ABSTRACT
This study was performed to determine the prevalence of Campylobacter species in retail chicken meat and chicken by-product, determine their in vitro cytotoxicity, as well as, examine their susceptibility to different antimicrobials. A total of 300 raw chicken meat samples were collected from different retail chicken meat outlets located at Mansoura city, Egypt classified into 120 thighs, 120 breasts, and 60 livers. All samples were subjected to conventional culture techniques and confirmed as Campylobacter jejuni by real time polymerase chain reaction (PCR). Antimicrobial susceptibility of Campylobacter species was determined using disc diffusion method to determine their susceptibility to 12 different antimicrobial agents. In addition, C. jejuni isolates were examined for their cytotoxicity against Vero cells. The overall prevalence of Campylobacter spp. was 10.3% (31/300) classified into 20 (18.2%) C. jejuni and 11 (10.7%) Campylobacter coli. Among C. jejuni isolates (n=20), 15 strains belonged to biotype I and 5 isolates belonged to biotype II. The isolation rate from chicken thighs, breasts and livers was 12.5, 10 and 6.6%, respectively. A total of 15 (75%) C. jejuni strains revealed cytopathic effect (CPE) against Vero cells. Campylobacter spp. displayed a high antimicrobial resistance against penicillin G, gentamicin, trimethoprim-sulfamethoxazole, cephalothin, erythromycin, and chloramphenicol. On the other hand, Campylobacter spp. displayed high sensitivity to ciprofloxacin and nalidixic acid. Multidrug resistance was observed in 85 and 81.82% of C. jejuni and C. coli isolates, respectively High frequency of cytotoxicity and multidrug resistance in Campylobacter spp. from chicken meat indicates an important epidemiological role of Campylobacter spp. in human infections which necessitate proper hygienic measures on poultry farms and control measures during carcass slaughtering and processing.
Key words: Campylobacter; retail chickens meat, real time polymerase chain reaction (PCR), cytotoxicity, antimicrobial susceptibility.
Campylobacter jejuni is a major zoonotic pathogen that causes food-borne gastroenteritis worldwide (Bronowski et al., 2014). Poultry meat and poultry by-products are the major sources of infection to humans (
EFSA, 2016).
Campylobacter is considered a part of saprophytic microflora in the digestive tract of poultry and frequently transmitted from contaminated chicken meat either by ingestion of undercooked or raw chicken meat contaminated with the
Campylobacter or handling of poultry meat and meat products during food processing procedures (
EFSA, 2016).
Campylobacter infections in human are self-limiting and the infection usually lasts for only one week, but the illness may relapses in some untreated cases. Campylobacter infections symptoms range from mild to severe symptoms. It usually started after 2 to 5 days after ingestion of the contaminated food including, fever, headache, nausea and diarrhea (Campbell et al., 2006). Infrequently, Campylobacter infections cause life threating infection if it spread to blood stream and causes multiple diseases all over including, pseudoappendicitis, abdominal cavity, central nervous system, gallbladder, or urinary tract infection (Campbell et al., 2006). C. jejuni infection may result in serious post-infectious sequelae, the most important sequel are Reiter’s syndrome or Hemolytic Uremic Syndrome (HUS), and rarely resulted in neurological disorder known as Guillain-Barré syndrome (GBS), which manifests as sever neurological signs and paralysis which may result in respiratory dysfunction, and eventually death (Murray et al., 2007; Nachamkin, 2008).
Campylobacter has numerous virulence factors which contribute to its survival and establishment of the disease, but four major virulence factors have been identified which include motility, adherence, invasion and toxin production and these toxins had biological activity on tissue culture cell lines (Wassenaar, 1997).
The excessive and misuse of antibiotics in the treatment of infections, prophylaxis, as well as a growth promoters in Egypt has resulted in the development and spread of drug resistances which represents a public health problem (Levy and Marshall, 2004). Hence, the aims of this study were to recognize the prevalence and antimicrobial susceptibility of Campylobacter species isolated from retail chicken meat and chicken products sold in Mansoura city outlets, and to investigate the cytolethal distending toxin (CDT) activity of C. jejuni against Vero cells.
Collection of samples
A total of 300 raw chicken meat samples and chicken products classified into 120 thighs, 120 breasts and 60 livers were collected from retail outlets in Mansoura city, Dakahlia Governorates, Egypt. Samples were obtained from three street markets (n = 100), four supermarkets (n =100), and two slaughterhouses (n = 100). Poultry samples were collected, 20 specimens by visit during the period between March and August, 2016. Each chicken sample was individually packed into a clean polyethylene bag and transferred directly to the laboratory in an ice box under aseptic conditions.
Isolation of Campylobacter spp.
Isolation of Campylobacter from the chicken meat samples was performed according to ISO 10272-1:2006 (ISO, 2006); briefly, 10 g of chicken meat was aseptically taken and placed into a clean sterile plastic bag. The plastic bag was filled with 90 ml of Bolton broth (CM0983, Oxoid) with selective supplement (SR0183, Oxoid), and the samples were mixed in a stomacher for 1 min and incubated at 37°C for 4 to 6 h under microaerobic conditions (Campygen, Oxoid) followed by 41.5 ± 0.5°C for 48 h. Approximately, 10 μl of the previous enrichment broth was streaked into the surface of mCCDA (PO5091A, Oxoid, Basingstoke, UK) with supplement (SR0155, Oxoid) plates media and incubated under microaerobic conditions at 41.5 ± 0.5°C for 48 h. Presumptive colonies of Campylobacter were purified onto Columbia blood agar (Oxoid) plates and incubated under microaerobic conditions at 41.5 ± 0.5°C for 24 h. Presumptive colonies that displayed typical growth on the mCCDA, Gram-negative with corkscrew-like darting motility, oxidase-positive were considered to be Campylobacter Biotyping of C. jejuni were performed according to Benjamin and Skirrow (1980). To confirm the biochemical identification, the isolates were subjected to Real Time PCR targeted hippuricase enzyme encoded by hipo gene.
Real time PCR for C. jejuni
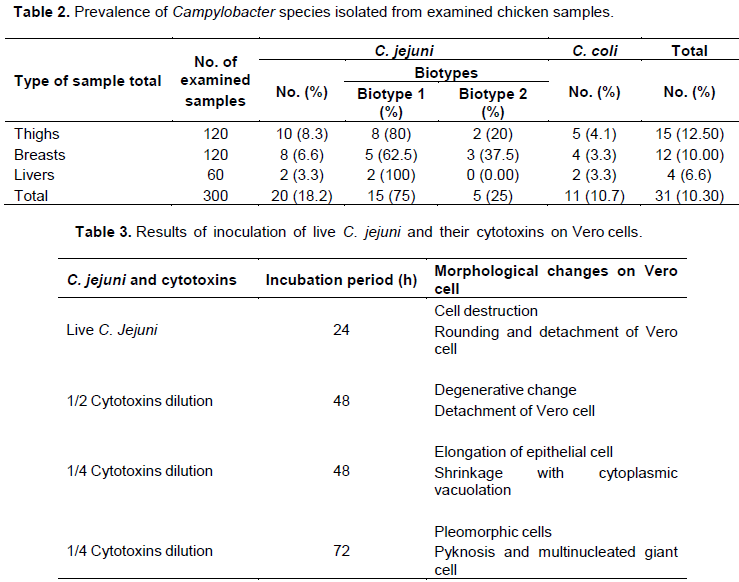
A real-time probe based quantitative PCR (qPCR) reaction was used for the confirmation of C. jejuni isolates. DNA extraction of C. jejuni was performed using boiling method according to De Medici et al. (2003). The sequences of primers and probe used for amplification of hippurhipO gene specific for C. jejuni are listed in Table 1 (Benson et al., 2002). PCR reaction was performed in a total volume of 25 μl containing 0.4 mM of each dNTP, 1× reaction buffer, 2.5 mM MgCl2 (Thermo Fisher Scientific, USA), and 1 U Platinum Taq DNA Polymerase (Life Technologies, USA). A positive control tube was also included. PCR condition concerning the MX3005P of Stratagene Cycle included initial denaturation at 94°C for 30 s followed by denaturation at 94°C for 30 s (40 cycles), annealing and extension ranged from 57 to 64°C for 60 s (Benson et al., 2002). The probes were conjugated with the fluorescent reporter dyes FAM and VIC (DNA Technology, Aarhus, Denmark) for C. jejuni, respectively, at the 5‘ ends and with the quencher dye MGBNFQ (Minor groove binder-non fluorescent quencher) at the 3’ends. The nucleotide sequences were retrieved from the Gene Bank sequence database under accession numbers Z36940 (HipO).
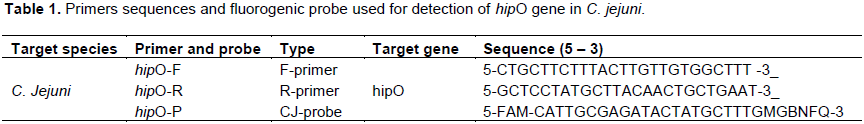
Cytopathic effect of C. jejuni live cells and their cytotoxins on Vero cells
Extraction of cytotoxins
Campylobacter cells growth were harvested in 10 ml of Brucella broth (Difco) supplemented with Campylobacter growth supplements (Oxoid), and then incubated at 42°C for 48 h (Misawa et al., 1994). Bacterial density was determined from absorbance measurement at 55 nm (Schmicz, Germany) and correlated to colony forming units (cfu). Then, cultures were centrifuged at 5000 g/15 min and the supernatant was filtrated through a 0.22 Mm Nitrocellose Membrane filter (Millipore). The resultant supernatant was tested for sterility using Campylobacter selective media (mCCD).
Vero cells
Determination of cytotoxicity of C. jejuni isolates obtained was performed on Vero cells. They were used for live bacterial cells as well as for cytotoxins assay and they were from African green monkey kidney (Vero) cells which is being supplied from Animal Health Research Institute, Dokki, Giza, Egypt. The cell viability was determined by trypan blue dye uptake. Suspension of all cell lines were prepared in eagle minimal essential media (MEM, Sigma) supplemented with 7.5% sodium bicarbonate, 10% fetal calf serum, 3% glutamine, 100 I.U/ml penicillin and 100 Mg/ml streptomycin. Cells were seeded in sterile screw capped glass Leighton tube (KIMAX) and incubated at 37°C for 24 h to allow adhering under normal atmosphere condition. When the cells become confluent, the growth medium was removed. Confluent monolayer of these cell lines were incubated with bacterium free supernatant fluids of various dilutions at 37°C (two fold dilutions of supernatant tested 1/2 to 1/4). The control groups included sterile Brucella broth was inoculated into cell culture and incubated at 42°C for 48 h, under the same condition described earlier. The cover slip was fixed with 95% methanol for 5 min and stained with 10% Giemsa stain for 20 min, then, it was washed with water and used cover slips were air-dried (Al-Delaimi, 2009).
Antimicrobial susceptibility of Campylobacter isolates
Campylobacter isolates were subjected to antimicrobial susceptibility testing including 20 C. jejuni and 11 Campylobactercoli strains tested using agar disk diffusion method (CLSI, 2014) on Muller-Hinton agar (Oxoid, CM0337) supplemented with 5% defibrinated horse blood. Plates were incubated at 42°C for 48 h under microaerobic condition. Antimicrobial agents used in this study included 12 different antimicrobials belonging to different classes including, penicillin (10 μg), ampicillin (10 μg), amoxicillin/clauvlanic acid (30 μg), erythromycin (15 μg), oxytetracycline (30 μg), nalidixic acid (30 μg), ciprofloxacin (5 μg), cephalothin (30 μg), gentamicin (10 μg), streptomycin (10 μg), and sulphamethoxazole/trimethoprim (25 μg) and chloramphenicol (30 μg).
Poultry and meat products represent the main vehicle for the distribution of Campylobacter infection (Pitk¨anen, 2013). In this study, the prevalence rate of Campylobacter spp. in chicken meat was 10.3% (31/300) classified into C. jejuni 64.5% (20/31) and C. coli 35.48% (11/31) which was in agreement with Nisar et al. (2018). All C. jejuni isolates were confirmed by real time PCR (Figure 2). The distribution of Campylobacter spp. in thighs, breasts and livers samples was 12.5, 10 and 6.6%, respectively (Table 2). Among C. jejuni samples, 15 strains belonged to biotype I and five strains belonged to biotype II (Table 3). The occurrence of Campylobacter spp. in chicken meat could be explained by unhygienic slaughter techniques including searing of carcasses with feces and rinsing, which leads to contamination of carcasses. A higher prevalence of Campylobacter spp. (56.0%) in poultry meat was recorded by Bardoˇn et al. (2011) in Czech Republic. Moreover, Strachan et al. (2012) recorded a high prevalence of Campylobacter spp. (81.0%) of chicken livers in broiler chickens at retail. In Northern Poland, Andrzejewska et al. (2015) assessed the prevalence of Campylobacter spp. in poultry meat and recovered a total of 309 (41.6%) Campylobacter isolates. in France, Guyard-Nicod`eme et al. (2015) examined 361 chicken products samples and recovered Campylobacter from 76.0% of the examined samples. In Estonia, M¨aesaar et al. (2014) reported 89.0% prevalence rate of C. jejuni. In addition, a literature survey conducted by Suzuky and Yamamoto (2009) on the presence of Campylobacter in retail poultry meats and meat by-products, the results showed high detection frequencies ranging between 28.1% in South Africa and 100% in Argentina, Belarus and Russia. Diversity in the prevalence rates of Campylobacter from retail chicken meat may result from the difference in the sanitation level during handling and processing of chicken, the sampling time of the year (hot or cold season), the sampling design, as well as diagnostic methods followed (Shin, 2000; Willis and Murray, 1997); which is definitely, the first contamination rate of poultry meat depending on post slaughter treatments, temperature control and hygiene management during the food processing or storage (Campbell et al, 2006). In the present study, C. jejuni was the most prevalent species identified from chicken samples which is in close agreement with those reported world-wide in different studies in which C. jejuni was the most prevalent than other Campylobacter spp. (Andrzejewska et al., 2015; Guyard-Nicodème et al. 2015; Suzuki and Yamamoto, 2009; Whyte et al., 2004).
Among C. jejuni positive samples, C. jejuni biotype I was the predominant biotype detected in the current study shown in Table 2. Similarly, Adesiyun et al. (1992) and Shaheen et al. (1994) concluded that C. jejuni biotype I was the predominant C. jejuni biotype isolated from poultry meat and poultry meat products and being frequently associated with human enteric infection.
In vitro demonstration of cytotoxins produced by C. jejuni isolates suggests a correlation between pathogenic virulence factors and clinical symptoms. Vero cells are employed to study the effect of microbial toxins and provide a useful, sensitive and reproducible experimental method for the study of pathogenic mechanism. Other investigators referred to association of cytotoxins production with a clinical history of bloody diarrhea, but enterotoxin production with watery diarrhea (Lee et al., 2000; Prasad et al., 2006). In this study, cytotoxin-producing capacity was detected in most of the strains tested (75%, 15/20). Moreover, Vero cells cytotoxicity was represented by rounding and detachment of cells. This activity was observed after 24, 48 and 72 h after incubation with titers which varied from 1/2 to 1/4 for cytotoxic isolates (Table 3 and Figure 1). High prevalence of cytotoxicity in Campylobacter spp. indicates a significant epidemiological role of Campylobacter in human infections which was in agreement with many previous investigators (Klipstien et al., 1985; Johnson and Lior, 1986; Florin and Antillon, 1992).
In Egypt, due to the excessive use of antibiotics for treatment and prophylaxis as well as growth promotion in chickens, poultry meat is considered a serious vehicle of antimicrobial-resistant Campylobacter transmission to human. Antibiotic susceptibility rates of Campylobacter isolates are shown in Table 4. There was a remarkably high resistance rate displayed by C. jejuni and C. coli to penicillin (95 and 90.1%), chloramphenicol (90 and 90.1%) and gentamicin (80 and 81.81%), respectively. Campylobacters also revealed a high antibiotic resistance against trimethoprim- sulfamethoxazole (85 and 81.81%), cephalothin (75 and 72.72%), erythromycin (75 and 72.72%), ampicillin (70 and 54.5%), amoxicillin-clauvlanic acid (65 and 63.63%), oxytetracycline (65 and 63.63%), and streptomycin (65 and 63.63%), while they revealed a lower resistance against nalidixic acid (30 and 36.36%) and ciprofloxacin (10 and 18.81%) for C. jejuni and C. coli, respectively. Multidrug resistance (Resistance to three or more classes of antimicrobials) was observed in 85% (17/20) and 81.82% (9/11) of C. jejuni and C. coli isolates respectively. While, none of the Campylobacter isolates were resistant to all of the antimicrobials tested. In this study C. jejuni showed remarkable higher incidence in antimicrobial resistance than C. coli. These findings were in agreement with that previously reviewed in many studies (Saleha, 2002; Sáenz et al., 2000; Aarestrup and Engberg, 2001; Taremi et al., 2006). In contrary, C. coli showed a higher prevalence of antimicrobial resistance than C. jejuni by Signorini et al. (2018).
In this study, Campylobacter isolates were more sensitive to ciprofloxacin which is in agreement with McDermott et al. (2002) and Moore et al. (2005) who stated that ciprofloxacin was the drug of choice for empirical therapy of bacterial food borne diarrhea, including that caused by Campylobacter. In addition, Kassa et al. (2007) found that C. jejuni, C. coli and C. lari isolated from food animals were sensitive to chloramphenicol and ciprofloxacin. Finally, a complete comparison in the susceptibility of Campylobacter to different antimicrobials is impossible as the strains numbers examined differ from one study to another.
The results obtained from this study suggest an important role of chicken meat as a source of cytotoxic and multidrug resistant Campylobacter spp.; therefore, there is a possible risk to human when dealing with the raw poultry carcass or consumption of undercooked chicken products. So effective vaccine against Campylobacter infection should be recognized to protect against infection by this group of organisms with appropriate hygienic measures during carcass slaughtering and processing. In addition, one of our future studies will be focused on developing strategies to decrease Campylobacter colonization in broilers chicken.
The authors have not any conflict of interests.
REFERENCES
|
Aarestrup FM, and Engberg J (2001). Antimicrobial resistance of thermophilic Campylobacter. Veterinary Research 32:311-321.
Crossref
|
|
|
|
Adesiyun AA, Ojo MO, Webb L, Paul C (1992). Isolation of Campylobacters, Salmonellae and Escherichia coli from broilers in commercial poultry processing plants in Trinidad. in Proceedings, The 3rd World Congress on Foodborne Infections and Intoxications. Vol.1. Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany pp. 468-473.
|
|
|
|
|
Al-Delaimi MS (2009). Cytopathic effect of cytotoxin produces by C. Jejuni On tissue culture cells. Journal of Duhok University 12(1):275-281.
|
|
|
|
|
Andrzejewska M, Szczepa’nska B, Spica D, Klawe JJ (2015). Trends in the occurrence and characteristics of Campylobacter jejuni and Campylobacter coli isolates from poultry meat in Northern Poland. Food Control 51:190-194.
Crossref
|
|
|
|
|
Bardoˇn J, Kolar M, Karpiskova R, and Hricova K (2011). Prevalence of thermotolerant Campylobacter spp. in broilers at retail in the Czech Republic and their antibiotic resistance. Food Control 22:328-332.
Crossref
|
|
|
|
|
Benjamin J, Skirrow MB (1980). 1001 Campylobacter: cultural characteristic of intestinal Campylobacter isolated from man and animals. J Food Hygiene (85):427-442. PMCID:PMC2134020
|
|
|
|
|
Bronowski C, James CE, Winstanle C (2014). Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiology Letters 356(1):8-19
Crossref
|
|
|
|
|
Campbell LK, Havens JM, Scott MA, Lamp LW (2006). Molecular detection of Campylobacter jejuni in archival cases of acute appendicitis. Modern Pathology 19:1042-1046.
Crossref
|
|
|
|
|
CLSI (2014) Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI Document M100-S24 Wayne, PA.
|
|
|
|
|
De Medici D, Croci L, Delibato E, Di Pasquale S, Filetici E, Toti L. (2003). Evaluation of DNA extraction methods for use in combination SYBR green I real-time PCR to detect Salmonella enterica serotype Enteritidis in poultry. Applied and Environmental Microbiology 69:3456-3461.
Crossref
|
|
|
|
|
European Food Safety Authority (EFSA) (2016) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. European Food Safety Authority Journal 14(12):4634.
|
|
|
|
|
Florin I, Antillon F (1992). Production of enterotoxin and cytotoxin in C. jejuni strain isolated in Costa Rica. Journal of Medical Microbiology 37:22-29.
Crossref
|
|
|
|
|
Guyard-Nicodème M, Rivoal K, Houard E, Rose V, Quesne S, Mourand G, Rouxel S, Kempf I, Guillier L, Gauchard F, Chemaly M (2015). Prevalence and characterization of Campylobacter jejuni from chicken meat sold in French retail outlets. International Journal of Food Microbiology 203:8-14.
Crossref
|
|
|
|
|
International Organization for Standardization (ISO) (2006). ISO 10272 Microbiology of Food and Animal Feeding Stuff - Horizontal Method for Detection and Enumeration of Campylobacter spp. - Part 1: Enrichment Method; Part 2: Enumeration Method. ISO, Geneva, Switzerland.
|
|
|
|
|
Johnson WM, Lior H (1986). Cytotoxic and cytotonic factors produced by C. jejuni, C. coli and C. lari. Journal of Clinical Microbiology 24:275-281.
|
|
|
|
|
Kassa T, Gebre-Selassie S, Asrat D (2007). Antimicrobial susceptibility patterns of thermotolerant Campylobacter strains isolated from food animals in Ethiopia. Veterinary Microbiology 119:82-87.
Crossref
|
|
|
|
|
Klipstien FA, Eugert RF, Short H, Schenk EA (1985). Pathogenic properties of C. jejuni assay and correlation with clinical manifestation. Infection and Immunity 58:43-49.
|
|
|
|
|
Lee A, Smith S, Coloe PJ (2000). Detection of a novel Campylobacter cytotoxin. Journal of Applied Microbiology 89:719-725.
Crossref
|
|
|
|
|
Levy SB, Marshall B (2004). Antibacterial resistance worldwide: causes, challenges and responses. Nature Medicine 10:S122–S129.
Crossref
|
|
|
|
|
M¨aesaar M, Praakle K, Merem¨ae K, Kramarenko T, S˜ogel J, Viltrop A, Muutra K, Kovalenko K, Matt D, H¨orman A, H¨anninen M-L, Roasto M (2014). Prevalence and counts of Campylobacter spp. in poultry meat at retail level in Estonia. Food Control 44:72-77.
Crossref
|
|
|
|
|
McDermott PF, Bodeis SM, English LL, White DG, Walker RD, Zhao S, Simjee S, Wagner DD. (2002). Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with Fluorquinolones. The Journal of Infectious Diseases 185:837-840.
Crossref
|
|
|
|
|
Misawa N, Ohnishi T, Itoh K, Takahashi E (1994). Development of a tissue culture assay system for C. jejuni cytotoxins and the influence of culture condition on cytotoxin production. Journal of Medical Microbiology 41:224-230.
Crossref
|
|
|
|
|
Moore JE, Corcoran D, Dooley JSG, Fanning S, Lucey B, Matsuda M, McDowell DA, Megraud F, Millar BC, O'Mahony R, O'Riordan L, O'Rourke M, Rao JR, Rooney PJ, Sails A, Whyte P (2005). Campylobacter. Veterinary Research 36:351-382.
Crossref
|
|
|
|
|
Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (2007). Manual of Clinical Microbiology, 9th ed. ASM Press, American Society of Microbiology. Washington, DC. pp. 1995-2012.
|
|
|
|
|
Nachamkin I, Szymanski CM, Blaser MJ (2008). Campylobacter, 3rd ed. ASM Press, American Society for Microbiology, Washington, DC. pp. 376-388.
|
|
|
|
|
Nisar M, Ahmad MUD, Mushtaq MH, Shehzad, W, Hussain A, Nasar M, Nagaraja KV, Goyal SM (2018). Occurrence of Campylobacter in retail meat in Lahore, Pakistan. Acta Tropica 185:42-45
Crossref
|
|
|
|
|
Pitk¨anen T (2013). Review of Campylobacter spp. in drinking and environmental waters. Journal of Microbiological Methods 95:39-47.
Crossref
|
|
|
|
|
Prasad KN, Dhole TN, Ayagari A (2006). Adherence, invasion and cytotoxin assay of C.jejuni in Hela and HEP-2 cell. Journal of Food Protection 69:768-774.
|
|
|
|
|
Sáenz Y, Zarazaga M, Lantero M, Gastanares MJ, Baquero F, Torres C (2000). Antimicrobial resistance in Campylobacter strains isolated from animals' foods and humans in Spain in 1997-1998. Antimicrobial Agents and Chemotherapy 44:267-271.
Crossref
|
|
|
|
|
Saleha AA (2002). Isolation and characterization of Campylobacter jejuni from broiler chickens in Malaysia, International Journal of Poultry Science 1(4):94-97.
Crossref
|
|
|
|
|
Shaheen AA, EL-Khashab N, Saad SM (1994). Listeria monocytogenes, Yersina enterocolitica and dressed poultry marked. Science Medicine Journal Cai Medical syndrome 4(6):1-8.
|
|
|
|
|
Shin DY (2000). Isolation and identification of Enteropathogenic Campylobacter spp. From samples in Taipei. Journal of Food Protection 63:304-308.
Crossref
|
|
|
|
|
Signorini ML, Rossler E, Díaz David DC, Olivero CR, Romero-Scharpen A, Soto LP, Astesana DM, Berisvil AP, Zimmermann JA, Fusari ML, Frizzo LS, Zbrun MV (2018). Antimicrobial Resistance of thermotolerant campylobacter species isolated from humans, food-producing animals, and products of animal origin: A worldwide meta-analysis. Microbial Drug Resistance P 30.
Crossref
|
|
|
|
|
Strachan NJC, Macrae AM, Thompson BA (2012). Source attribution, prevalence and enumeration of Campylobacter spp. from retail liver. International Journal of Food Microbiology 153:234-236.
Crossref
|
|
|
|
|
Suzuki H, Yamamoto S (2009). Campylobacter contamination in retail poultry meats and by-products in the world: A literature survey. The Journal of Veterinary Medical Science 71:255-261.
Crossref
|
|
|
|
|
Taremi M, Dallal MS, Gachkar L, MoezArdalan S, Zolfagh- arian K, Zali MR (2006). Prevalence and antimicrobial resistance of Campylobacter isolated from retail raw chiken and beef meat, Tehran, Iran. International Journal of Food Microbiology 108: 401-403
|
|
|
|
|
Wassenaar TM (1997). Toxin production by Campylobacter spp. Clinical Microbiology Reviews 10: 466-476.
|
|
|
|
|
Whyte P, McGill K, Cowley D, Madden RH, Moran L, Scates P, Carroll C, O'Leary A, Fanning S, Collins JD, McNamara E, Moore JE, Cormican M (2004). Occurrence of Campylobacter in retail foods in Ireland. International Journal of Food Microbiology 95:111-118.
Crossref
|
|
|
|
|
Willis W, Murray C (1997). Campylobacter jejuni seasonal recovery observation of retail market broilers The Journal of Poultry Science 76:314-317.
Crossref
|
|