ABSTRACT
The Brazilian Ministry of Health determined in 2012 that the official protocol for diagnosis of Canine Visceral Leishmaniasis (CVL) would be the Dual-Path Platform (DPP) for screening, followed by enzyme-linked immunosorbent assay (ELISA) for confirmation. This study evaluated serum samples from 426 dogs from a region in northern Brazil. All samples were tested according to the Official Protocol and the sequence inverting (ELISA followed DPP). Regardless of the protocol adopted, prevalence (14.7%) has not changed. The approach using ELISA followed by DPP state that, the number of positive animals in screening was higher compared to the official protocol. Screen the ELISA test could be more appropriate.
Key words: Canine visceral leishmaniasis, Dual-Path Platform (DPP), enzyme-linked immunosorbent assay (ELISA), tocantins.
Canine visceral leishmaniasis (CVL) is a potentially fatal disease caused by the intracellular protozoan parasite Leishmania infantum, which is endemic in South and Central America, Mediterranean basin and parts of Asia. Dog is the most important reservoir host, and infection is maintained by transmission between dogs by phlebotomine sandfly species (Quinnell and Couternay, 2009).From an epidemiological point of view, the canine disease is more important than the human disease because, besides being more prevalent, it has large numbers of asymptomatic dogs with parasites in the dermis, and has the potential of transmitting the parasite to sand-fly (Laurenti et al., 2013).
Recently, to improve accuracy in the diagnosis of CVL in Brazil, the Visceral Leishmaniasis Control and Surveillance Program (VLCSP) has recommended the immunochromatographic rapid test comprising rK26 and rK39 recombinant antigens, the Dual-Path Platform (DPP; Bio- Manguinhos/Fiocruz, Rio de Janeiro, Brazil), for the screening of L. infantum-infected dogs and enzyme-linked immunosorbent assay (ELISA) to confirm the positive results (Ministério da Saúde, 2011). In this sense, the present study aimed to carry out the first seroepidemiological survey for CVL in the city of Gurupi, Tocantins, northern Brazil between 2013 and 2015. For this, we used the Brazilian official protocol (DPP and ELISA), and the reversal order in serologic techniques, investigating whether changing the protocol could change the animals positive rate.
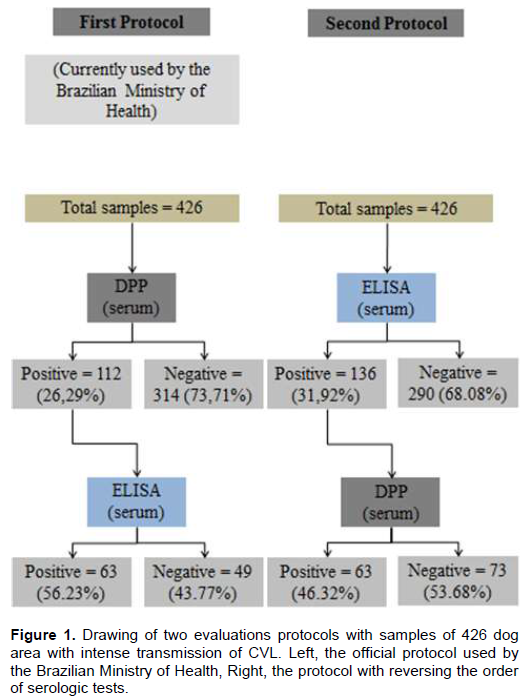
The present study consist a cross-sectional survey carried out in Gurupi (latitude 11° 43’ 45’’S, longitude 49° 04’ 07’’W, altitude 287 m), a municipality located in the southwest of Tocantins, Brazil.For random sampling calculation, we used official data expected prevalence of 20%, 95% confidence interval (95% CI) and maximum acceptable error of 0.05, totaling 246 samples. Furthermore, 10% samples were added, amounting to 271 samples. However, more samples were collect over a period of time, reaching 426 blood samples from asymptomatic and symptomatic dogs between September 2013 and November 2015.Each sample was tested using two approaches, the first using the protocol recommended by the Brazilian Ministry of Health, and the second, reversing the order of the tests. The first protocol used DPP CVL rapid test (Bio-Manguinhos/Fiocruz) for screening and ELISA (Canine Leishmaniasis EIE Kit, Biomanguinhos/Fiocruz) as a confirmatory test. This protocol used serum for serological tests while both protocols followed the manufacturer’s instructions. The second protocol used ELISA (Canine Leishmaniasis EIE Kit) for screening and DPP CVL rapid test for confirmation.
The cut-off of the EIE Kit was defined based on the manufacturer’s instructions, which consider the mean of the optical density of the negative controls multiplied by two. Statistical analysis was performed using Stata software (version 11.0; Stata Corp, College Station, TX). The prevalence rates indicated by DPP and ELISA were estimated using 95% CI.
In the first approach, following the Brazilian Ministry of Health protocol, of the 426 serum samples evaluated by both methods, 112 (26.29%) were positive in DPP and from this initial screening, 63 (56.23%) were positive by ELISA. While in the second proposal, out of the 426 samples screened in the ELISA test, 136 (31.92%) were positive, and from this screening, 63 (46.32%) samples were positive to purified protein derivative (PPD) test. For both protocols, the prevalence was 14.7%, with no differences in the final number of positive animals in the two serologic techniques (Figure 1). Sensitivity and specificity were 82.3 and 92.8% at DPP test and 85 and 92.3% in the ELISA test, respectively.
Official data indicate that, the city of Gurupi has an intense transmission rate of CVL, with a prevalence of 23% in 2013 and 23.5% in 2014 (official unpublished data). These results are favored by the climate of the region and the constant degradation of native areas housing construction and agricultural activities. The rates of positive animals found in an urban area in the State of Pernambuco (Brazil), has an overall seroprevalence which was 40.3% (Dantas-Torres e Brandão-Filho 2006). However, the results found in this study, is in line with the average in Brazil, ranging from 5.9 to 51.35% (Franca-Silva, 2003; Monteiro et al., 2005; Morais et al., 2013). It notes that, the current official protocol has to be implemented in 2012. The sensitivity of the DPP test depends on the clinical condition of the animal. However it is known that, the DPP is more sensitive when used in symptomatic dogs, and lower the income in asymptomatic animals (Grimaldi et al., 2012).
In a previous state developed in other regions of Brazil, this was bought for the first time to change the protocol for diagnosis of CVL. A survey was conducted with 1226 dogs, followed by a cohort study using 447 dogs. Results showed that the protocol using DPP and ELISA detected a higher prevalence (8.1%) of infected dogs than the protocol using ELISA and IFAT (prevalence, 6.2%). However, regardless of the test sequence (DPP followed by ELISA or ELISA followed by DPP), the number of positive animals is the same in both tests (Coura-Vital et al., 2014). Positive serum samples for Ehrlichia canis, Babesia canis, Toxoplasma gondii, Neospora caninum and Trypanosoma cruzi were tested using three serological methods ELISA, indirect immunofluorescent antibody test (IFAT) and Kalazar Detect™, for CVL. Of the 57 dog samples tested, 24 (42.1%) tested positive using one of the three serological methods: 10/57 (17.5%) for ELISA, 11/57 (19.3%) for IFAT and 3/57 (5.3%) for Kalazar Detect™. Results demonstrated that the presence of other infectious agents may lead to cross-reactivity on leishmaniasis serological tests. (Zanette et al., 2014). Moreover, in another study using DPP and ELISA, cross-reactivity was obtained with only Babesia (Laurenti et al., 2014).
Among DPP using and ELISA for screening of dogs in endemic areas, the DPP have advantages by being easy and practical easier to handle, with the result been ready in 15 min after blood collection. Further, laboratory equipment is not necessary for diagnosis. On the other hand, if the animal is positive, spend more time in collecting more samples to be sent to, the Central Public Health Laboratories (LACENS). As the ELISA detects more positive animals in screening, it is interesting that in areas of high prevalence and incidence, the ELISA will be used for screening and DPP for confirmation, given that there was no difference in the final number of animals positive.
The authors have not declared any conflict of interests.
REFERENCES
|
Coura-Vital W, Ker HG, Roatt BM, Aguiar-Soares RD, Leal GG, Moreira N, Oliveira, LA, de Menezes Machado EM, Morais MH, Corrêa-Oliveira R, Carneiro M, Reis AB (2014). Evalution of change in canine diagnosis protocol adopted by the visceral leishmaniasis control program in Brazil and a new proposal for diagnosis. Plos One. 7(3):e91009.
Crossref
|
|
|
|
Dantas-Torres F. de Brito MEF, Brandão-Filho SP (2006). Seroepidemiological survey on canine leishmaniasis among dogs from an urban area of Brazil. Vet. Parasitol. 140:54-60.
Crossref
|
|
|
|
Franca-Silva JC, da Costa RT, Siqueira AM, Machado-Coelho GL, da Costa CA (2003). Epidemiology of canine visceral leishmaniosis in the endemic area of Montes Claros Municipality, Minas Gerais State, Brazil. Vet Parasitol. 111:161-173.
Crossref
|
|
|
|
Grimaldi GJr, Teva A, Ferreira AL, dos Santos CB, Pinto IS (2012). Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP(R) CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans. R Soc. Trop. Med. Hyg. 106:54-59.
Crossref
|
|
|
|
Laurenti MD, Leandro Jr MVS, Tomokane TY, De Lucca HRL, Aschar M, Souza CSF, Silva RM, Marcondes M, da Matta VLR (2014). Comparative evaluation of the DPP® CVL rapid test for canine serodiagnosis in area of visceral leishmaniasis. Vet. Par. 205:444-450.
Crossref
|
|
|
|
Laurenti MD, Rossi CN, da Matta VL, Tomokane TY, Corbett CE, Secundino NF, Pimenta PF, Marcondes M. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet. Parasitol. 23:296-300..
Crossref
|
|
|
|
Ministério da Saúde (2011). Esclarecimento sobre substituição do protocolo Diagnóstico da leihsmaniose visceral canina; Nota técnica conjunta nº 01/2011, CGDT-CGLAB/DEVIT/SVSE/MS. Monteiro EM, da Silva JC, da Costa RT, Costa DC, Barata RA, de Paula EV. Visceral leishmaniasis: a study on phlebotomine sand flies and canine infection in Montes Claros, State of Minas Gerais. Rev. Soc. Bras. Med. Trop. 38:147-152.
|
|
|
|
Morais NA, Sousa MG, Meireles LR, Kesper JrN, Umezawa ES (2013). Canine visceral leishmaniasis and Chagas dsease among dogs in Araguaína, Tocantins. Rev. Bras. Parasitol. Vet. 22:225-229.
Crossref
|
|
|
|
Quinnell RJ, Couternay O (2009). Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 136:1915-1934.
Crossref
|
|
|
|
Zanette MF, Lima VM, Laurenti MD, Rossi CN, Vides JP, Vieira RF, Biondo AW, Marcondes M. Serological cross-reactivity of Trypanosoma cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi tests in dogs. Ver. Soc. Bras. Med. Trop. 47:105-107.
|