ABSTRACT
Antimicrobial resistance by bacteria isolates continues to receive attention globally. This investigation looks into the antibiotic susceptibility pattern of Gram negative bacteria isolated from intensive care unit patients in Al-Ahsa, KSA. Bacteria samples were classified based on the CDC criteria for the definition of ICU infections. Gram negative bacteria had been isolated on MacConkey agar using basic bacteriological technique. Identification and antimicrobial susceptibility was carried out using the GN cards of the Vitek 2 compact system. The results showed non-ESBL producing Klebsiella pneumoniae to be the most frequently encountered, isolated from 21% (n=23) of the patients. Other isolates were ESBL producing Escherichia coli (9.47%) and K. pneumoniae (3.77%), E. coli (15.09%), Pseudomonas aeruginosa (10.38%), Proteus mirabilis (9.43%), Acinetobacter baumannii (8.5%), and Carbapenem resistant K. pneumoniae amongst others. Resistance to five antibiotic groups was seen in A. baumannii, Enterobacter, E. coli, K. pneumoniae ESBL K. pneumoniae, non ESBL K. pneumoniae and P. aeruginosa. The association between bacteria resistance to antibiotic groups was statistically significant with a p-value of 0.00001. The encountered isolates showed both multi-drug resistance as well as extensive drug resistance against the tested drug. This information is being provided for Al-Ahsa and would be important for regional surveillance.
Key words: Intensive care unit, Gram negative, patients, multi-drug resistance.
Hospital intensive care units are said to account for less than 10% of total hospital beds in most hospitals. However more than 20% of nosocomial infections are said to be gotten from ICUs (Dror and Keith, 2016). World Health Organisation (Ducel et al., 2013) report defines nosocomial infections as “those infections occurring in hospitalised patients or those in healthcare settings in whom the infection was neither present nor incubating at the time of admission”. With the rise in morbidity and mortality resulting from ICU infections all over the world, attention is being drawn by researchers (Iwuafor et al., 2016; Sugata et al., 2015; Molay et al., 2014) to the
reporting of pathogens associated with these infections. Also, besides the effect of ICU infections on morbidity and mortality, there is the effect on the cost of treatment to be considered (Blot, 2008). A wide range of organisms inclusive of Gram negative and Gram positive bacterial isolates have been associated with these infections (Maazuddin et al., 2014). Encountered pathogens have included both coagulase positive and negative Staphylococci, Proteus mirabilis and Klebsiella pneumoniae (Iwuafor et al., 2016). Other reports listed ICU infectious agents to include Acinetobacter baumannii, Pseudomonas aeruginosa, species of candida amongst other organisms (Kayaaslan et al., 2016; Zaman et al., 2015).
The antimicrobial susceptibility patterns of encountered ICU isolates have also been receiving much attention, particularly in this era of multidrug resistant bacterial superbugs (Theuretzbacher, 2013). According to Maazuddin et al. (2014), nosocomial infections are frequently caused by multidrug resistant (MDR) strains of bacteria such as methicillin resistant Staphylococcus aureus (MRSA), vancomycin resistant S. aureus (VRSA) and vancomycin resistant enterococci (VRE). This view was also supported by Zaman et al. (2015) who reported an increase in resistance by Gram negative bacteria (GNB) to antimicrobials used for their treatment. Also, it is stipulated (Brusselaers et al., 2011) that pathogens associated with ICU infections exhibited higher resistant rates to commonly used antimicrobials when compared to those in community or hospital wards. Antibiotics are generally used in the treatment of bacterial infections. However, the advantages of such usage is being hindered by an increase in the emergence of MDR bacteria strains worldwide (Yezli et al., 2014). In Saudi Arabia, there is a recorded increase in the prevalence of bacteria pathogen that are highly resistant to antimicrobial of choice (Yezli et al., 2014; Memish et al., 2012; Alsultan et al., 2014). High levels of antibiotic resistance by GNB such as A. baumannii and P. aeruginosa isolated from intensive care units have been known to lead to a significant morbidity and mortality (Al-Ahmadey et al., 2013).
There is an urgent need to reduce both morbidity and mortality rates caused by ICU infections. Iwuafor et al. (2016) however postulated that such a reduction would be dependent on the availability of adequate and accurate data for individual local regions. They were of the view that there was an over dependence on available data from different regions which they say is not a reflection of the realities at various local regions. They argued that aetiological resistance pattern differ even in units of the same hospital. Earlier reports (Barai et al., 2010) indicated that the organisms causing infections, along with their susceptibility to antibiotics not only varied from country to country but varied also from one hospital to another and even among ICUs within a hospital. As the world is gripped with thoughts of the possibility of returning to pre-antibiotic era, there is the need for regular surveillance of clinical isolates and their susceptibility to available antibiotics. Regional difference must also be taken into consideration. The present investigation looks into the GNB isolates associated with ICU infections in Al-Ahsa, south-eastern region of Saudi Arabia with a view to providing information of such in this region.
Description of the study setting
The isolates were collected from the laboratories of five hospitals for a period of six months, from January 2016 to June 2016. Four of the hospitals were in Al-Ahsa and one in Al-Khobar. All of the hospitals are located in the South eastern region of Saudi Arabia.
Ethics approval and consent to participate
Ethical approval was not required as the samples were part of the routine in standard care of patients.
Sample collection and criterial for collection
Selection of ICU samples was based on the criteria by Centre for Disease Control and Prevention (CDC) definition for ICU infections. This stipulates that ICU associated infections are those that occur after 48 h of ICU admission or within 48 h after transfer from an ICU (Deep et al., 2004). For exclusion criteria, samples from patients who had stayed in ICU for less than 48 h were not included in the study.
Laboratory samples included endo tracheal aspirates, surgical abdominal site swabs, catheter tips, sputum, diabetic wounds, urine, type 1 necrotising fasciitis, ear swabs and vagina swabs. A total of 93 samples were collected and used for the investigation.
Processing of samples and antimicrobial susceptibility test
Only Gram negative isolates were used for the investigation. They had been isolated and identified in the respective hospital laboratories using basic microbiological and biochemical identification test. Samples were transported to the College of Medicine, Microbiology Laboratory on ice bed and stored at -80°C until required. At the College of Medicine Laboratory, they were cultured using MacConkey agar following basic microbiological techniques for the preliminary identification of isolate. Confirmation of the isolates was by the Vitek 2 compact system (bioMe´rieux) according to the manufacturer’s guidelines using the GN ID and AST Cards.
The antimicrobial susceptibility profile of the isolates was tested against the following antibiotics: Augmentin (AUG), ceftriaxone (CRO), cefotaxime (CTX), ceftazidime (CAZ), Cefepime (FEP), gentamicin (GM), amikacin (AK), Imipenem (IMP), Meropenem (MEM), Ciprofloxacin (CIP), Levofloxacin (LEVO), piperacillin-Tazobactam (TZP), Colistin (CS), Tigecycline (TG), and Sulphamethoxazole/trimethoprim (SXT) using the GN cards of the Vitek 2 compact system.
ESBL producing isolates were detected using the Vitek 2 compact automated system based on the antimicrobial susceptibility pattern. ESBL production confirmation was carried out using the cefepime/cefepime plus clavulanic acid Etest strips (AB Biodisk. Solna, Sweden),following manufacturer’s guidelines.
Multi-drug resistance (MDR) was assessed based on the method of Zhanel et al. (2008) as resistance to 3 or more of the following antibiotic groups; Aminoglycosides, Cepharlosporins, Carbapenems, Fluoroquinolones and Penicillin. While extreme drug resistance (XDR) was classified as defined by Magiorahos et al. (2012), it was not only being resistant to multi antimicrobials but also exhibiting the likelihood of being resistant to all.
Statistical analysis
Data was analysed with excel Microsoft software and GraphPad Prism. One-way ANOVA test was used to compare treatment of ICU isolates to the different antibiotic groups. Statistical significance was taken at p<0.05.
A total of 93 specimens analysed were collected from both male and female patients. Based on gender, 28 (30.12%) were females while 65 (69.89%) were males. The age of patients ranged from 6 months to 98 years old. Table 1 shows the percentage distribution of these ages. Six (6.45%) of the patients were below 20 years, 3 (3.23%) were in the age range of 20 - 30, while 7 (7.53%) were between 31 - 40 years of age. The patient age breakdown continues as follows: 41 - 50 years, 16 (17.20%) patients, 51 - 60 years, 19(20.43%), 61 - 70 years 9 (9.78%), 71 - 80 years had 17 (18.28%) of the patients while those between 81 - 90 years of age were 16(17.2%).
The encountered bacterial isolates and their characteristics
K. pneumoniae were the most frequently ICU bacteria isolates with 23(21%) being non ESBL producing. The ESBL producing K. pnemoniae constituted 3.77% of all the ICU isolates while an equal percentage (3.77%) were carbapenem resistant (CRE). ESBL - producing Escherichia coli made up 9.43% of the total ICU isolates while non ESBL E. coli producers made up 15.09% of the total isolates and the results are presented in Figure 1. Also, encountered ICU bacteria isolates were P. aeruginosa (10.38%), P. mirabilis (9.43%), A. baumannii (8.5%), and Proteus vulgaris (6.6%). Enterobacter constituted 5.66% of the total isolate, while Serratia marcescens and Stenotrophomonas maltophilia made up 2.83 and 0.94% of the total isolates respectively (Figure 1).
Characteristics of patient specimens
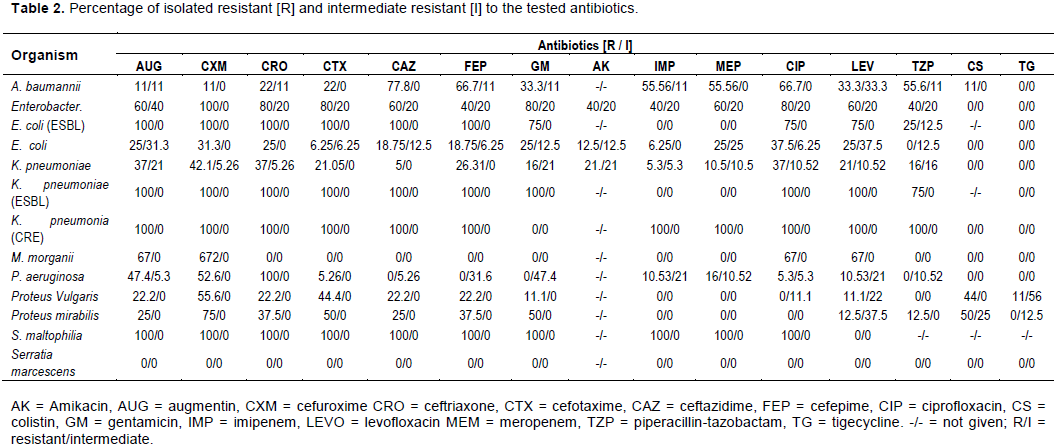
Of the confirmed infections, 15.5% of the patient isolates were from the sputum. Infections associated with urinary tract were 10.7% of the total ICU samples while bloodstream infections were from 11.9% of the patients. The most common infections were from wound swabs constituting 32.1% of patients with diabetic wounds, bed sores as well as post-surgical abdominal swabs. Tissue specimens were from 7.1% of patients with necrotising fasciitis while endotracheal aspirates made up 4.8% of the total ICU samples. Catheter related infection were 2.4% of total isolates, with infections from ear and throat making up 2.4 and 1.2% respectively of the total isolates. The infection sites were not given for 11.5% of the total patient samples, and the results are presented in Figure 2.
Antimicrobial susceptibility of the isolates
The results on the antimicrobial susceptibility of the Gram negative ICU isolates are presented in Table 2. For A. baumannii, resistance was highest with ceftazidime (77.8%), followed by Cefepime and ciprofloxacin with a 66.7% resistance each. Imipenem, meropenem and piperacillin-tazobactam recorded 55.56% each. One (11%) isolate was resistant to Colistin, Augmentin and Cefuroxime. There was a 33.33% resistance against gentamycin and levofloxacin. While being sensitive to tigecycline, intermediates were seen in all the antibiotics with the exception of the following: Cefuroxime, Cefotaxime, Cefepime, Meropenem and Ciprofloxacin. A high antimicrobial resistance was seen among the Enterobacter. All (100%) were resistant to Cefuroxime and 80% resistant to the following; Ceftriaxone, Cefotaxime, Gentamicin and Ciprofloxacin. There was no resistance to Colistin and Tigecycline, while for other antimicrobials, resistance was as follows: 60% for Ceftazidime, Meropenem and Levofloxacin. Also, there were intermediates to all the tested drugs with the exception of cefuroxime.
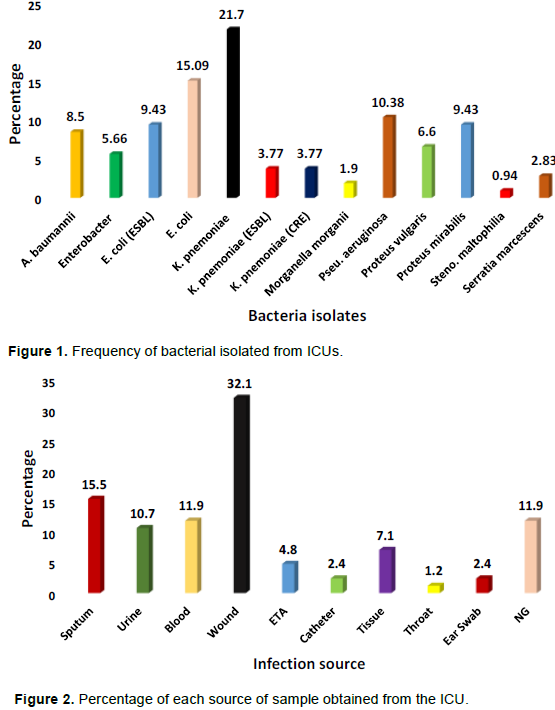
Table 3 also shows that carbapenem resistant K. pneumoniae exhibited 100% resistance against all the tested antibiotics with the exception of Colistin and Tigecycline. A similar resistant pattern is seen with ESBL producing K. pneumoniae. For this isolate, there was 100% resistance against the tested antibiotics with the exception of piperacillin-tazobactam (75%) and sensitive to the carbapenems. For E. coli, there was no resistance against Colistin and Tigecycline while ESBL E. coli showed resistance against the Carbapenems and Tigecycline.
Fifty percent (50%) of the isolated P. mirabilis were resistant to Colistin, with a further 25% being intermediate to this drug. Resistance rates for P. aeruginosa were as follows: Augmentin (47.4%), Cefuroxime (52.6%), Ceftriaxone (100%). This isolate was sensitive to Ceftazidime, Cefepime, and Gentamicin amongst others as shown in Table 2. The only Stenotrophomonas maltophilia isolate was resistant to all the antimicrobials with the exception of Levofloxacin. The Enterobacter were sensitive to Colistin and Tigecycline with a resistance pattern for other antimicrobials as follows: AUG (60%), CXM (100%), CRO (80%), CTX (80%), GM (80%), CIP (80%) and the results are shown in Table 2.
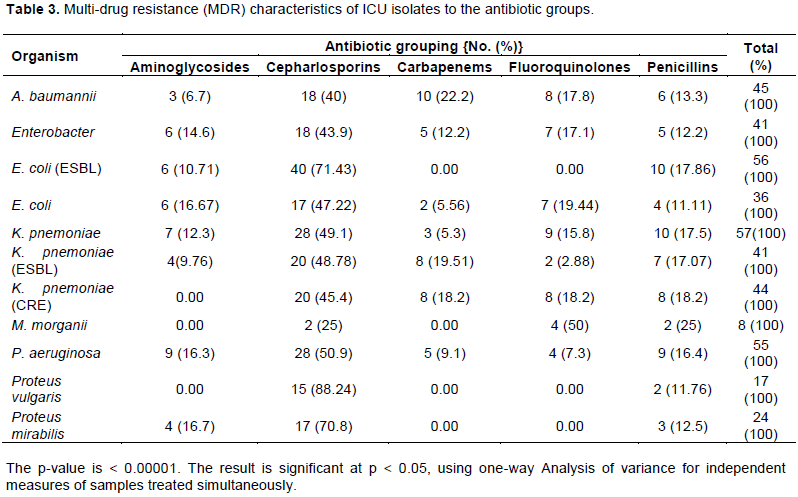
From the results presented in Table 2, resistance to five antibiotic groups is seen in the following isolate: A. baumannii, Enterobacter, E. coli, K. pneumoniae, ESBL K. pneumoniae, and P. aeruginosa. However, K. pneumoniae (CRE) was resistant to four antibiotic groups while Proteus species were each resistant to three groups. The result is statistically significant with a p-value of 0.00001 as shown in Table 3.
The encountered GNB isolates in the present findings as well as their antimicrobial susceptibility further highlights the healthcare problem facing the world in general and the need for both regional and global surveillance on the susceptibility of these bacteria isolates to commonly used, as well as last line antimicrobials. The monitoring of ICU isolates has become the norm in this era of evolving bacteria that are difficult to treat due to the enormous public health problem caused by these bacteria supper bug. No one is spared for this problem as ICUs are visited by all gender and all ages. In this study, 93 Gram negative isolates were from 28 (30.12%) females and 65 (69.89%) males. This simply implies that there were more males than females in ICUs during the period of this study. Zaman et al. (2015), from a study in Jeddah Kingdom of Saudi Arabia (KSA), reported a ratio of 41.14% males to 58.86% females. However, their study comprises both Saudi and non-Saudi nationals while the ICU isolates in the present study were from Saudi nationals only as there were no available data for non-Saudis in this region at the time of the study. Also, similar to the report in the present investigation are those from a previous report (El-Amin and Faidah, 2012) in which there was a higher number of ICU males than females. This therefore indicates that there would be variations in different hospitals, within a country as well as around different regions of the world.
Age-wise, most of the ICU patients in the present study belong to varying age groups with majority of them being between the ages of 51 - 60 years and above. Therefore, age could be a contributing risk factor to ICU infections in this region. Similar findings had earlier been reported in KSA (Zaman et al., 2015; Al-Anazi, 2009). However, there are reports (Kayaaslan et al., 2016) that advanced age was not found to be a risk factor in ICU mortality cases, thus suggesting non-specific pattern in patient age variations. The aetiological agents responsible for the ICU infections as seen in the present investigation appears to represent the general reflection of GNB isolates as had been reported by other researchers such as Kayaaslan et al. (2016) in Turkey, Zaman et al. (2015) in Jeddah, KSA as well as in many regions of the world with slight variations. In this study, K. pneumoniae was the most encountered bacterial pathogen followed by E. coli, P. aeruginosa, ESBL E. coli and P. mirabillis. In Kayaaslan et al. (2016) report, pneumonia was considered to be the frequent ICU infection followed by urinary tract infections without stating the prevalent causative organism. However, Pradhan et al. (2014), while indicating respiratory tract infections to be the commonest encountered in their study, indicated that the most encountered GNB isolate was A. baumannii. Moreso, study by Zaman et al. (2015) that reported E. coli as the most frequently encountered ICU isolate followed by K. pneumoniae, shows that there are variations in results. This could be attributed to a number of reasons which might include the time of study, the place, hospital, country as well as the region of the world. Thus, results could reflect what is obtainable differently for individual countries and hospitals.
As there are differences in aetiological GNB responsible for ICU infections, so it would be expected that their response to treatment may differ. The high level of resistance to antimicrobials shown by the isolates in the present report further highlights the difficulty in treating bacteria supper bugs in the 21st century. While all the isolates were MDR, and based on the definition of extreme drug resistance (XDR) by Magiorakos et al. (2012), the following isolates in the present study were considered to be XDR: K. pneumoniae (ESBL), E. coli (ESBL), K. pneumoniae (CRE), Enterobacter and S. maltophilia. These findings are similar to those of Zaman et al. (2015) who reported ESBL strains to be most resistant to commonly used antibiotics. Also from the same region of the present study, earlier reports (Khanfar et al., 2009) showed high resistance to Ciprofloxacin in ESBL producers while other reports (Al-Ahmadey et al., (2013) found much lower rates in ESBL K. pneumoniae in different regions of KSA. To this effect, earlier reports (Yezli et al., 2014) pointed to the fact that ESBLs showed wide variations from one country to another and also within the same country. Therefore, all the results either in similarities or in differences help in emphasizing the need for a constant and regular monitoring of antibiotic susceptibility of bacteria pathogen in different regions of the world. Such is needed as the indiscriminate use of antibiotics by patients and healthcare givers continue all over the world. It might also be necessary to look into all types of clinical isolates as Zhanel et al. (2008) indicated from their investigations that there was a high resistance to antimicrobials in ICU patients than in patients in other regions of hospital.
The antimicrobial resistance seen in P. aeruginosa in this study is similar to those from previous studies (Zaman et al., 2015; Memish et al., 2012; Al-Ahmadey et al., 2013) in KSA as well as in other regions of the world (Benachinmardi et al., 2014).
A. baumannii is seen to be resistant against the tested antibiotics, with one isolate being resistant to Colistin in the present study. The MDR of ICU, A. baumannii, from KSA has been reported by researchers (Zaman et al., 2015; Memish et al., 2012; Al-Ahmadey et al., 2013). However, contrary to the findings in the present study, the A. baumannii in their study were sensitive to Colistin. There is therefore the possibility of an emerging strain of A. baumannii that is resistant to Colistin. It is obvious that there is an urgent and continuous need for surveillance of bacteria antimicrobial susceptibility pattern all over the world. To this effect, Ramsamy et al. (2016) were of the view that the “knowledge of inherent flora and their antimicrobial susceptibility pattern are crucial.”
The single S. maltophilia isolate encountered in the ICU isolates in the present investigation was resistant against all the tested antimicrobials with the exception of levofloxacin. This bacterium might be of the strain as that encountered in an earlier report (Zaman et al., 2015) in Jeddah. This XDR S. maltophilia might be the strain circulating in ICU units in KSA. There is therefore need to trace the source of this bacterium in ICUs in KSA for possible control measures.
The present investigation shows that bacterial isolates from ICU continue to show high antimicrobial resistance patterns. This calls for concerted effort at stemming the tide of regional surveillance for MDR bacteria in the Kingdom of Saudi Arabia”.
The authors have not declared any conflict of interests.
REFERENCES
|
Al-Ahmadey ZZ, Mohamed SA (2013). Antimicrobial susceptibility pattern of bacterial isolates in the intensive care unit of Al-Ansar Hospital, Saudi Arabia. European Journal of Advanced Research in Biological and Life Sciences (EJARBLS) 1(1):17-27.
|
|
|
|
Al-Anazi AR (2009). Prevalence of methicillin-resistant Staphylococcus aureus in a teaching hospital in Riyadh, Saudi Arabia. Biomedical Research 20(1):7-14.
|
|
|
|
|
Alsultan AA, Elsayed A, Benjamin AE, Sebastian GBA (2014). Clonal diversity of Acinetobacter baumannii from diabetic patients in Saudi Arabian hospitals. Journal of Medical Microbiology 63:1460-1466.
Crossref
|
|
|
|
|
Barai L, Fatema K, Ashraful HJ, Omar FM, Areef Ahsan ASM, Golam MMAH, Hossain Md B (2010). Bacterial profile and their antimicrobial resistance pattern in an intensive care unit of a tertiary care hospital in Dhaka. Ibrahim Medical College Journal 4(2):66-69.
Crossref
|
|
|
|
|
Benachinmardi KK, Padmavathy M, Malini J, Naveneeth BV (2014). Prevalence of non-fermenting Gram-negative bacilli and their in vitro susceptibility pattern at a tertiary care teaching hospital. Journal of the Scientific Society 4(3):162-166.
Crossref
|
|
|
|
|
Blot S (2008). Limiting the attributable mortality of nosocomial infection and multidrug resistance in intensive care units. Clinical Microbiology and Infection 14:5-13.
Crossref
|
|
|
|
|
Brusselaers N, Vogelaers D, Blot S (2011). The rising problem of antimicrobial resistance in the intensive care unit. Annals of Intensive Care 1:47.
Crossref
|
|
|
|
|
Deep A, Ghildiyal R, Kandian S, Shinkre N (2004). Clinical and microbiological profile of nosocomial infections in the pediatric intensive care unit (PICU). Indian Pediatrics 41(12):1238-1246
|
|
|
|
|
Dror M, Keith K (2006). Infections and antimicrobial resistance in the intensive care unit: Epidemiology and prevention.
View
|
|
|
|
|
Ducel G, Fabry J, Nicolle L (2013). A Practical Guide Prevention of hospital acquired infecions. WHO/CDS/CSR/EPH/2002.12.
View
|
|
|
|
|
El Amin NM, Faidah HS (2012). Methicillin-resistant Staphylococcus aureus in the Western region of Saudi Arabia: prevalence and antibiotic susceptibility pattern. Annals of Saudi Medicine 32(5):513-516.
Crossref
|
|
|
|
|
Iwuafor AA, Ogunsola FT, Oladele RO, Oduyebo OO, Desalu I, Egwuatu CC, Nnachi AU, Akujobi CN, Ita IO, Ogban GI (2016). Incidence, clinical outcome and risk factors of intensive care unit infections in the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria. PLoS ONE 11(10):e0165242.
Crossref
|
|
|
|
|
Kayaaslan B, Ayse B, Aliye B, Ahmet S, Halide A, Meltem AY, Esragul AHB (2016). Intensive care unit-acquired infections and association of these infections with mortality: A prospective study in a Turkish Tertiary Care Hospital. Journal Microbiology and Infectious Disease 6(2):53-59.
Crossref
|
|
|
|
|
Khanfar HS, Bindayna KM, Senok AC, Botta GA (2009). Extended spectrum beta-lactamases (ESBL) in Escherichia coli and Klebsiella pneumoniae: trends in the hospital and community settings. Journal of Infection in Developing Countries 3(4):295-299.
|
|
|
|
|
Maazuddin M, Arshad HM, Misba ABM, Azizullah G (2014). Nosocomial infections: An overview. International Research Journal of Pharmacy 5(1):7-12.
|
|
|
|
|
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection 18: 268-281.
Crossref
|
|
|
|
|
Memish ZA, Shibl AM, Kambal AM, Ohaly YA, Ishaq A, Livermore DM (2012). Antimicrobial resistance among non-fermenting Gram-negative bacteria in Saudi Arabia. Journal of Antimicrobial Chemotherapy 67(7):1701-1705.
Crossref
|
|
|
|
|
Molay B, Abhishek A, Sandeep KG, Ashok KM, Abhilasha G (2014). Pattern of pathogens and their sensitivity isolated from nosocomial infections in a tertiary care hospital. International Journal of Current Microbiology and Applied Sciences 3(12):398-403
|
|
|
|
|
Pradhan NP, Bhat SM, Ghadage DP (2014). Nosocomial infections in the medical ICU: a retrospective study highlighting their prevalence, microbiological profile and impact on ICU stay and mortality. Journal of the Association of Physicians of India 62(10):18-21.
|
|
|
|
|
Ramsamy Y, Hardcastle TC, Muckart DJ (2016). Surviving Sepsis in the Intensive Care Unit: The Challenge of Antimicrobial Resistance and the Trauma Patient. World Journal of Surgery 41:1165-1169.
Crossref
|
|
|
|
|
Sugata D, Soumi D, Neeraj SC, Avijit H (2015). Nosocomial infections in the intensive care unit: Incidence, risk factors, outcome and associated pathogens in a public tertiary teaching hospital of Eastern India. Indian Journal of Critical Care Medicine 19(1):14-20.
Crossref
|
|
|
|
|
Theuretzbacher U (2013). Global antibacterial resistance: The never-ending story. Journal of Global Antimicrobial Resistance 1(2):63-69.
Crossref
|
|
|
|
|
Yezli S, Shibl AM, Livermore DM, Memish ZA (2014). Prevalence and antimicrobial resistance among Gram-negative pathogens in Saudi Arabia. Journal of Antimicrobial Chemotherapy 26:257-272.
Crossref
|
|
|
|
|
Zaman RM, Magda MA, Noof RH (2015). Antimicrobial susceptibility pattern of Gram-negative bacilli isolated from a Teaching Hospital in Jeddah, Saudi Arabia. African Journal of Microbiology Research 9(41):2145-2158.
Crossref
|
|
|
|
|
Zhanel GG, DeCorby M, Laing N, Weshnoweski B, Ravi V, et al., (2008). Antimicrobial-Resistant Pathogens in Intensive Care Units in Canada: Results of the Canadian National Intensive Care Unit (CAN-ICU) Study, 2005-2006. Antimicrobial Agents and Chemotherapy 52(4):1430-1437.
Crossref
|
|