ABSTRACT
Biosafety has currently become an important public health issue in seafood industry of Thailand. In order to enhance quality of seafood products, three phases of study were established. In the first phase, biosafety of traditional processed squid sold in Chon Buri province, Thailand compiled from literatures between 2002 and 2019 was evaluated. The squid products represented a health hazard due to 86.5% samples, having total viable count (TVC) over the allowable limits imposed by Thailand and international food administration agencies. In the second phase, bacterial contamination during multiple steps related to dried seasoned squid preparation and storage was determined. TVC were low at approximately 102 CFU/g during a multi-step of preparation, and increased significantly (P < 0.05) to 103 CFU/g in post-prepared products during 25-day storage. Lastly, a novel preservation strategy was developed. A combination of partially purified solution from Bacillus velezensis BUU004 with nisin and the mixed herb extracts from hot pepper and lemongrass exhibited the strongest antibacterial efficacy observed by a significantly (P < 0.05) low TVC in the post-prepared squid within allowable limits recommended by the food administration authorities. This study suggests that the novel mixture has a preservative potential to improve biosafety of dried seafood products.
Key words: Squid, bacteriocin, nisin, herb extract, Bacillus velezensis, synergy, biosafety.
Thailand is one of the major countries in production, consumption and trade of seafood products. A divergence of traditional seafood-based product with varying unique flavors and textures is produced in response to an increased demand of domestic consumption. Annual per capita consumption of seafood in Thailand is approximately 33 kg in 2016 (Pisuthipan, 2019). Biosafety has currently become a significant public health issue in seafood industry of Thailand (Thungkao and Muangharm, 2008; Nimrat et al., 2019). In spite of an advanced technology in food preservation being applied to extend the shelf life, seafood products have been threatened by pathogens and pose a serious risk of food-borne diseases like diarrhea, gastritis, and food poisoning illnesses (Butkhot et al., 2019a). The most frequently isolated food-borne pathogens in dried seafood products are Bacillus cereus, Escherichia coli, Staphylococcus aureus and Salmonella enterica (Thungkao and Muangharm, 2008; Butkhot et al., 2019a; Nimrat et al., 2019). This encourages food producers, food-safety inspectors and researchers to develop an effective biopreservative-based technology for improving the biosafety of food products. Among different approaches, supplementation of natural antimicrobial compounds, such as bacteriocins, bacteriocin-like substances, and plant extracts, has promising potential for controlling pathogen growth in food systems in response to an increased pressure from consumers to purchase more “natural-green” processed products (Burt, 2004; Butkhot et al., 2019a).
Nisin is a lanthionine-containing peptide produced by certain strains of Lactococcus lactis subsp. lactis (Ettayebi et al., 2000). It is thermally stable and easily degraded in digestive system. Despite being granted as “generally regarded as safe” for use as food additive, such limitation of nisin in many food industries includes its expensiveness, especially when high concentration is required to gain satisfactory inhibitory activity against food-borne pathogens (Ettayebi et al., 2000). In addition, its use as biopreservative in commercial foods is compromised by the lack of inhibitory actions against Gram negative bacteria and development of nisin-resistant strains of various pathogens (Zhou et al., 2014). The problems could be overcome by the administration of nisin together with other natural substances having a synergistic action. Several authors claimed that the inhibitory spectrum of nisin in food systems can be potentiated to Gram negative bacteria with the presence of plant-derived extracts and other bioactive compounds (Govaris et al., 2010; Turgis et al., 2012; Shahbazi, 2015; Shahbazi et al., 2016). In our recent study, bacteriocin produced by Bacillus velezensis BUU004 demonstrated thermoresistance, stability under wide range of pH, very low cytotoxicity, and broad-spectrum antibacterial activity against important food-associated pathogens in vitro and food system studies, thereby having a promising potential as natural preservative in food industry (Butkhot et al., 2019a; 2019b).
The use of plant essential oils and extracts as biopresevative to inhibit growth of pathogenic and food-spoilage bacteria is of great interest in dried seafood industry owing to harmless side effects, broad spectrum of antibacterial activity, economic benefits and a long history of safe use in foods (Nazarizadeh et al., 2013). However, high concentration of plant extracts/essential oils required to achieve satisfactory inhibitory effects in food matrix as observed in in vitro experiment may be problematic because of degenerative sensorial quality of food products and unacceptability of consumers (Burt, 2004). Undesirable consequences of herb extracts/ essential oil supplement in foods could be obviated simultaneously with securing antibacterial efficacy by the combined addition of antimicrobial peptides. Until now, antibacterial activity of nisin and/or other bacteriocins in combination with Thai herb extracts against food-borne and spoilage bacteria in processed seafood products have not been investigated. Therefore, this study aim to compile the literatures focused on bacteriological quality of processed squid-based products, evaluate bacterial contamination in multiple steps related to dried seasoned squid preparation and storage, and extend the antibacterial efficacy of bacteriocin produced by B. velezensis BUU004 by combination with nisin and mixture of hot pepper and lemongrass extracts for controlling growth of contaminated bacteria in dried seasoned and crushed squid.
Bacteriological quality of traditional processed squid
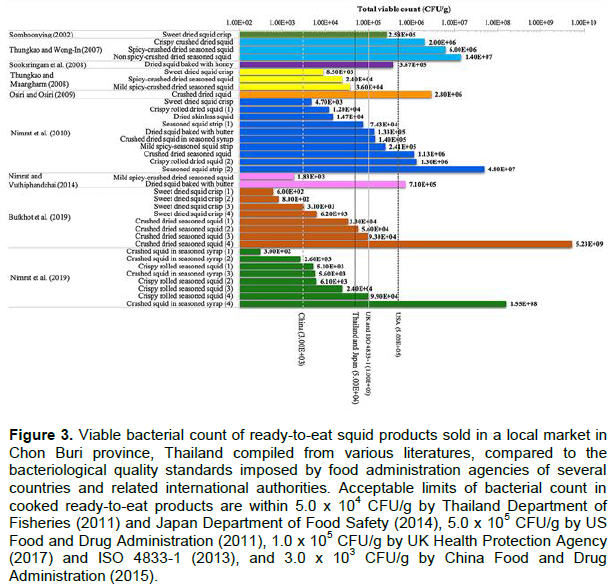
A bacteriological quality of a variety recipe of traditional ready-to-eat squid sold in Chon Buri province, Thailand was compiled from several sources as follows: authoritative collections of online journals, books, and research resources, e.g. Pubmed, Sciencedirect, Wiley online library, SpringerLink, ThaiJO, and Google scholar; manual compilation of published and unpublished data from the library of Burapha University; and research articles, review articles, academic reports, conference proceedings, and thesis from Thai Library Network (cooperation network among university libraries in Thailand). Keywords used for searching the targeted data in all available collections were viable bacteria, heterotrophic bacteria, bacteriological quality, biosafety, seafood products, processed squids, ready-to-eat squids, and Chon Buri. The criteria used for selection of articles were the findings focused on bacteriological quality in ready-to-eat and processed traditional squid products in Chon Buri province, Thailand, and published or unpublished articles in Thai and/or English before 2020. Total viable counts (TVC) in the squid product samples were extracted from each article and compared to the allowable values imposed by food administration agencies of Thailand, USA, UK, Japan and China, and related international authorities to indicate biosafety quality of the Thai traditional processed seafood products (China Food and Drug Administration, 2015; Thailand Department of Fisheries, 2011; International Organization for Standardization ISO 4833-1, 2013; Japan Department of Food Safety, 2014; UK Health Protection Agency, 2017; US Food and Drug Administration, 2011).
Bacterial contamination during preparation steps of dried seasoned and crushed squid
Dried seasoned and crushed squid was prepared in a small household facility located in a fishing village in Chon Buri province, Thailand, to imitate a real situation of the dried squid production in Thailand. Briefly, fresh splendid squids (Loligo duvauceli) were purchased from a local market in Chon Buri province, Thailand. Medium-sized squids (15-18 cm long) were cut longitudinally in the center of the abdomen using a sharp knife. Then, manual cleaning of the squid was performed by thoroughly removing head, tentacles, skin, eyes, soft shell, beak and internal organs using tap water (Figure 1a). The 5-kg rinsed squids were marinated in brown seasoning sauce composed of chili sauce (1.5 L), sugar (500 g), chili pepper powder (80 g), chopped garlic (10 g), and vinegar (50 mL) for 1 h (Figure 1b). Subsequently, flattened squids were laid on a steel wire grate, and sun-dried from 8 am to 4 pm for two successive days (Figure 1c). After approximately 2 min grilling on an electric grill (Figure 1d), the squids were individually crushed using a rolling machine (Figure 1e) and sun-dried again until dry. Dried seasoned and crushed squids (Figure 1f) were packed into a plastic bag and stored at room temperature for 25 days. During multistep related to preparation and storage, random sampling was taken in rinsed squid, marinated squid, first sun-dried squid, grilled squid, crushed squid, second sun-dried squid, 10-day stored squid, and 25-day stored squid to evaluate bacterial quality and abundance.
Bacteriological quality was evaluated based on a protocol recommended by U.S. Food Drug Administration (1998) with some modifications. A portion (50 g) of samples collected at defined intervals was homogenized with Butterfield’s phosphate-buffered water (450 mL) using a stomacher for 2 min. To enumerate TVC, homogenate was 10-fold diluted in same solution and an aliquot (0.1 mL) of each dilution was spread-plated onto Plate Count Agar (Becton BD, Sparks, Maryland, USA). All petri dishes were incubated at 35 ± 2 ºC for 24 h. All colonies were counted and expressed as colony forming unit (CFU) per g of sample. Bacterial identification was conducted following a basis of Gram-staining, cell morphology, and biochemical characteristics recommended by Winn et al. (2006) and API test kits (bioMerieux, Marcy I’ Etoile, France). All measurements were achieved in triplicate.
Preparation of partially-purified solution containing bacteriocin from B. velezensis BUU004 (PPS-BV)
The partially-purified solution containing bacteriocin was obtained from the culture of B. velezensis BUU004 (Butkhot et al., 2019a). The strain was grown in a 250-mL flask containing 100 mL of Trypticase Soy Broth (Becton BD) and incubated at 30°C, 200 rpm for 18 h in a shaking incubator. Cell suspension was centrifuged at 8,000 g, 4°C for 10 min. The supernatant containing bacteriocin was collected, and then added with ammonium sulfate at 80% saturation to precipitate proteins with gentle stirring overnight at 4°C. Protein precipitate was harvested by centrifuging at 10,000 g at 4°C for 30 min (Figure 2a), re-suspended in 50 mM sodium phosphate buffer (pH 7.0), and dialyzed using a dialysis membrane tubing (1 kDa cutoff, Spectrum Laboratory, New Brunswick, New Jersey, USA) at 4 °C overnight. Following passing through a 0.45 mm syringe filter (Whatman, Kent, UK), the PPS-BV was tested for bacteriocin activity in arbitrary unit (AU) against Bacillus cereus TISTR 687 using an agar well diffusion method (Figure 2b; Butkhot et al., 2019b). The PPS-BV (800 AU/mL) was prepared by dissolving in 50 mM sodium phosphate buffer (Figure 2c) and frozen at -20 °C until use.

Herb extraction
Lemongrass stems (Cymbopogon citratus (DC) Stapf.) and hot pepper fruits (Capsicum frutescens L.) were purchased from a local spice store in Chon Buri province, Thailand. Herb preparation and extraction were followed a protocol described by Soodsawaeng et al. (2021a). The herbs were chopped using a table knife, dried in a plant drier at 35 °C for 72 h, and then ground using an electronic blender. The dried herb powders were individually submerged with 95% ethanol at a ratio of 1:10 of material to extractant, and agitated in a shaking incubator (JSR, JSSI-100C model, Cheongju, South Korea) at 120 rpm, 30 °C for 72 h. The supernatants were vacuum-filtered using a Whatman filter membrane no.1 and evaporated at 40°C and 175 mbar, using a rotary evaporator (Buchi R-215, Flawil, Switzerland). The ethanolic extract stock was made, sterilized using a 0.45 mm syringe filter, and stored in an amber bottle at -20°C.
Nisin preparation
Nisin contained 2.5% active nisin with minimum potency of 106 International Units (IU/g) was purchased from Sigma-Aldrich Chemical Co, Darmstadt, Germany. Nisin solution was prepared by dissolving nisin powder (100 mg) in 0.02 N HCl, adding with sterile distilled water to produce a final concentration of 103 IU/mL, filtering using a 0.45 mm syringe filter, and storing in a 4°C refrigerator.
Synergistic study of the PPS-BV in combination with nisin and the mixed herb extracts on viable bacteria in dried seasoned and crushed squid
Antibacterial efficacy of the PPS-BV in combination with nisin and the mixed herb extracts on viable bacterial count was examined according to the method described by Butkhot et al. (2019a). Due to having a significantly increased TVC compared to other stages, dried seasoned and crushed squid during a storage period was used as a food model in this experiment. The squids were produced following procedures mentioned earlier and stored in a plastic bag at 4 ºC for 28 days. A 2 x 2 cm piece of the squid was made using a sterile scissors. Treatment of the samples included supplementation of 1) sterile distilled water (control), 2) nisin (103 IU/mL), and 3) a combined additive of the PPS-BV (800 AU/mL), nisin (103 IU/mL) and the mixture of herb extracts (160 mg/mL). A minute volume (0.1 mL) of the supplements was introduced onto whole surface of the squid samples following the respective treatments. After air-drying in a biosafety cabinet for 15 min, the sample in each treatment was placed in a sterile plastic bag (3 x 5 inches; 1 sample: 1 bag) to prevent cross contamination, and then stored in a 4ºC refrigerator. Enumeration of viable bacteria in the squid samples was conducted at 15 min, 1, 7, 14, 21 and 28 days post-inoculation using a spread plate technique as aforementioned. TVC of the control and treated groups was compared to the allowable limits imposed by food administration agencies of Thailand, USA, UK, Japan and China, and related international authorities as mentioned previously to evaluate the efficacy of the tested additives.
Data analysis
Data are represented as mean ± standard deviation (S.D.). The numbers of bacteria were 10-log transformed to normalize distribution when needed prior to statistical analyses. Differences were analyzed using a One-way ANOVA following the Tukey’s multiple comparison test at a significant level of P < 0.05. All statistical analyses were performed using a Minitab Version 18.1.0.
Bacteriological quality of processed squid products distributed in Chon Buri province, Thailand based on literatures
TVC of traditional processed squid complied from literatures was in the ranges of 3.0 × 102 to 5.2 × 109 CFU/g (Figure 3). Of all samples compiled, 27.0% (10/37), 40.5% (15/37), 51.4% (19/37) and 86.5% (32/37) samples contained TVC over the maximum limits acceptable for ready-to-eat fishery products set by US Food and Drug Administration (2011; 5 × 105 CFU/g), UK Health Protection Agency (2017) and ISO 4833-1 (2013; 1 x 105 CFU/g), Thailand Department of Fisheries (2011) and Japan Department of Food Safety (2014; 5 × 104 CFU/g), and China Food and Drug Administration (2015; 3 × 103 CFU/g), respectively (Figure 3). Overall, a public health issue of traditional processed squid distributed in Chon Buri province, Thailand is alarming due to 86.5% samples compiled between 2002 and 2019, containing TVC over the acceptable value of at least one criterion imposed by food administration agencies.
Bacterial abundance during various steps related to production of dried seasoned and crushed squid
Squid samples contained low TVC in the ranges of 1.2 ± 0.7 × 102 to 2.6 ± 0.9 × 102 CFU/g during the steps between rinsing and second sun-drying (Figure 4a). In post-prepared products, TVC appeared to increase significantly during 25 days of storage at room temperature. TVC increased significantly to 2.0 ± 0.2 × 103 CFU/g at 10 days of storage and 2.1 ± 0.5 × 103 CFU/g at 25 days of storage (Figure 4a).
Bacterial composition of squid samples changed towards a multistep of production and storage. In rinsed squid, six bacterial species were isolated including Bacillus pasteurii (30.8%), Micrococcus luteus (15.4%), B. brevis (15.4%), B. badius (15.4%), Bordetella parapertussis (15.4%), and B. insolitus (7.6%; Figure 4b). In marinated squid, bacterial component was changed to Staphylococcus carnosus subsp. utilis (63.6%), B. pasteurii (18.2%), S. piscifermentans (9.1%) and B. badius (9.1%). Thereafter, isolated bacteria increased to eight species belonging to S. piscifermentans, M. luteus, B. pasteurii, B. brevis, B. firmus, B. pantothenticus, B. insolitus, and Bo. parapertussis in first sun-dried squid.
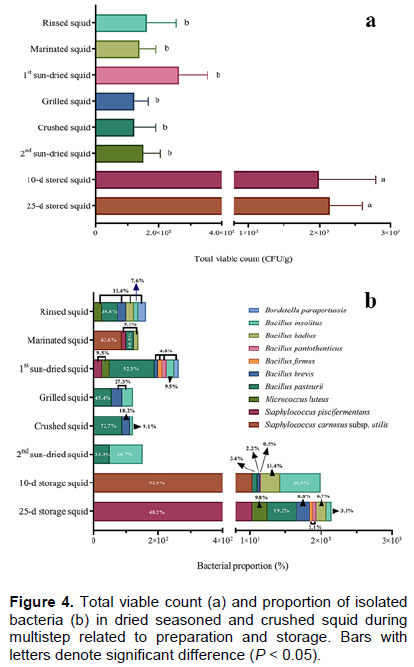
Bacillus species (B. pasteurii, B. brevis, and B. insolitus) were predominantly isolated in squid during the grilling and crushing steps. Then, two Bacillus species, such as B. pasteurii and B. insolitus, were present in the 2nd sun-dried squid. At 25 days of storage, eight bacterial isolates were recovered with the main bacterial species of S. piscifermentans (48.2%), and B. pasteurii (19.2%; Figure 4b).
Efficacy of the PPS-BV in combination with nisin and the mixed herb extracts against TVC in dried seasoned and crushed squid
High TVC ranging from 4.27 ± 0.33 × 103 to 1.37 ± 0.02 × 104 CFU/g was observed in the control squids during 28-day trial. At 15-min post-exposure, the lowest TVC was produced with the presence of the PPS-BV in combination with nisin and the mixed herb extracts in dried seasoned and crushed squid (1.75 ± 0.43 × 102 CFU/g) and, then TVC remained in the range of 2.33 ± 0.82 × 102 to 1.53 ± 0.11 × 103 CFU/g and dropped to 7.50 ± 4.33 × 101 CFU/g at the end of experiment. TVC in squid samples introduced with nisin was also significantly different (P < 0.05), compared to the control, but significantly (P < 0.05) higher than that of the dried squid added with the PPS-BV plus nisin and the mixed herb extracts during 28-day refrigerated storage, except at day 7 and 14 of storage (Figure 5).
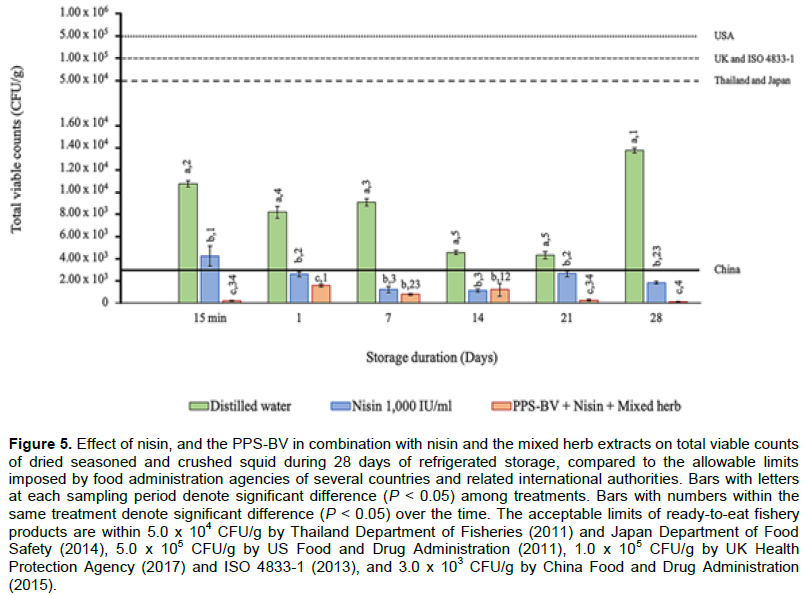
In comparison with the acceptable values, TVC in the control and the dried squid introduced with nisin during 28-day refrigerated storage met the standards for processed seafood products announced by Thailand Department of Fisheries (2011), ISO 4833-1 (2013), Japan Department of Food Safety (2014), UK Health Protection Agency (2017) and US Food and Drug Administration (2011), except China Food and Drug Administration (2015). Interestingly, TVC in the squid samples added with a combination of the PPS-BV with nisin and the mixed herb extracts was within allowable limit of all criteria throughout 28-day trial (Figure 5).
Bacteriological quality and bacterial abundance during multi-steps related to prepared and post-prepared dried seasoned squid
Deteriorated bacteriological quality of food products is a serious safety issue. Although the growth of most microorganisms can be limited by high salt and low moisture contents, almost all processed squid products (86.5%) compiled from the literatures contained TVC over the allowable limits declared by Thailand and international food administration agencies. This suggested that a health hazard from consumption of traditional processed squid products distributed in Chon Buri, province, Thailand is indeed alarming. In general, several factors throughout all stages of the food production and distribution systems affect food safety. Food-borne pathogenic and spoilage bacteria can enter food products during: primary production (in the farm/sea where animals are raised or caught); transportation; food processing; storage; distribution; and preparation and serving (both outside and inside the home; Bintsis, 2018). Thus, investigations of bacteriological quality involved production of dried seafood products would assist in identifying certain sources of contamination. In this study, predominant bacteria isolated along a multistep production of dried seasoned and crushed squid were Bacillus, Staphylococcus, and Micrococcus. Similarly, Butkhot et al. (2019a) reported that dried seafood products were composed mainly of several species in the genera of Staphylococcus, Bacillus, and Pantoea. Due to their water and environmental origins, the presence of Bacillus, Micrococcus and Bordetella as dominant species in post-rinsed squid is possibly associated with poor personnel hygiene and inadequate handling (Moon et al., 2017). In the next step, bacterial abundance in the squid marinated in brown seasoning sauce was changed to S. carnosus subsp. utilis and S. piscifermentans and a few species of Bacillus remained survived, namely B. pasteurii and B. badius. The shifting of bacterial composition is perhaps expected due to antimicrobial feedback of spices, such as chili pepper, garlic and other additives, used as ingredients of brown seasoning sauce. Multiple bioactive phytochemicals present in garlic and chili pepper have been reported to have a strong antibacterial potential against Aeromonas, Bacillus, Citrobacter, Clostridium, Enterobacter, Escherichia, Klebsiella, Lactobacillus, Leuconostoc, Micrococcus, Mycobacterium, Proteus, Providencia, Pseudomonas, Salmonella, Serratia, Shigella, Staphylococcus, Streptococcus, Listeria, and Vibrio (Shokrzadeh and Ebadi, 2006; Omolo et al., 2014). However, re-emergence of several species belonging to Bacillus, Staphylococcus, Micrococcus and Bordetella was observed in 1st sun-dried products in this study. In Thailand, the operation and processing conditions of traditional dried seafood products in small factories or household facilities are primitive with rudimentary hygienic practices (Butkhot et al., 2019a). The facilities have free access of biotic and non-biotic agents, such as pathogens, flies, insects, rodents, dust, soil, and hazardous chemicals and pollutants into the products. These reasons may account for a deteriorative bacteriological quality of the traditional Thai squid products observed in this study. Interestingly, viable bacterial count in dried squid products appeared to significantly increase during storage and distribution periods for 25 days in this study. Such a phenomenon may reflect a poor handling practice of post-prepared products supported by the presence of S. carnosus subsp. utilis and S. piscifermentans as the predominant flora due to their human skin origin (Becker et al., 2014). In retail stores in Thailand, the products are usually stored at room temperature and handled without suitable hygienic awareness during being portioned from bulk containers into small plastic bags for sale (Butkhot et al., 2019a). Of all bacteria isolated, Bo. parapertussis causes a pertussis-like syndrome in human (Masin et al., 2015). M. luteus is considered as an opportunistic pathogen associated with meningitis, septic arthritis, endocarditis, chronic cutaneous infections and catheter infections in immunocompromised patients (Becker and von Eiff, 2011).
In order to minimize the risk of microbial contamination and secure food safety from farm to fork of the traditional Thai squid products, the factories should follow the microbiological guidance, such as. Good Hygiene Practices (GHPs), Good Manufacturing Practices (GMPs) and Hazard Analysis Critical Control Point (HACCP) systems developed by World Health Organization and United States Food and Drug Administration. Stakeholders, such as government and food industry should promote education programs based on basic principles for microbiological food safety to create biosafety awareness of the food personnel and consumers. In addition, due to a markedly increased TVC in dried seasoned squids during storage and distribution processes observed in this study, it is imperative to implement an effective technique for minimizing the bacterial growth in the products to avoid the outbreak of food-borne illnesses.
Antibacterial efficacy of the PPS-BV with the presence of nisin and the mixture of herb extracts
Safety issue of chemical preservatives has been questioned by consumers in terms of the potential carcinogenic and toxic effects on human health. In our recent study, the PPS-BV was inactive to control the growth of spoilage bacteria in the dried seasoned squid (Soodsawaeng et al., 2021b). We postulated that such a phenomenon may be due to easy degradation of bacteriocin by indigenous and/or microbial proteolytic enzymes, its interactions with the food components, like proteins, carbohydrates and fats in squid, and biofilm formation of bacterial flora. Likewise, the mixed herb extracts from hot pepper and lemongrass demonstrated low inhibitory activity against food spoilage bacteria in food system trial (Soodsawaeng et al., 2021b). Therefore, in the present study, we introduced the PPS-BV in combination with nisin and the mixed herb extracts into dried seasoned and crushed squid during storage and distribution processes. The novel combination represented a great potential as biopreservative supported by a significant decrease in TVC in squid samples to below allowable limits of all food administration agencies during 28 days of chilled storage. Our results are similar to several previous reports. Field et al. (2015) reported that a solution containing a bioengineered derivative nisin V in combination with low concentration of either carvacrol or trans-cinnamaldehyde was synergistically effective to delay lag phase and markedly reduce viable cells of L. monocytogenes in laboratory media, chocolate milk drink and chicken noodle soup. Bag and Chattopadhyay (2017) claimed that the activity of nisin was significantly enhanced against planktonic cells and biofilm formation of food-borne pathogenic B. cereus and S. Typhimurium when nisin was combined with p-coumaric acid. Similarly, administration of nisin simultaneously with essential oil (0.1-0.2%) from Ziziphora clinopodioides resulted in a significant decrease in E. coli O157:H7 count in raw beef patty during 9 days of refrigerated storage (Shahbazi et al., 2016), and complete mortality of E. coli O157:H7 in doogh yoghurt drink (Shahbazi, 2015). Antilisterial activity of enterocin AS-48, a cyclic bacteriocin produced by Enterococcus feacalis, was significantly extended by combined addition of one of these essential oils (thyme verbena, thyme red, Spanish oregano, ajowan, tea tree, clove, and sage) into the Russian type salad (Molinos et al., 2009).
A significant reduction in TVC of the squid samples introduced with the PPS-BV in combination with nisin and the mixed herb extracts in this study is likely to involve in a synergistic activity between the components in the combined additives. Lemongrass extract/essential oil has been reported to contain citral chemotype: geranial, neral and limonene with a wide inhibitory spectrum against pathogenic E. coli O157:H7, Salmonella Typhimurium, S. aureus and L. monocytogenes (Oussalah et al., 2007). Cinnamic acid and m-coumaric acid are predominantly present in chili pepper extract contributing to the inhibition of food-borne pathogens (Dorantes et al, 2000). A recent study revealed the bacteriocin produced by B. velezensis BUU004 capable of killing food-borne pathogens through membrane pore formation (Butkhot et al., 2019a). The actual mode of mechanisms of the bacteriocins combined with nisin and naturally herb-based substances is not fully understood. Ettayebi et al. (2000) observed the complete mortality of L. monocytogenes and B. subtilis by a combination of sub-inhibitory concentrations of thymol, a major active component of thyme, and nisin Z. They hypothesized that the bacterial death might be due to intracellular metabolite dissipation caused by changes in the cytoplasmic membrane structure and permeability, and the leakage and/or passage of a variety of molecules and ions. Hyldgaard et al. (2012) also pointed out the bactericidal effects of the combined agents thought to be associated with destabilization of the bacterial membrane structure by attacking the cytoplasmic membrane, enhancing the uptake the antimicrobial peptides, changing in the proton motive force, and inhibiting enzymatic systems. Similarly, we observed that the PPS-BV acted synergistically with the mixture of lemongrass and hot pepper extracts against pathogenic E. coli ATCC 25922 and S. Typhimurium TISTR 292 evident from formation of the membrane pores and cell lysis (Soodsawaeng et al., 2021a). An increased antibacterial efficacy of the combined additives observed in this study may not be arisen from only one factor. Therefore, additional future studies are needed to identify the active constituents present in the combined additive and elucidate their mechanisms of the antimicrobial actions. This would be useful for technological applications in food industry. This study may spotlight in the field of study to use the bacteriocins simultaneously with naturally plant-based substances as biopreservative in dried seafood products. Combinatorial strategy of bacteriocins and plant-derived components can broaden antibacterial spectra, enhance quality of processed seafood products, and reduce the potential adverse hazards of synthetic counterparts. It also has financial benefits by reducing the costs of treatment due to an expensive commercial preservative.
The authors declare that there is no conflict of interest.
The authors are grateful for the financial support by the Royal Golden Jubilee Ph.D. Program (RGJ-Ph.D.) of The Thailand Research Fund (TRF) (PHD/0109/2556) and the Research Grant of Burapha University through National Research Council of Thailand (grant no. 65/2558) S. Nimrat. We are also grateful to Biological Science Program, Department of Microbiology, Faculty of Science, Burapha University, Thailand, for providing experimental equipment and facilities.
REFERENCES
|
Bag A, Chattopadhyay RR (2017). Synergistic antibacterial and antibiofilm efficacy of nisin in combination with p-coumaric acid against food-borne bacteria Bacillus cereus and Salmonella typhimurium. Letters in Applied Microbiology 65:366-372.
Crossref
|
|
|
|
Becker K, Heilmann C, Peters G (2014). Coagulase-negative staphylococci. Clinical Microbiology Reviews 27:870-926.
Crossref
|
|
|
|
|
Becker K, von EC (2011). Staphylococcus, Micrococcus, and other catalase-positive cocci. In Versalovic J, Carroll KC, Funke G., Jorgensen JH, Landry ML, Warnock DW (eds.), Manual of Clinical Microbiology (10th ed). ASM Press, Washington DC, USA pp. 692-713.
|
|
|
|
|
Bintsis T (2018). Microbial pollution and food safety. AIMS Microbiology 4(3):377-396.
Crossref
|
|
|
|
|
Burt S (2004). Essential oils: their antibacterial properties and potential applications in Foods: a review. International Journal of Food Microbiology 94(3):223-253.
Crossref
|
|
|
|
|
Butkhot N, Soodsawaeng P, Samutsan S, Chotmongcol K, Vuthiphandchai V, Nimrat S (2019a). New perspectives for surveying and improving Thai dried seafood qualities using antimicrobials produced by Bacillus velezensis BUU004 against foodborne pathogens. ScienceAsia 45:116-126.
Crossref
|
|
|
|
|
Butkhot N, Soodsawaeng P, Vuthiphandchai V, Nimrat S (2019b). Characterisation and biosafety evaluation of a novel bacteriocin produced by Bacillus velezensis BUU004. International Food Research Journal 26(5):1617-1625.
|
|
|
|
|
China Food and Drug Administration [CFDA]. (2015). National food safety standard aquatic products of animal origin. Standardization Administration of China, Beijing, China.
|
|
|
|
|
Dorantes L, Colmenero R, Hernandez H, Mota L, Eugenia-Jaramillo M, Fernandez E, Solano C (2000). Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. International Journal of Food Microbiology 57(1-2):125-128.
Crossref
|
|
|
|
|
Ettayebi K, Yamani JE, Rossi-Hassani BD (2000). Synergistic effects of nisin and thymol on antimicrobial activities in Listeria monocytogenes and Bacillus subtilis. FEMS Microbiology Letters 183(1):191-195.
Crossref
|
|
|
|
|
Field D, Daly K, O'Connor PM, Cotter PD, Hill C, Ross RP (2015). Efficacies of nisin A and nisin V semipurified preparations alone and in combination with plant essential oils for controlling Listeria monocytogenes. Applied and Environmental Microbiology 81(8):2762-2769.
Crossref
|
|
|
|
|
Govaris A, Solomakos N, Pexara A, Chatzopoulou PS (2010). The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. International Journal of Food Microbiology 137(2-3):175-180.
Crossref
|
|
|
|
|
Hyldgaard M, Mygind T, Meyer RL (2012). Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology 3:12.
Crossref
|
|
|
|
|
ISO 4833-1. (2013). Microbiology of the food chain. Horizontal method for the enumeration of microorganisms, part 1: colony count at 30 °C by the pour plate technique. Retrieved October 8, 2019, from
View
|
|
|
|
|
Japan Department of Food Safety (2014). Food sanitation act.
|
|
|
|
|
Masin J, Osicka R, Bumba L, Sebo P, Locht C (2015). Bordetella protein toxins. In Alouf JE, Ladant D, Popoff MR (eds.), The Comprehensive Sourcebook of Bacterial Protein Toxins. Elsevier, Oxford, Oxfordshire, England pp. 161-194.
Crossref
|
|
|
|
|
Molinos AC, Abriouel H, López RL, Omar NB, Valdivia E, Gálvez A (2009). Enhanced bactericidal activity of enterocin AS-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad. Food and Chemical Toxicology 47(9):2216-2223.
Crossref
|
|
|
|
|
Moon HJ, Min KJ, Park NY, Park HJ, Yoon KS (2017). Survival of Staphylococcus aureus in dried fish products as a function of temperature. Food Science and Biotechnology 26(3):823-828.
Crossref
|
|
|
|
|
Nazarizadeh A, Mikaili P, Moloudizargari M, Aghajanshakeri S, Javaherypour S (2013). Therapeutic uses and pharmacological properties of Plantago major L. and its active constituents. Journal of Basic and Applied Scientific Research 3(9):212-221.
|
|
|
|
|
Nimrat S, Butkhot N, Samutsan S, Chotmongcol K, Boonthai T, Vuthiphandchai V (2019). A survey in bacteriological quality of traditional dried seafood products distributed in Chon Buri, Thailand. Science and Technology Asia 24(4):102-114.
|
|
|
|
|
Nimrat S, Vuthiphandchai V, Thongniam P (2010). Contamination of heterotrophic bacteria in dry seafood products distributed in Chon Buri province. Panyapiwat Journal 2(1):70-84.
|
|
|
|
|
Nimrat S, Vuthiphandchai V (2014). Novel antimicrobial agents from bacterial probiotics for controlling bacterial standard of dried and processed seafood products in Chon Buri province. Department of Microbiology, Faculty of Science, Burapha University, Chon Buri, Thailand.
|
|
|
|
|
Omolo MA, Wong ZZ, Mergen AK, Hastings JC, Le NC, Reiland HA, Case KA, Baumler DJ (2014). Antimicrobial properties of chili peppers. Journal of Infectious Diseases and Therapy 2(4):145.
|
|
|
|
|
Osiri S, Osiri P (2009). Seafood safety situation in the East. Thailand Journal of Health Promotion and Environmental Health 32(4):74-86.
|
|
|
|
|
Oussalah M, Caillet S, Saucier L, Lacroix M (2007). Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 18(5):414-420.
Crossref
|
|
|
|
|
Pisuthipan A (2019). Practising seafood safety: how to avoid what's toxic in food pulled from the ocean. Bangkok Post, Life, Social and Lifestyle, Retrieved October 8, 2019, from
View
|
|
|
|
|
Shahbazi Y (2015). Ziziphora clinopodioides essential oil and nisin as potential antimicrobial agents against Escherichia coli O157:H7 in doogh (Iranian yoghurt drink). Journal of Pathogens: article ID 176024.
Crossref
|
|
|
|
|
Shahbazi Y, Shavisi N, Mohebi E (2016). Effects of Ziziphora clinopodioides essential oil and nisin, both separately and in combination, to extend shelf life and control Escherichia coli O157:H7 and Staphylococcus aureus in raw beef patty during refrigerated storage. Journal of Food Safety 36:227-236.
Crossref
|
|
|
|
|
Shokrzadeh M, Ebadi AG (2006). Antibacterial effect of garlic (Allium sativum L.) on Staphylococcus aureus. Pakistan Journal of Biological Sciences 9:1577-1579.
Crossref
|
|
|
|
|
Somboonying S (2002). Microbiological quality examination of flavoured squid products from Nongmon market, Chon Buri province. Bachelor's thesis: Department of Microbiology, Faculty of Science, Burapha University, Chon Buri, Thailand.
|
|
|
|
|
Soodsawaeng P, Butkhot N, Boonthai T, Vuthiphandchai V, Nimrat S (2021a). Synergistic antibacterial effects of bacteriocin produced by Bacillus velezensis BUU004 and medicinal plant extracts against Escherichia coli and Salmonella Typhimurium in dried, crushed and seasoned squid. International Food Research Journal, In-press article.
|
|
|
|
|
Soodsawaeng P, Rattanamangkalanon N, Boonthai T, Vuthiphandchai V, Nimrat S (2021b). Preservative potential of Thai herb extracts combined with bacteriocin from Bacillus velezensis BUU004 for controlling food spoilage and pathogenic bacteria in dried crushed seasoned squids. Science and Technology Asia, In-press article.
|
|
|
|
|
Sooksringam B, Hrimpeng K, Krairak N, Nupasant P, Thungkao S, Siripermpool P, Ua-aungkoon K, Homthong S, Nimrat S, Jongyota W, Suanjit S, Pilantanapak A (2008). Situation of microbial contamination and technique development for pathogen detection in dried seafood towards the centre for detection and inspection of dried seafood. Department of Microbiology, Faculty of Science, Burapha University, Thailand.
|
|
|
|
|
Thailand Department of Fisheries (2011). Microbiology reference criteria for fishery products, April 2011; revision 5. Retrieved June 24, 2017, from
View
|
|
|
|
|
Thungkao S, Muangharm S (2008). Prevalence of Bacillus spp. and Bacillus cereus in dried seasoned squid products. In Proceedings of 46th Kasetsart University Annual Conference: Agro-industry. Kasetsart University, Bangkok, Thailand pp. 138-146.
|
|
|
|
|
Thungkao S, Wong-In K (2007). Microbiological quality of retail ready-to-eat dried seasoned squids from Nongmon market, Chon Buri. In Proceedings of 45th Kasetsart University Annual Conference: Agro-industry. Kasetsart University, Bangkok, Thailand pp. 750-755.
|
|
|
|
|
Turgis M, Vu KD, Dupont, Lacroix M (2012). Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Research International 48:696-702.
Crossref
|
|
|
|
|
UK Health Protection Agency (2017). Guidelines for assessing the microbiological safe of ready-to-eat foods placed on the market. Retrieved October10, 2019, from
View
|
|
|
|
|
US Food and Drug Administration (US FDA) (1998). Bacteriological Analytical Manual, 8th ed. AOAC International, Gaithersburg, Maryland, USA.
|
|
|
|
|
US Food and Drug Administration (US FDA) (2011). Fish and fisheries products hazards and controls guidance.
|
|
|
|
|
Winn W, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, Woods G (2006). Koneman's color atlas and textbook of diagnostic microbiology 6th ed. Lippincott Williams and Wilkins, New York, USA.
|
|
|
|
|
Zhou H, Fang J, Tian Y, Lu XY (2014). Mechanisms of nisin resistance in Gram-positive bacteria. Annals of Microbiology 64(2):413-420.
Crossref
|
|