ABSTRACT
This research work was executed to isolate potential Lactobacillus spp. from local raw goat milk samples for their antimicrobial activities against different human intestinal pathogens. Isolation of such bacteria was carried out by Man Rogosa Sharpe (MRS) agar media. Identification of the isolated strain was conducted according to the morphological and biochemical tests. Furthermore, growth optimization and their antimicrobial activity also studied against the pathogenic bacteria (Escherichia coli ATCC 25921, Pseudomonas aeruginosa ATCC 9027, Vibrio cholera ATCC 14035 and Salmonella enteric ATCC 14028). A total of 23 isolates were obtained from the raw goat milk samples, among them four isolates were selected on the basis of their antimicrobial activities against pathogenic bacteria. The Lactobacillus spp. showed better growth in 6.5 to 7.5 pH range and temperature range was 35 to 40°C. Thus it can be said that the goat milk provides a natural dwelling place for Lactobacillus spp., which are beneficial to human health.
Key words: Antimicrobial activity, growth optimization, intestinal pathogens, Lactobacillus spp., Man Rogosa Sharpe (MRS) agar media, pH, raw goat milk.
Lactobacillus is one of the most important genera of lactic acid bacteria (LAB) (Coeuret et al., 2003). They are considered as generally recognized as safe (GRAS) organisms and can be safely used as probiotics for medical and veterinary purposes (Fuller, 1989). Probiotics, as defined in the FAO/WHO (2002) report, are “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. Probiotics are beneficial bacteria in that they favorably alter the intestinal microflora balance, inhibit the growth of harmful bacteria, promote good digestion, boost immune function and increase resistance to infection (Helland et al., 2004). Other physiological benefits of probiotics include removal of carcinogens, lowering of cholesterol, immunostimulating and allergy lowering effect, synthesis and enhancing the bioavailability of nutrients, alleviation of lactose intolerance (Parvez et al., 2006).
Study on lactic acid bacteria specially Lactobacillus spp. are drawing a worldwide interests significantly in worldwide due to its applied beneficiary effects in the prevention, control and treatment of diseases and health maintenance (Osuntoki et al., 2008). Now, Lactobacillus spp. are widely used as probiotics which play a key role in enhancing resistance to colonization by exogenous, potentially, pathogenic organisms in the intestinal tract. They produce a variety of substances e.g., bacteriocin, nisin, lacticin etc. that are effective against different types of enteric pathogens like Escherichia coli, Salmonella spp., Shigella spp., Vibrio spp., Bacillus spp., Klebsiella spp., Staphylococcus spp., Pseudomonas spp., Proteus spp. etc. These effects can be described as the improvement of lactose digestion and the treatment of diarrheal disorders (Abdullah and Osman, 2010).
Lactobacillus spp. is widely distributed in nature and found as indigenous microflora in raw milk (Rodriguez et al., 2000) and fermented milk with spontaneous fermentation. LAB mostly found in milk of human (Martin et al., 2003) and other animals (Fujisawa and Mitsuoka, 1996). The present study was designed to characterize some Lactobacillus spp. from various goat milk samples collected from different regions of Chittagong district to investigate their optimum growth conditions, and some probiotic properties like sensitivity to antibiotics and antagonistic activity to pathogenic bacteria.
Sample collection
Five different raw goat milk samples were collected from different local area of Noakhali zone under the Chittagong division in Bangladesh in sterile bottles and packed in sterile polythene bags. After packing the bags, the milk samples were carried to microbiology laboratory of globe pharmaceuticals LTD, Bangladesh in an ice box contained ice which maintained the temperature at 4°C within four hours, and used for further studies (isolation, identification and antimicrobial activity). The remaining milk samples were stored at 0°C for further use.
Isolation of Lactobacillus spp. from goat milk
Samples were cultured by pour plat method on the Man Rogosa Sharpe (MRS) agar media after serial dilution and incubated at 37°C for 24 to 48 h. The selected strains on the bases of their activity in MRS media were then subcultured onto MRS agar slants which were incubated at 37°C for 24 h and preserved in 20% glycerol (Oxoid, Canada) at -20°C until further used.
Identification of isolated Lactobacillus spp.
The selected isolates were examined for their morphological properties, such as size, shape, cell arrangement and gram-staining properties. Cultural properties including form, colour, elevation, margin, surface of colonies on MRS agar plate and slant were also recorded. Physiological and biochemical characteristics of the isolates were evaluated by Voges-proskauer, methyl red, indole, and citrate utilization (IMViC) catalase and oxidase tests. The ability of the isolates to ferment a number of sugars including glucose, xylose, arabinose, lactose, glycerol, starch, and manitol were also tested. The isolates were identified up to species based on comparative analysis of the observed characteristics with the standard description of bacterial strains in Bergey’s Manual of Determinative Bacteriology.
Optimization of growth parameters
Optimum temperature and pH were determined. Optimum growth temperature was determined by growing the selected isolates in MRS broth and incubating at different temperatures (20 to 45°C) for 24 h. MRS broth of different pH (pH 4.5 to 9) were inoculated and incubated for 24 h to determine the optimum pH. The optimum parameters for highest growth of the identified Lactobacillus spp. were determined by measuring and comparing the optical density (OD) at 600 nm (OD600) (Barua et al., 2015).
Anti-microbial activity of isolated Lactobacillus spp.
Cross-streak method was used to detect the anti-microbial activity of Lactobacillus spp. (Lertcanawanichakul and Sawangnop, 2008). against selected gram negative pathogenic bacteria such as Salmonella spp., Vibrio cholerae, Pseudomonas aeruginosa and Enterotoxigenic E. coli (ETEC). Each pure isolated Lactobacillus spp. culture from previous MRS agar slant was individually streaked in half portion of different MRS agar plates with a smear line. Then, all plates were incubated at 37°C for 3 days. After 2 days incubation period, the isolates secreted anti-metabolites into the medium and then, test pathogens were cross-streaked along the line of fully grown isolates following Cross-streak method. Each streaking was started from near the edge of the plates and was streaked toward the growth line of the isolated Lactobacillus spp. After streaking into the medium, the plates were incubated at 37°C for 24 h. After overnight incubation, a clear zone of inhibition along the growth line of the isolates ware observed which indicated growth inhibition of pathogenic test bacteria due to anti-microbial activity of isolated Lactobacillus spp. The microbial interactions were analyzed by the observation of the size of the inhibition zone (Madigan et al., 1997).
Assay of antibiotic sensitivity pattern
To assess the antibiotic sensitivity pattern, disk diffusion method was followed (Ivanova et al., 2000). Culture inoculums of the isolates grown in MRS broth was taken as amount of 0·5 ml, and was mixed in 5 ml of the same medium containing 0·5% agar, and aseptically poured into glass Petri dishes containing MRS agar medium. The antibiotic disks (Ampicillin, Amoxicilline, Tetracycline, Erythromycin and Gentamicin) were placed on the surface of the plate at equidistance. The plates were then kept at 4°C for 1 h for proper diffusion of antibiotics. The plates were incubated at 37°C for 24 h. The antibiotic sensitivity or resistance was determined by observing the presence of zone of inhibition. The zone of inhibition was measured by a millimeter scale.
Isolation
After primary screening of five goat milk samples by MRS agar media, 23 isolates were found, all the isolates produced small, irregular and round shape with shiny whitish cream or brownish colored which were morphologically similar to Lactobacillus spp. and among these isolets only 4 isolates were selected for further study according to their growth inhibition properties against four references pathogenic microorganisms Salmonella spp., Vibrio cholerae, Pseudomonas aeruginosa and Enterotoxigenic E. coli (ETEC).
Identification
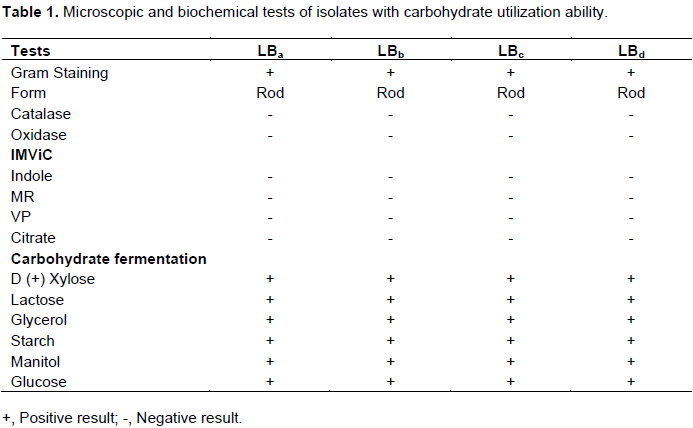
All isolates were examined under microscope to observe their microscopic properties. These isolates were found gram positive, short and medium rod shaped non-spore forming bacteria. Furthermore, some biochemical tests such as catalase test, oxidase test, IMViC tests (Indole test, Methyl Red (MR) test, Voges Proskauer (VP) test, citrate utilization test) and carbohydrate fermentation patterns were performed as depictured by Bergey’s manual systematic bacteriology (Hensyl, 1994). The isolates were found catalase and oxidase negative and in indole, methyl-red, voges proskauar, citrate utilization (IMViC) tests all isolates were also found negative. In this study, all the four isolates were able to ferment 6 different carbohydrates, that is, Xylose, Lactose, Glycerol, Starch, Mannitol and Glucose indicating that they are able to grow in variety of habitats utilizing different type of carbohydrates. The summarized results of all bacteriological and biochemical tests are presented in Table 1.
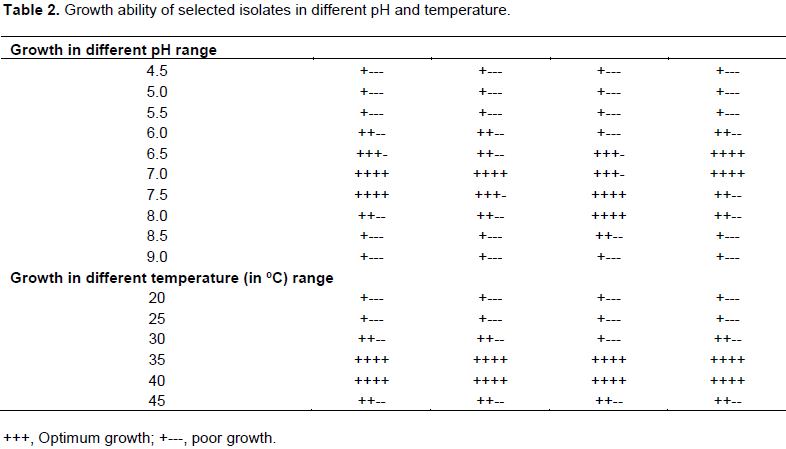
Optimum growth parameters for the selected Lactobacillus spp.
Growth of selected isolates (four isolates) studied on different growth parameters like pH and temperature to reveal their optimum growth parameter. Most of the isolates showed the best growth at neutral pH and the less growth observed when pH increased and decreased. Furthermore, it also found that the best bacterial growing temperature was between 35 to 40°C. Cellular growth was indicated by turbidity after incubated for 24 h on broth media. The results of morphological and physiological tests over four isolates were shown in the Table 2.
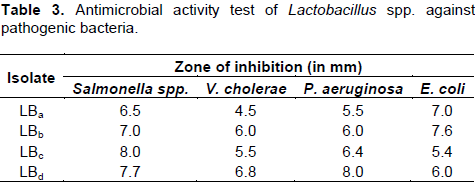
Assay of anti-bacterial activity by cross-streak method
Zone of inhibition produced by the isolates against target pathogens were indicated their antimicrobial properties and identified isolates of Lactobacillus spp. showed that inhibitory properties against Salmonella spp., Vibrio cholerae, Pseudomonas aeruginosa and Enterotoxigenic E. coli (ETEC) (Table 3).
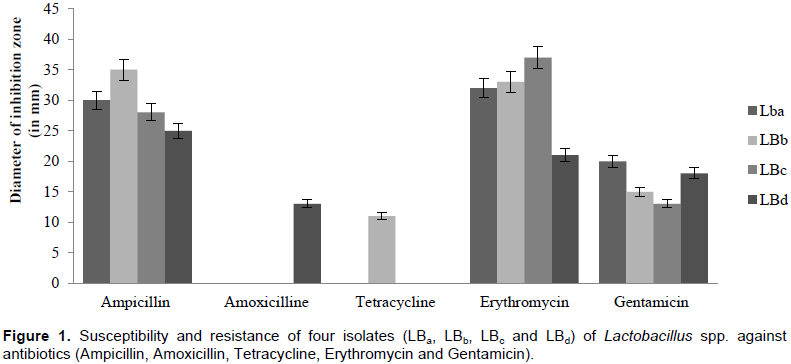
Susceptibility and resistance to antibiotics
Four potential isolates (LBa, LBb, LBc and LBd) were subjected to the antibiotic resistance study, where we use five antibiotics (Ampicillin, Amoxicilline, Tetracycline, Erythromycin and Gentamicin). The result showed that all isolates were susceptible to Erythromycin, Gentamicin and Ampicillin. Whereas most of these isolates were resistant against remaining antibiotics (Figure 1).
Milk and milk products are usually associated with probiotics bacteria, which provide supplements in maintaining beneficial intestinal balance (Tambekar and Bhutada, 2010). This study was designed to isolate and identify Lactobacillus spp. using MRS media and from bacterial morphological and biochemical characteristics. Moreover to determine probiotic activities of the isolates against various pathogens like Salmonella spp., Vibrio cholerae, Pseudomonas aeruginosa and Enterotoxigenic E. coli (ETEC). Man Rogosa Sharpe (MRS) agar media is most commonly used for cultivation of Lactobacillus spp. (El-Moez et al., 2001). This is why, after isolation of 23 Lacobacillus spp. from five raw goat milk samples using MRS media, only four isolates showed better activities against pathogenic bacteria mentioned above. The Lactobacillus spp. can produce some metabolites such as Bacteriocins like Acidophilin, Acidolin, Lactocidin, Bulgarican, Lactolin, Lactobacillin and Lactobrevin which are antagonistic to various degrees against diarrheagenic intestinal pathogens (Tambekar and Bhutada, 2010). Four potential isolates (out of 23) were characterized during the isolation steps, according to their colony morphology (color, size, shape, appearance, gram staining) on MRS media, which showed better antibacterial activity against four reference strains. All the potential isolates showed moderate or strong inhibitory activity and zone of inhibition was measured. But the entire growth inhibition (in ml) was not same. Some isolates showed positive result against all reference strains but some isolates could not.
Selected isolates (LBa, LBb, LBc and LBd) found to be gram positive, short and medium rod shaped non-spore forming bacteria which indicated they are members of Lactobacillus spp. (Thamaraj and Shah, 2003). The isolates were showed catalase and oxidase negative and in IMViC tests all isolates were also found negative, thereby these might confirm the isolates were Lactobacillus spp. (Dhanasekaran et al., 2010). All these results are found relevant to the findings of Chowdhury et al. (2012).
These four isolates were also subjected to examine their optimum growth condition by allowing them to grow in different pH and temperature for 24 h in broth medium. They showed better result in 6.5 to 7.5 pH range and temperature range was 35 to 40°C. Findings of present study showed more or less similarities with the previous study where optimum pH and temperature for the growth of Lactobacillus spp. was neutral (7) and 37ºC respectively (Barua et al., 2015). Antibiotic susceptibility and resistance test also observed of these four bacterial isolates, the result showed that all isolates are susceptible to Erythromycin, Gentamicin and Ampicillin. But most of these strains were resistant against remaining antibiotics. Study of Barua et al. (2015) and Anas et al., (2014) also support the present research.
It is clear that these isolates may produce some metabolites that play role on growth inhibition of pathogens. So, isolated Lactobacillus spp. might have a further research value.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdullah SA, Osman MM (2010). Isolation and identification of lactic acid bacteria from raw cow milk, white cheese and Rob in Sudan. Pak. J. Nutr. 9(12):1203-1206.
Crossref
|
|
|
|
Anas M, Ahmed K, Mebrouk K (2014). Study of the Antimicrobial and Probiotic Effect of Lactobacillus plantarum Isolated from Raw Goat's Milk from the Region of Western Algeria. Int. Res. J. Appl. Basic Sci. 13(1):18-27.
|
|
|
|
Barua R, Al Masud HA, Mahmud MN, Hakim MA (2015). Assessment of Potential Probiotic Lactobacillus Strains Isolated from Goat Milk. IOSR J. Pharm. Biol. Sci. 10(4):9-15.
|
|
|
|
Chowdhury A, Hossain MN, Mostazir NJ, Fakruddin M, Billah MM, Ahmed MM (2012). Screening of Lactobacillus spp. from buffalo yoghurt for probiotic and antibacterial activity. J. Bacteriol. Parasitol. 3(8):156.
Crossref
|
|
|
|
Coeuret V, Dubernet S, Bernardeau M, Gueguen M, Vernoux JP (2003). Isolation, characterisation and identification of lactobacilli focusing mainly on cheeses and other dairy products. Le Lait 83:269-306.
Crossref
|
|
|
|
Dhanasekaran D, Saha S, Thajuddin N, Rajalakshmi M, Panneerselvam A, (2010). Probiotic effect of Lactobacillus isolates against bacterial pathogens in fresh water fish. J. Coast. Dev. 13:103-112.
|
|
|
|
Fujisawa TT, Mitsuoka T (1996) Homofermentative Lactobacillus species predominantly isolated from canine feces. J. Vet. Med. Sci. 58:591-593.
Crossref
|
|
|
|
Fuller R (1989). Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378.
Crossref
|
|
|
|
Helland MH, Wicklund T, Narvhus JA (2004). Growth and metabolism of selected strains of probiotic bacteria in maize porridge with added malted barley. Int. J. Food Microbiol. 91:305-313.
Crossref
|
|
|
|
Hensyl WR (1994). Bergey's Manual of Systematic Bacteriology. 9th Edn. Williams and Wilkins, Baltimore, Philadelphia, Hong Kong, London, Munich.
|
|
|
|
Ivanova I, Kabadjova P, Pantev A, Danova S, Dousset X (2000). Detection, purification and partial characterization of a novel bacteriocin substance produced by Lactococcus lactis subsp. lactis b14 isolated from BozaBulgarian traditional cereal beverage. Biocatalysis 41:47-53.
|
|
|
|
Lertcanawanichakul M, Sawangnop S (2008). A Comparison of Two Methods Used for Measuring the Antagonistic Activity of Bacillus Species. Walailak J. Sci. Technol. 5(2):161-171.
|
|
|
|
Madigan MT, Martiko JM, Parker J (1997). Antibiotics: Isolation and Characterization. In. Madigan MT (ed). Brock Biology of Microorganisms. 8th ed. Prentice-Hall International Inc., New Jersey, Pp. 440-442.
|
|
|
|
Martin R, Langa S, Reviriego C, Jiminez E, Marin ML, Xaus J, Fernandes L, Rodriguez JM (2003). Human milk is a source of Lactic Acid Bacteria for the infant gut. J. Pediatr. 143:754-758.
Crossref
|
|
|
|
Osuntoki AA, Ejide OR, Omonigbehin EA (2008). Antagonistic effects on Enteropathogenic and plasmid analysis of Lactobacilli isolated from fermented Dairy products. Biotechnology 7(2):311-316.
Crossref
|
|
|
|
Parvez S, Malik KA, Ah Kang S, Kim HY (2006). Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 100:1171-1185.
Crossref
|
|
|
|
Rodriguez E, Gonzalez B, Gaya P, Nunez M, Medina M (2000). Diversity of bacteriocins produced by lactic acid bacteria isolated from raw milk. Int. Dairy J. 10:7-15.
Crossref
|
|
|
|
Tambekar DH, Bhutada SA (2010). An evaluation of probiotic potential of Lactobacillus sp. from milk of domestic animals and commercial available probiotic preparations in prevention of enteric bacterial infections. Recent Res. Sci. Technol. 2:82-88.
|
|
|
|
Thamaraj N, Shah NP (2003). Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus and propionibacteria. J. Dairy Res. 86:288-2296.
|