaluated by determining the minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC) and diameter zones of inhibition. Hexane extract from soxhlet method of extraction showed higher diameter zone of inhibition against isolated dermatophytes (Trichophyton mentagrophytes, Trichophyton rubrum and Microsporum canis), ranging from 14.33 – 17.33 mm. The MIC and MFC values of the extract range from 3.13 to > 50% v/v and 50 to >50%v/v respectively. The oils and fractions recorded class of compounds which include alkyl, alkanes, alkenes, aliphatic esters, ketone, carboxylic acid, amide and alkyl halide. Hexane extract of seed of A. indica extracted by soxhlet and cold maceration methods had inhibitory activities against the causative agents of T. corporis (ringworm) when tested.
Key words: Dermatophytes, Azadirachta indica, antifungal, Fourier-transform infrared (FT-IR).
Dermatophytes are fungi that require keratin for growth. These fungi can cause superficial infection of the skin, hair and nails. Dermatophytes are spread by direct contact from other people, animals and soil. Dermatophytes which comprise a group of closely related fungi made up of three genera; Trichophyton, Microsporum, and Epidermatophytes, have the ability to invade the stratum corneum of the epidermis and
keratinized tissues derived from it, such as skin, hair, and nail of humans and other animals. They cause superficial fungal infections that pose public health problems to man and animals. Dermatophytes infections can be disfiguring and recurrent and generally need long-term treatment with antifungal agents (Tortorano et al., 2014).
The use of plants in the alleviation and cure of bodily ills goes as far back as the history of the human race itself. The universal role of plants in the treatment of disease is exemplified by their employment in all the major systems of medicine irrespective of the underlying philosophical premise (Evans, 2002).
Use of herbal medicines in the developed world has continued to rise because they are rich source of novel drugs and their bioactive principles form the basis in medicine, pharmaceutical intermediates and lead compounds in synthetic drugs (Akula et al., 2003). More importantly in Africa, particularly West Africa, new drugs are often beyond the reach of the poor such that up to 80% of the population uses medicinal plants as remedy against infections and diseases (Thomford et al., 2015).
Various parts of neem tree are claimed to have significant medicinal importance: which include uses in leprosy, malaria, asthma, intestinal worms. Topical application of neem seed oil can cure dermatological diseases within 3-4 days (Akula et al., 2003). Neem oil used in cosmetic industries is a product of the neem tree and is employed in soap making (Edwards, 2015). Nimbolide, gedunin and nimbin (triterpenoids) are chemical compounds with antifungal activity from neem seed oil (Hashmat et al., 2012). The objective of this research is to determine the in-vitro antifungal activity of n-hexane extracts and fractions of Azadirachta indica against clinical isolates. The work aimed at a therapeutic alternative against dermatophytes and the functional groups responsible for such activity.
Media used were: Sabouraud Dextrose Agar (Oxoid, Basingstoke, U.K.), Sabouraud Dextrose Liquid Medium (SDLM: Oxoid, Basingstoke, U.K.) and Sabouraud Dextrose Agar + Chloramphenicol + Cycloheximide (Cat No.21089.00 Deben Diagnostics Limited, U.K). The media were prepared according to the manufacturer’s instruction.
Chemical and reagents used include: Tween 80 (Sigma Aidrich- Missouri, U.S.A.), Dimethylsulfoxide (DMSO: Sigma Aidrich- Missouri, U.S.A.), n-Hexane (Sigma Aidrich- Missouri, U.S.A.), Chloroform (Sigma Aidrich- Missouri, U.S.A.), Christensen’s urea agar medium (Sigma Aidrich- Missouri, U.S.A.) and Lactophenol cotton blue (Sigma Aidrich- Missouri, U.S.A).
Antifungal used: Terbinafine powder (Cat No. F8929, Sigma Aidrich, U.S.A.).
Instrument
Spectrophotometer, Single-beam, Spectronic 20D; Milton Roy Company, Madrid, Spain.
Collection, extraction and fractionation
Plant seeds were obtained from National Research Institute for Chemical Technology (NARICT), Zaria, Kaduna State, Nigeria. Identification and authentication was done in the herbarium of the Department of Biological Sciences, Ahmadu Bello University, Zaria, Kaduna State, Nigeria with a voucher specimen number 900151.
The seeds were washed, sun-dried and foreign materials removed by winnowing. The cleaned neem seeds were oven dried at 50°C and then grinded into powder using milling machine at National Research Institute for Chemical Technology (NARICT), Zaria, Kaduna State.
Oil extraction
Soxhlet extraction: Soxhlet extraction method was used to extract the oil from the processed seeds. One thousand five hundred (1500 g) of the neem seed powder was packed inside a muslin cloth and placed in a thimble of Soxhlet extractor. A round bottom flask containing n-hexane was fixed to the end of the extractor and a condenser was tightly fixed at the bottom end of the extractor. Extraction was done each time with n- Hexane. The flask was then heated at 60°C with the use of a heating mantle. The solvent was vaporized and condensed into the evaporator. The process continued for 4 h. Oil was recovered from the mixture (oil and solvent) by the use of rotary evaporating process. The oil was obtained and stored in a bottle for further processes (Awolu et al., 2011).
Cold maceration: In this process, 1500 g of the coarsely powdered crude plant seed was placed in a stoppered container (conical flask) with 1000 ml of n- hexane and was allowed to stand at room temperature for a period of 3 days with intermittent agitation (stirring) until the soluble matter dissolved. The mixture was then strained, the marc (the damp solid material) pressed, and the oil extract was clarified by filtration after standing. The oil was recovered from the mixture (oil and solvent) by the use of rotary evaporator.
Physicochemical analysis
The following physicochemical analysis which involved organoleptic properties, density, viscosity, saponification value, iodine value, acid value, peroxide value, and free fatty acid of the neem oil were carried out as described by Association of Official Analytical Chemists methods (AOAC, 1990).
Fractionation of oil extract
A portion (50 ml) of crude n-hexane oil extract was subjected to column chromatography using silica gel G as an absorbent. The column was successively eluted with hundred milliliters (100 ml) of n-hexane (100%), hexane: chloroform mixture (75:25 and 50:50%) and 100% chloroform.
Spectroscopic analysis of A. indica oil and its fractions
Infra-red (I.R) analysis
Infra-red (I.R) analysis of the absorption spectra of
A. indica oil and the fractions that have activity against dermatophytes was conducted at National Institute for Chemical Technology (NARICT), Basawa, Zaria, Kaduna state of Nigeria. A
Fourier- transform infrared (FTIR)
spectrometer was used in which the sample was placed. The spectrometer directed beams of IR at the sample and measured how much of the beam and at which frequencies the sample absorbs the infrared light. The molecular identities were determined through a reference database which houses thousands of spectra, so samples can be identified. The functional groups present in the oil and its fractions were determined by comparing the vibration frequencies in wave numbers of the samples spectrograph obtained from an FT-IR spectrophotometer (Coates, 2000).
Isolation and identification of dermatophytes
Thirty swab samples of Tinea corporis (ringworm) were collected from infected skins of pupils at Kudan, Kaduna State using sterile scalpels. Affected areas were cleansed with 70%v/v ethanol, allowed to dry and light scrapings from the edge of the lesions were taken using a blunt sterile scapel blade. The specimens were placed in well labelled clean white envelopes. Mycological analysis of the 30 specimens from the suspected infected sites of the participants was carried out in the Pharmaceutical Microbiology Laboratory at the Department of Pharmaceutics and Pharmaceutical Microbiology, Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Kaduna State, Nigeria.
The specimens were inoculated into 10 ml Sabouraud Dextrose Liquid Medium (SDLM) and incubated for 48 h. Growth from the broth was then streaked on Sabouraud Dextrose Agar containing chloramphenicol and cycloheximide and incubated at 30°C for 14 days. Cultures were examined weekly for sporulation. Colonies of dermatophytes were later subculture on respective petri-dishes containing SDA and incubated at 30°C for 21 days. The isolates were identified by routine mycological and biochemical procedures (Urease Test, Corn meal test and Nutritional test) as modified by Refai et al. (2013).
Standardization of the innoculum
Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis, Candida albican and Aspergillus niger fungal spores were harvested from 7 day old Sabouraud dextrose agar (SDA) slant cultures by washing with 10 ml sterile normal saline containing 3% v/v Tween 80 with aid of sterile glass beads to help in dispersing the spores. Thereafter, the spore suspension were standardized to 1.0 × 105 spores / ml by using a single-beam spectrophotometer at 530 nm (OD530) adjusted to 70-72% transmittance for T. mentagrophytes, T. rubrum and M. canis. All adjusted suspensions were quantified by spreading 100 µl on Sabouraud dextrose agar plate and incubated at 30°C for 72 h for dermatophytes (Aberkane et al., 2002). Standardized isolates were maintained at 4°C (in the refrigerator) until required for use.
Determination of antifungal activity of A. indica A. Juss
The plates were allowed to dry at 30°C temperature in a sterilized incubator. Using the agar diffusion cup plate method, a sterile cork borer (6 mm) was used to bore wells in the agar plates. The bottoms of the wells were each sealed with a drop of molten SDA. Using micropipette, 0.1 ml each of the different graded concentrations of the extract (soxhlet and cold maceration) were dispensed into the wells marked 100 and 90%v/v and 10% DMSO (used in diluting the extracts and a negative control). These were allowed to diffuse into the agar at room temperature for an hour before incubation at 30°C for 72 h. The diameter of zones of inhibition of the test organisms were measured to the nearest millimetre using a well-calibrated meter ruler. The experiment was carried out in triplicate.
Determination of minimum inhibitory concentration (MIC)
The MIC was determined by agar dilution method as modified by Serban et al. (2011). Ten millilitres of the graded concentration of the crude extracts was mixed with 10 ml of double strength SDA supplemented with 0.5%v/v Tween-80 and poured aseptically into sterile plates. The plates were allowed to set. Ten microlitres of the standardized organisms containing 106 CFUmL-1 was inoculated on the equidistantly placed sterile filter paper disc. The plates were allowed to stand for one hour and then incubated at 30°C for 48 h. The same procedure was repeated using Terbinafine. The lowest concentration of the agent that inhibits the visible growth of the test organisms was taken as the MIC. The experiment was carried out in triplicate.
Determination of minimum fungicidal concentration (MFC)
The filter paper discs showing visible growth at two test concentrations below MIC, discs at MIC and discs at two concentrations above MIC were aseptically removed with the aid of a sterile forceps and transferred into 5 ml sterile SDLM supplemented with 0.3%v/v Tween 80 and incubated at 30°C for 48 h. Minimum fungicidal concentrations were determined as the lowest concentration resulting in no growth on subculture.
Statistical analysis
All the data obtained from the studies were expressed as mean ± standard deviation (SD).
Dermatophytes isolated with T. corporis infections
A total of 18 dermatophytes consisting of Trichophyton species (16 isolates) (Table 1).
T. mentagrophytes 10 Isolates
T. rubrum 6 Isolates
M. canis 2 Isolates
Extraction of oil from A. indica A. Juss seeds
The percentage yields of the oils from A. indica seeds using n-hexane and extraction methods are shown in Table 2. Soxhlet method of extraction yielded more oil than the cold maceration method; about 4% higher than cold maceration.
Physicochemical characteristics of A. indica seeds oil
The physicochemical and organoleptic characteristics of the oils obtained via the two methods of extractions (soxhlet and maceration) using n-Hexane is shown in Table 3. Hexane oil from maceration recorded higher acid, iodine, saponification and free fatty acid values compared to values for the other oil. Oil obtained by soxhlet method was generally lighter in colour; more viscous, denser with lower peroxide and acid values. Oil obtained by maceration was associated with lower viscosity higher acid, iodine, and free fatty acid values. The variations can be attributed to the different methods involved in the oil extraction as reported by Jessinta et al. (2014).
Antifungal activities of the oil fractions
A total of 8 fractions were collected from the elution of the n-hexane and chloroform of the two oil extracts. The results of their antifungal test are presented in Table 4. Antifungal activities of the fractions varied among the fractions. None of the fractions obtained from oil extracted by soxhlet method using Hexane as solvent had inhibitory activity against the test isolates. On the other hand, fractions from the oil obtained by maceration had activity only against the Trichophyton species (T. mentagrophytes).
Determination of the functional groups present in the oils and its fractions
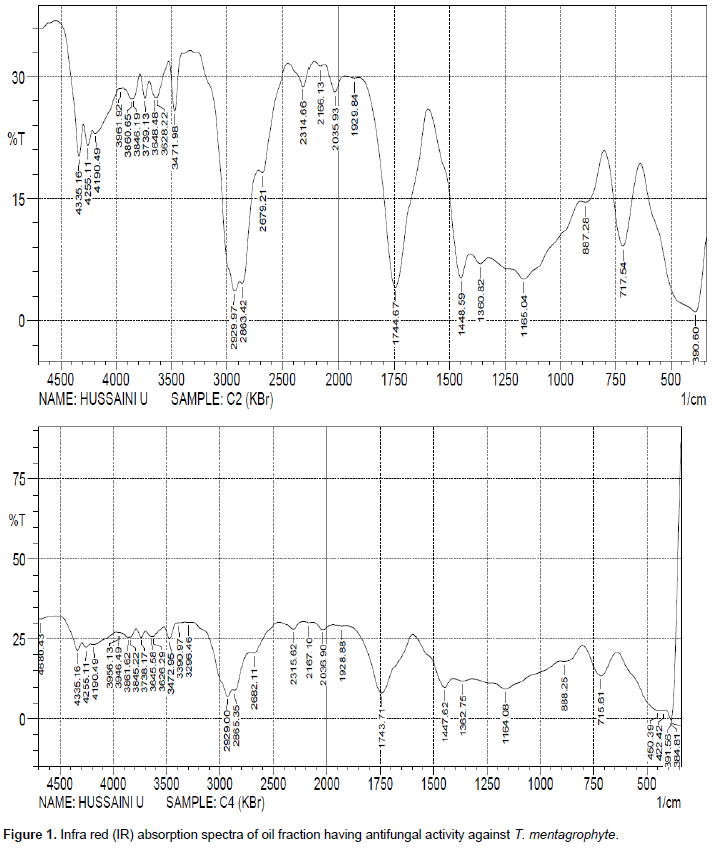
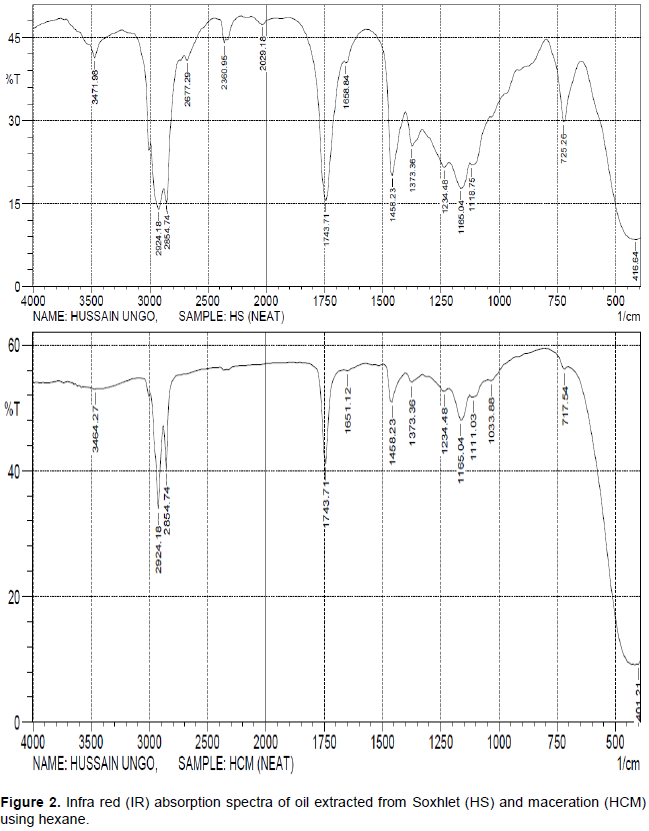
The spectroscopic analysis (FT-IR) interpretation detecting functional groups of the oils and the fractions having antifungal activity is shown in Tables 5 and 6 respectively. Both the oils and fractions recorded class of compounds which include alkyl, alkanes, alkenes, aliphatic esters, ketone, carboxylic acid, amide and alkyl halide. However, there was a significant difference in FT-IR of the oil extract and its fractions, where the oil extract recorded the presence of aromatic compounds.
A strong and broad absorption band or frequency from 2500- 3500 cm-1 showed the presence of O – H stretch from carboxylic acid as seen in all the spectra, a strong absorption band from 1740- 1755 cm-1 revealed the presence of C = O bond for a five member cyclic ketone. A weak absorption band from 3400-3500 showed the presence of N – H stretch from an amides. The absorption frequency with a strong band from 1160-1210 cm-1 revealed the presence of O = C – O – C from an aliphatic esters. A medium absorption band 1450-1600 cm-1 showed the presence of C = C ring from an aromatic compounds and frequency bands of 200-500 cm-1 revealed the presence of an alkyl halide.
Antifungal activities of n-hexane extract of A. indica
Table 7 shows the results of the susceptibility of the isolates to the undiluted and diluted with DMSO in a ratio of 90 to 10%v/v extracts of A.indica. Hexane extract from soxhlet method of extraction had shown higher diameters of zones of inhibition against the isolated dermatophytes. Activity was exerted mostly on T. mentagrophytes and M. canis. However, the levels of inhibition against the dermatophytes were far less compared those exerted by terbinafine. The level of inhibitory also changed following dilution of the n-hexane extracts with DMSO. The results of the susceptibility of the isolates from soxhlet showed higher activities against isolated dermatophytes.
Minimum Inhibitory Concentration and Minimum Fungicidal Concentration values
The MIC of n-hexane extract of A. indica is as shown in Table 8. The MIC against T. mentagrophytes and T. rubrum was 50%v/v by both extraction methods but M. canis was as low as 3.13%v/v (by soxhlet) and 12.5%v/v (by cold maceration). The MFC against T. mentagrophytes and T. rubrum is 50%v/v and above by both extraction methods while against M. canis, it was as low as 12.5%v/v (by Soxhlet and 25%v/v (by cold maceration).
Plants have been known to contain bioactive constituents with inhibitory substances against bacteria and fungi (Kadhim et al., 2016). Results from this study reveal a degree of antifungal activities of the plant seed oil which varied from one clinical isolate to another. The gradual increase in the diameter zones of inhibition with increase in concentration of the oil shows that the inhibitory action on the fungi clinical isolates is dependent on the amount of drug used (Table 8). Generally, oil obtained from soxhlet method of extraction had better antifungal activity on T. rubrum, T. mentagrophytes, M. canis. The zones of inhibition of the oil against all the clinical isolates tested at 100% v/v was comparable to the standard drugs (Terbinafine) used. The antifungal activity of the oil and its fraction can be linked to the presence of secondary metabolites which have been shown to possess bioactive properties. The seed oil which is usually extracted by steam or solvents from crushed seed consists mainly of triglycerides and large amounts of triterpenoids (Takase et al., 2015). The oil extracts and some fractions revealed an antifungal activity against dermatophytes, the classes of compounds present include, alkyl, alkanes, alkenes, aliphatic esters, ketone, carboxylic acid, amide and alkyl halide and aromatic compounds (Supplementary Figures 1 and 2). The presence of ketone C = O functional group from a five membered cyclic ring and O = C – O – C functional group from an aliphatic esters revealed that gedunin, nimbin, nimbinin and nimbolide constituents are likely present in the oil extracts and its fractions. Thus, these functional groups of ketone, hydroxyl, carboxyl and aliphatic ester may be responsible for the antidermatophytic activity of neem seed oil. The constituents nimbinin, gedunin, nimbin and nimbolide are terpenoid compounds and a work done by Nuzhat and Vidyasagar (2014) revealed that terpenoid compounds are responsible for antidermatophytic activity.
The predominance of Trichophyton species as the causative agent of Tinea corporis (Ringworm of the body) is not unexpected. Most studies found T. rubrum and T. mentagrophytes as the commonest etiological agents of dermatomycosis (Hayette and Sacheli, 2015). Trichophyton spp accounted for 76.2% of T. corporis in India (Harinath, 2016). T. mentagrophytes has been cited as the major causative agent for T. corporis and is known to account for as much as 47.6- 69.5% of all dermatophytic infections (Pranab et al., 2003).
The key discovery that has emerged from this research is that the oil extracted from A. indica A Juss seeds using both soxhlet and maceration methods of extraction inhibited the growth of clinical isolates of dermatophytes (T. mentagrophytes, T. rubrum and M. canis). This study has established the in-vitro activity of A. indica seed oil against dermatophytes isolated from clinical sample. It has also shown that the oil extract can be used to carry out further studies (bioassay guided) so as to isolate lead compounds responsible for such activity and enable its redeployment as potent antidermatopytic agent.
The authors have not declared any conflict of interests.
REFERENCES
|
Aberkane A, Cuenca-Estrella, Gomez-Lopez A, Petrikkou E, Mellado E, Monzón A, Rodriguez-Tudela J, Eurofung LN (2002). Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. Journal of Antimicrobial Chemotherapy 50(5):719-722.
Crossref
|
|
|
|
Akula C, Akula A, Drew R (2003). Somatic embryogenesis in clonal neem, Azadirachta indica A. Juss. and analysis for in vitro azadirachtin production. In Vitro Cellular and Developmental Biology-Plant 39(3): 304-310
Crossref
|
|
|
|
|
Awolu OO, Obafaye RO, Ayodele BS (2011). Optimization of solvent extraction of oil from neem (Azadirachta indica) and Its Characterizations, Journal of Scientific Research and Reports 2(1): 304-314
Crossref
|
|
|
|
|
Edwards VH (2015). The Aromatherapy Companion: Medicinal Uses/Ayurvedic Healing/Body-Care Blends/Perfumes & Scents/ Emotional Health & Well-Being. Storey Publishing.
|
|
|
|
|
Evans WC (2002). Trease and evans. WB Saunders Harcourt Publishers Ltd. 292:357-375.
|
|
|
|
|
Harinath BC (2016). Mycobacterial excretory secretory-31 protein with serine protease and lipase activities: An immunogen and drug target against tuberculosis infection. International Journal of Mycobacteriology 5:S86-S87.
Crossref
|
|
|
|
|
Hayette MP, Sacheli R (2015). Dermatophytosis, trends in epidemiology and diagnostic approach. Current Fungal Infection Reports 9(3):164-179.
Crossref
|
|
|
|
|
Hashmat I, Azad H, Ahmed A (2012). Neem (Azadirachta indica A. Juss)-A nature's drugstore: an overview. International Research Journal of Biological Sciences 1(6):76-79.
|
|
|
|
|
Jessinta S, Azhari NT, Abdurahman HN (2014). Impact of geographic variation on physicochemical properties of neem (Azadirachta indica) seed oil. International Journal of Pharmaceutical Sciences and Research 5(10):4406-4413
|
|
|
|
|
Coates J (2000). Interpretation of Infrared Spectra, A practical Approach, R.A Meyers (Ed) Encyclopaedia of Analytical Chemistry, Published by John Wiley and Son Limited, Chichester pp. 10815-10837
|
|
|
|
|
Kadhim MJ, Sosa AA, Hameed IH (2016). Evaluation of anti-bacterial activity and bioactive chemical analysis of Ocimum basilicum using Fourier transform infrared (FT-IR) and gas chromatography-mass spectrometry (GC-MS) techniques. Journal of Pharmacognosy and Phytotherapy 8(6):127-146.
Crossref
|
|
|
|
|
Refai M, El-Yazid HA, El-Hariri M (2013). Monograph on Dermatophytes, A guide for isolation and identification of dermatophytes, diseases and treatment.
View
|
|
|
|
|
Nuzhat T, Vidyasagar GM (2014). Antifungal investigations on plant essential oils. A review. International Journal of Pharmacy and Pharmaceutical Sciences 5(2):19-28
|
|
|
|
|
Serban ES, Ionescu M, Matinca DO, Maier CS, Bojiţă MT (2011). Screening of the antibacterial and antifungal activity of eight volatile essential oils. Farmacia 59(3):440-446
|
|
|
|
|
Takase M, ZhaoT, Zhan M, Chen Y, Liu H, Yang L, Wu X (2015). An expatiate review of neem, jatropha, rubber and karanja as multipurpose non-edible biodiesel resources and comparison of their fuel, engine and emission properties. Renewable and Sustainable Energy Reviews 43:495-520.
Crossref
|
|
|
|
|
Tortorano AM, Richardson M, Roilides E, Diepeningen AV, Caira M, Munoz P, Verweij P (2014). ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clinical Microbiology and Infection 20(3):27-46.
Crossref
|
|
|
|
|
Thomford NE, Dzobo K, Chopera D, Wonkam A, Skelton M, Blackhurst D, Chirikure S, Dandara C (2015) . Pharmacogenomics implications of using herbal medicinal plants on African populations in health transition. Pharmaceuticals 8(3):637-663.
Crossref
|
|