ABSTRACT
A novel ‘dilution tube method’ (DTM) which is a modification of the ‘dilution method’ (DM) is hereby described for the determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). This new DTM uses only broth medium in tubes and the required antibiotic. MIC and MBC for Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus were determined in tubes by double diluting (or higher dilutions), broths containing gentamicin concentrations that inhibit bacterial growth, and incubating at 37°C for 18 to 24 h. The tube for MIC showed growth and appeared turbid after incubation while that for MBC remained clear. The results obtained using DTM agrees completely with those obtained with DM. The advantages of this novel DTM include the elimination of extra stress, time and costs associated with preparing and inoculating agar medium as done in DM.
Key words: Antibiotics, minimum inhibitory concentration, minimum bactericidal concentration, dilution tube method, dilution method.
Antibiotics are antimicrobial agents produced by microorganisms that inhibit the growth or kill other microorganisms while being harmless to the host cells. The determination of the susceptibility of pathogens to antibiotics is necessary for the selection of the most appropriate one for treating microbial infections. Antibiotics which kill bacteria are said to be bactericidal, while those that only prevent their multiplication are referred to as bacteriostatic. However, some antibiotics can act as both bacteriostatic and bactericidal depending on their concentration. Antibiotics are evaluated for their inhibitory potentials. A few methods used for evaluating antibiotics include the filter paper disc (Kirby-Bauer) method (Bauer et al., 1966), agar and broth dilution method (Wiegand et al., 2008), and the dilution method (Brown and Young, 1947; Bradshaw, 1979; Owuama, 2015). The dilution method is mainly useful in determining minimum inhibitory concentration (MIC), which is the least concentration of antimicrobial agent that prevents microbial growth, as well as the determination of minimum bactericidal concentration (MBC), which is the least concentration of antimicrobial agent required to kill microorganisms (Andrews, 2001). Microdilution and macrodilution methods have been used in the determination of MIC and MBC (Lambert and Pearson, 2000; Eucast, 2003). Usually, the determination of MIC and MBC by the Dilution Method (DM), involves the inoculation of an indicator bacterium into various concentrations of an antibiotic, incubating for 18 to 24 h and thereafter testing for bacterial viability by sub-culturing on agar media prepared without the antibiotic (Andrews, 2001; CLSI, 1998). Usually, for MIC and MBC determinations, agar medium free of antibiotic is prepared and inoculated with samples from tubes which show no turbidity or growth. Preparation of agar medium involves extra time, additional stress and use of Petri dish. Hence, an improved new method, dilution tube method (DTM) which is easier and cheaper as it does not require agar medium but uses only broth medium in tubes for determining MIC and MBC is hereby described.
Bacterial species, Klebsiella pneumonia, Escherichia coli and Staphylococcus aureus were obtained from Microbiology Department, Modibbo Adama University of Technology, Yola. A modification of the dilution method for the determination of MIC and MBC was used. Briefly, gentamicin was diluted into various concentrations, 5, 6, 7, 7.5, 9.0, 9.5, 9.8, 10 and 15 µg/ml, in sterile nutrient broth in test tubes. Using standard wire loop (Merck), a loopful (10 µl) of E. coli culture, 0.5 McFarland standard (Eucast, 2003), was inoculated into test tubes containing 1 ml of the various concentrations of gentamicin in nutrient broth. Similarly, this was repeated for K. pneumoniae and S. aureus. The tubes were incubated at 37°C for 18 to 24 h and thereafter observed for growth or turbidity. Subsequently, a loopful of broth from each test tube not showing growth, was inoculated into nutrient agar plate. Thereafter, equal volumes of sterile nutrient broth were added into the test tube cultures and incubated further for 24 h at 37°C. Then, the tubes and agar plates were examined for growth or turbidity using unaided eye (CLSI, 2012). A repeat of the DTM with higher dilution (1:10 dilution, that is, 9 vol. of nutrient broth to 1 vol. of broth culture) instead of the double dilution with nutrient broth was done. These experiments were repeated three times.
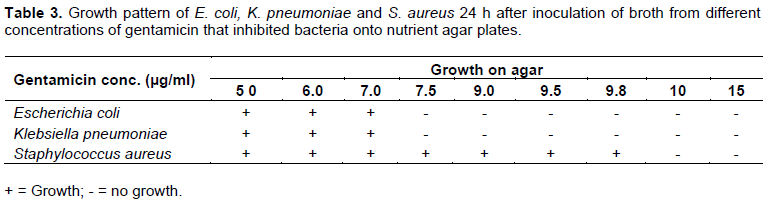
Similar results were obtained using the DM of determining the MIC and MBC (which requires inoculation onto agar plates devoid of antibiotic), and the new DTM described in the work. Tubes containing concentrations of antibiotic that showed no bacterial growth or turbidity after the first 24 h incubation but showed growth (on agar plate) or turbidity (in tube) after the addition of equal volumes of sterile nutrient broth and further 24 h incubation, were said to have minimum inhibitory concentration, while tubes with least antibiotic concentration that showed no growth or turbidity after the first 24 h and still no growth (on agar plate) or turbidity (in tubes) after further 24 h (that is, after the addition of equal volume of sterile nutrient broth), were regarded as minimum bactericidal concentration, MBC. In Table 1, both E. coli and K. pneumonia showed turbidity after incubation in nutrient broth with different gentamicin concentrations at 5 and 6 µg/ml, but not at higher concentrations, while Staphylococcus aureus showed turbidity at 9.5 µg/ml, but not at 9.8 µg/ml or higher gentamicin concentrations.
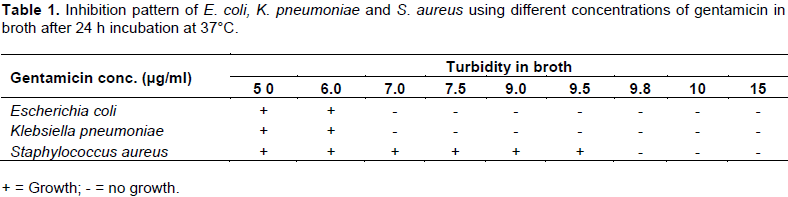
In Table 2, both bacterial species showed turbidity at 5 and 6 µg/ml, but not at 7.0 µg/ml concentrations suggesting that 7.0 µg/ml was the MIC for both E. coli and K. pneumonia, while S. aureus showed turbidity at 9.0 and 9.5 µg/ml, but not at 9.8 µg/ml suggesting that 9.8 µg/ml was the MIC for S. aureus. Inoculation of loopful of samples of the E. coli and K. pneumoniae from 7.0 µg/ml tubes onto nutrient agar without antibiotic, and addition of equal volume of sterile nutrient broth without antibiotic (that is, double dilution) into 7.0 µg/ml tube sample, showed growth in both the tubes and agar plates (after 24 h incubation at 37°C). Thus, confirming that 7.0 µg/ml is the minimum inhibitory concentration, MIC. For S. aureus, inoculation of a loopful of sample from 9.8 µg/ml tube onto nutrient agar without antibiotic, and addition of equal volume of sterile nutrient broth without antibiotic into 9.8 µg/ml tube sample, showed growth in both the tube and agar plate after 24 h incubation at 37°C. Thus, confirming that 9.8 µg/ml was the MIC for S. aureus.
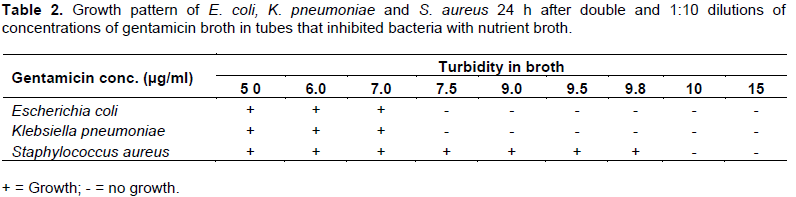
In Table 3, MBC is also the same for both bacterial species, that is, 7.5 µg/ml, using both the DM and the new DTM, as there were no growths in E. coli and K. pneumoniae tubes after 24 h incubation at 37°C, as well as in the tubes after double dilution and inoculation onto agar plates, following further 18 to 24 h incubation. However, for S. aureus, the MBC was 10 µg/ml using both the DM and new DTM, as there were no growths in their tubes after 24 h incubation at 37°C, as well as in the tubes after double dilution and inoculation onto agar plates after further 24 h.
In addition, adding sterile nutrient broth (to make 1:10 dilution) to tubes which were not turbid, that is, showed no growth, revealed similar results with those of double dilution, indicating that dilutions of the tube broth higher than double dilution will give same results.
Application of the DM (Bradshaw, 1979; CLSI, 1998) and the novel DTM yielded similar results for MIC for E. coli and K. pneumoniae (Table 2) and MBC (Table 3) of gentamicin. Observation of same results clearly indicated that both methods are interchangeable in the determination of MIC and MBC. The DM as described by Eucast (2003) and Bradshaw (1979) shared similarity at the first stage with DTM, but differed in the later stages particularly because of the use of agar in DM unlike the new DTM. It is known that the determination of MBC using DM, macrodilution or microdilution requires sub-culturing a sample from wells or tubes, yielding a negative microbial growth after incubation on the surface of non-selective agar plates to determine the number of surviving cells after 24 h of incubation (CLSI, 1998; Eucast 2003). In DTM, it is understandable that the addition of equal volume or higher volume of sterile nutrient broth to the broth in non-turbid tubes, invariably reduced the antibiotic strength below the MIC. Thus, for the gentamicin concentration (7.0 µg/ml) which only inhibited but not kill the bacteria, adding equal volume of broth devoid of antibiotic will doubly dilute and reduce the concentration medium to 3.5 µg/ml which is less than the MIC, and consequently permit the growth of the ‘dormant’ bacteria in the tube. Similarly, for 7.5 µg/ml concentration which killed the bacteria, that is, the MBC, killed bacteria will not grow even after reducing the antibiotic concentration by double dilution or even 1:10 dilution in the tube with sterile nutrient broth, to 3.75 or 0.75 µg/ml, because the bacteria were already dead and will no longer revive. Similarly, the interpretations for MIC and MBC of E. coli and K. pneumoniae explain the MIC and MBC of gentamicin for S. aureus.
In conclusion, the principle underlying the development of DTM is that bacteria made dormant (but not killed) by a particular concentration of antibiotic can be revived if that antibiotic concentration is remarkably reduced by dilution, while bacteria killed by antibiotic at a given concentration cannot be revived no matter the dilution. Interestingly, the new DTM and DM give same results, however, DTM has the advantage that it is less expensive, less time consuming and less stressful as no nutrient agar plates are required. Thus, the new method is recommended for MIC and MBC determination.
The author has not declared any conflict of interests.
REFERENCES
|
Andrews JM (2001). Determination of minimum inhibitory concentrations. Antimicrob. Chemother. 48(6):5-16.
Crossref
|
|
|
|
Bauer AW, Kirby WMM, Sherris JC, Turck M (1966). Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 45:493-496.
|
|
|
|
|
Bradshaw LJ (1979). Laboratory manual. W.B. Saunders Company, London. 3rd edition.
|
|
|
|
|
Brown AM, Young PA (1947). A dilution method for the assay of streptomycin. Microbiology 1:353-360.
Crossref
|
|
|
|
|
CLSI (1998). Methods for Determining Bactericidal Activity of Antimicrobial Agents. Approved Guideline, NCCLS document M26-A. Clinical and Laboratory Standards Institute, 950 West Valley Roadn Suite 2500,Wayne, Pennsylvania 19087, USA.
|
|
|
|
|
CLSI (2012). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed., CLSI document M07-A9. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
|
|
|
|
|
Eucast (2003). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 6(9):509-515.
|
|
|
|
|
Lambert RJ, Pearson J (2000). Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 88(5):784-790.
Crossref
|
|
|
|
|
Owuama CI (2015). Microbiology Laboratory manual. Microtrend Digital Press, Yola. ISBN 978-978-943-328-2.
|
|
|
|
|
Wiegand I, Hilpert K, Hancock RE (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3(2):163-175.
Crossref
|
|