Full Length Research Paper
ABSTRACT
Meningitis is a public health concern. It is caused by several etiologic agents that vary by age group and geographical area. This study aims to highlight the etiological and predictive factors of acute meningitis in hospitalized febrile patients in Mansoura Fever Hospital, Egypt. This study includes cases admitted with suspected meningitis. The study is conducted in the period between April 2019 and March 2020. Lumbar puncture, CSF examination and blood culture and sensitivity were done. Brain Magnetic Resonance Imaging (MRI) was performed before lumbar puncture in some patients. Detailed analysis of epidemiologic characteristics, clinical data, laboratory findings, the causative organisms and predictors of patients with Bacterial Meningitis (BM) were studied. This study included 110 patients had BM with CSF leukocytosis > 100 cells/mm3. Out of 110 CSF samples, 95 cases (86.4%) pathogens were detected by direct Gram-stained smear. Gram positive cocci were the commonest microorganism isolated. 66.4% of patients had blood culture growth of the same organism as the CSF culture. Reagent strip CSF examinations showed a positive correlation compared with laboratory tests. BM had 22.7% mortality rate. Predictive factors of poor outcomes include CSF/serum glucose ratio >0.6, CSF protein >80 mg/dl and Tonsillitis. Prognostic factors that are associated with poor outcome include old age, late presentation, delayed antibiotics treatment, neurologic complications and Glasgo Coma Scale (GCS). BM remains a leading cause of morbidity and mortality,so early diagnosis and treatment decrease both. Predictors of poor outcome of BM are CSF/serum glucose ratio >0.6, CSF protein >80 mg/dl and Tonsillitis.
Key words: Bacterial meningitis, lumbar puncture, cerebrospinal fluid (CSF) analysis.
INTRODUCTION
Acute infectious meningitis is a lethal infection of the central nervous system that leads to 422,900 deaths and 2628,000 disable patients all over the world (Portnoy et al., 2015). Microorganisms reach the meninges either by direct spread from the ears, nasopharynx, cranial injury or congenital meningeal defect, or via bacteremia (WHO, 2017). The annual global prevalence of bacterial meningitis is over 1.2 million patients (Mount et al., 2017). Bacterial menigitis has high mortality of 50% of patients if left untreated (CDC, 2015). Furthermore, patients who were rapidly diagnosed and early treated had a mortality rate ranged from 8 to 15% within 24-48 h of onset of meningitis symptoms (CDC, 2017; Thigpen et al., 2011). In addition 10-20% of survivors may develop neurological squalea that may include permanent brain damage, cognitive dysfunction in the form of reduced processing speed that was found in one third of patients in addition to hearing loss (Rosenstein et al., 2001).
Bacterial meningitis (BM), is a serious disease that is characterized by infection and inflammation of the meninges, spinal cord and may spread to brain parenchyma, resulting in significant morbidity and mortality (Abdelkader et al., 2017). The most common infective bacteria that cause bacterial meningitis include, Neisseria meningitis, Streptococcus pneumonia, Haemophilus influenza, Streptococcus Group B, Staphylococcus aureus, and Listeria monocytogenes (Afifi et al., 2007).
Viral meningitis is generally considered a benign, self-limited disease with low mortality. Certain viruses can meningitis such as varicella zoster virus (VZV), cytomegalovirus (CMV), 6 JC virus, herpes simplex (HSV), enterovirus, Epstein- Barr virus and human herpes virus. Atypical microorganisms were also found in immunocompromised patients at increased risk of bacterial meningitis (Van Veen et al., 2017). Non-communicable diseases that are characterized by inflammation could also lead to meningitis such as malignancy, certain drugs and blood after subarachnoid hemorrhage (Jarvis et al., 2010).
Meningitis is divided clinically into acute and chronic diseases. Acute meningitis develops over hours or days, and is caused by a variety of infectious agents. Chronic meningitis has an onset that may last for weeks to months, but is generally determined when symptoms, signs, and the CSF remain abnormal for at least 4 weeks (Nath, 2016). So several infectious and non-infectious diseases characterized by chronic meningitis also exist (McGill et al., 2016). The typical clinical features of acute meningitis include clinical trial of headache, neck stiffness and fever. Photophobia and vomiting are often present. In acute bacterial infection other constitutional symptoms may be present such as intense malaise, fever, rigors, severe headache, photophobia and vomiting. This develops within hours or minutes. The patient is irritable and often prefers to lie still. Neck stiffness and a positive Kernig's sign usually appear within hours (Sa?glam et al., 2013). The clinical manifestations and CSF examination should determine a presumptive cause of acute meningitis within few hours. Typically, treatment must be initiated rapidly before confirming diagnosis of the actual organism is identified to decrease morbidity and mortality of this serious infection (Amarilyo et al., 2011).
MATERIALS AND METHODS
Bacterial meningitis is still a life-threatening disease that is associated with significant mortality and morbidity. Prognostic factors associated with poor outcomes were old age, neurological complications, Glasgow Coma Scale and late administration of antibiotics so early therapeutic interventions are of utmost importance to save life.
Patients
This cross-sectional study was conducted in Mansoura fever hospital, in the period between April 2019 and March 2020. Informed consent obtained from all patients and study protocol was approved by the ethical committee of Faculty of Medicine, Zagazig University.
Study population
This study included 350 patients admitted to Mansoura fever hospital is specialized 50 bed fever hospital that is located on The Nile River 250 Km east of Cairo, Egypt with suspected meningitis during the period of the study.
Inclusion criteria
Any person with sudden onset of fever and one of the following signs: neck stiffness, altered consciousness, other meningeal signs or purpura fulminans. Any child under 2 years old age with sudden onset of fever and one of the following signs: neck stiffness, or flaccid neck, bulging fontanel, convulsion, other meningeal signs or purpura fulminans.
Exclusion criteria
Cases admitted with suspicious meningitis following head trauma or neurosurgical procedure, or cerebral abscess.
Methods
All patients underwent history taking, clinical examination. Laboratory studies included complete blood count, liver and kidney function tests.
Lumbar puncture
CSF was collected from the subarachnoid space by a sterile spinal needle (25 or 27 G) between the fourth and the fifth lumbar vertebrae. The CSF was collected in 3 sterile, screw-capped tubes. Two ml of CSF was collected in each tube. The first tube was labeled as No 1 (for direct Gram' stain, standard bacteriological culture methods and antibiotics sensitivity testing). The second tube was labeled as No 2 for physical (color, aspect), chemical (glucose level, protein concentration and cytological examination). The third tube was used to test CSF with reagent strips (Sharma et al., 2021).
Blood culture and antibiotic sensitivity test
An aseptic technique was used to collect the blood from patients. The top of the culture bottle was wiped using an ethanol swab, and 10-12 ml of blood was taken (Giuliano et al., 2019).
Testing the CSF with rapid reagent strips
Combur-10 (Roche) reagent strip is a 10-patch test strip that is used to test urine for specific gravity, pH, leukocytes, nitrites, protein, glucose, ketones, urobilinogen, bilirubin, and blood. These test strips were used in this study to measure CSF protein, glucose and leukocytes. A separate CSF sample was used and according to manufacturer instructions, the reagent strip was dipped directly into the tube for approximately one second making sure that all test areas are moistened. When withdrawing the test strip, its edge was wiped against the rim of the vessel to remove excess fluid. After 60 s (60 - 120 s for the leukocyte test area) the color change was red against the standards provided (Moosa et al., 1995).
CSF analysis
The specimen number one was centrifuged at 2000-3000 rpm for 20 min. The supernatant aspirated with a sterile pipette, leaving approximately 0.5 ml of fluid in the specimen tube (supernatant can be reserved for biochemical studies), the sediment shaken to re-suspend.
Physical characteristics of CSF: Color and aspect.
Chemical analysis of CSF: Glucose: negative < 25, 1+ 25 - 75, 2+ 76 - 200, 3+ 201 - 650 and 4+ > 650 mg/dL, Protein: negative < 15, 1+ (15 - 65, 2+ 66 - 300, and 3+ >300 mg/dL and Lactate.
Cytological examination: The presence of leukocytes was graded: negative < 10, 1+ 10 - 50, 2+ 51 - 290, and 3+ > 291 cells/µl (Hrishi and Sethuraman, 2019).
Microbiological examination: Direct smears stained by Gram were done according to microbiological standards. Microscopical examination of Gram-stained smears was performed. Ziehl Neelsen (ZN) staining was done when indicated clinically and according to microbiological standards (Gray, 1992).
Culture of CSF: Inoculation was done on blood, chocolate and Mac-Conkey's agar plates and incubated aerobically and anaerobically in presence of 5-10% CO2 at 37°C for 72 h. Identification of bacterial colony by colony morphology and biochemical reactions e.g: Coagulase test, Catalase test, Optochin test and bile solubility test, Indole production test and Oxidase test. Analytical Profile Index (API) 20 strep (BioMerieux, Germany) and (API) 20E (Bio-Merieux, France): an identification system using standardized miniaturized biochemical tests, used for biotyping and delineation of different species (Leazer et al., 2017).
Antibiotics sensitivity testing: Disk diffusion Kirby-Bauer method was used to determine the susceptibility of the recovered clinical isolates to antimicrobial agents. The diameters of inhibition zones were measured in mm using a ruler and classified as either susceptible, intermediate, or resistant to the agents that were tested (Assegu et al., 2020).
Brain MRI
MRI may be performed before the lumbar puncture in patients with neurological deficit, seizure, Glasgow score < 11.
According to the results of the above studies and discharge diagnosis, patients were categorized into three groups
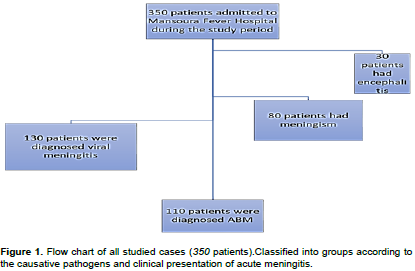
Meningitis patients, 240 patients: presented with fever, meningeal symptoms and altered mental status, and with an abnormal number of white blood cells in CSF.
Encephalitis patients 30 patients: presented with acute onset of fever and a change in mental status and/or new onset of seizures, and a clear CSF, leukocyte count < 80/mm3, all were lymphocytes or without CSF pleocytosis with no identification of bacteria by CSF culture or gram stain, and after exclusion of other causes of decreased mentation.
Meningism patients are 80 patients: presented with signs of meningeal irritation but CSF examination was normal and the subsequent investigations and evolution of the disease revealed the true diagnosis (Dian et al., 2020).
Confirmed meningitis patients were classified into two groups
Group I (bacterial meningitis): 110 patients with a positive Gram stain and/or CSF culture or positive blood culture with concurrent meningitis; or detection in the CSF of >100 white blood cells per ml.
Group II (aseptic meningitis): This group included 130 patients with CSF pleocytosis (≤100 WBCs), negative Gram stain; and the CSF and blood cultures were negative for bacterial meningitis.
Inpatient follow-up and outcome assessment
Patients were evaluated daily for symptom improvement or occurrence of new symptoms. Vital signs were assessed every four hours for the first 48 h and based on the need thereafter. Daily follow-up with a neurosign chart that included the following variables: Glasgow coma scale (GCS), seizures, headache, and nuchal rigidity was done during the inpatient treatment.
Patients were also assessed at discharge for gross neurologic deficits (visual problems, hearing deficits, and body weakness) and mini-mental state examination. Detailed analysis of epidemiologic characteristics, clinical data and laboratory findings, the causative organisms, prognosis of all the patients with BM were studied.
Statistical analysis
Data was collected and tabulated. IBM’s SPSS statistics (Statistical Package for the Social Sciences) for windows, version 25, 2017 was used for statistical analysis of the collected data. Shapiro-Wilk test was used to check the normality of the data distribution. All tests were conducted with 95% confidence interval. P (probability) value < 0.05 was considered statistically significant. Charts were generated using SPSS’ chart builder and Microsoft Excel for windows 2019.
Quantitative variables were expressed as mean and standard deviation, median, inter-quartile range, minimum and maximum values as appropriate while categorical variables were expressed as frequency and percentage. Cohen's kappa (κ) was run to measure reliability and agreement between the studied diagnostic methods and BM diagnosis.
RESULTS
This study included 110 patients with confirmed diagnosis bacterial meningitis (BM). BM occurred in males more than females. It affected patients from rural much more commonly than patients from urban communities (Figure 1).
The clinical presentation of BM was characterized mainly by fever (90.9%) and headache (88%). Signs of meningeal irritation that is, neck rigidity (90.9%), Kernig sign (72.7), and Brudziniski sign (69%) are significantly higher in patients with meningitis. Once neck rigidity, headache, or both are present with fever meningitis is highly suspected.
Antecedent illnesses (that is, diseases diagnosed at the time or shortly before the diagnosis of meningitis) were found in 74.5% of patients with BM. Pneumonia was the most common predisposing condition. A large percentage of meningitis patients, 33.6% reported a positive history of antibiotic intake in the few days before admission to the hospital.
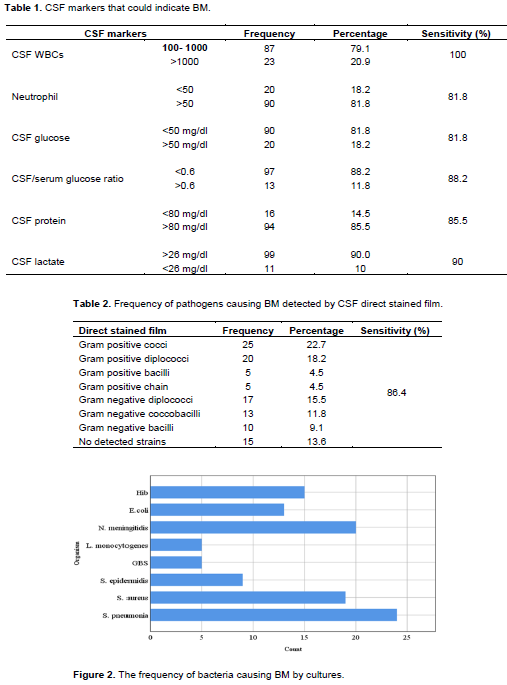
The study shows that 79.1% of BM patients had a CSF leukocyte count in the range of > 100 up to 1,000 cell/mm3 and 80% of BM patients had CSF neutrophil percentage > 50%. 85.5% of BM patients had elevated CSF protein (> 80mg/dl). While 81.8% of BM patients had decreased CSF glucose < 50 mg/dl. It was found that 90% of BM patients had elevated CSF lactate> 26 mg/dl.
Out of 110 CSF samples, 95 cases, 86.4% of pathogens were detected by direct Gram-stained smear. Gram positive bacteria were detected in 55/110, 50% of cases. Gram negative bacteria were detected in 40/110 (36.4%) of cases. Gram positive cocci was the commonest 25/110 microorganism isolated in the present study. S. pneumonia was the most common isolated pathogen accounting for 24% of BM (Table 1).
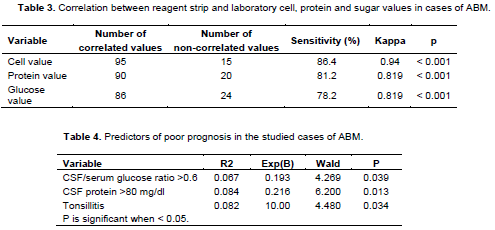
Table 2 shows rapid reagent strip and CSF examination showed a positive correlation with laboratory test results that include, laboratory cell, protein and sugar values in cases of BM. This study shows a sufficient degree of agreement between CSF and blood culture results. Out of 110 patient 73 (66.4%) had blood culture growth of the same organism as the CSF culture. Furthermore, 23(20.9%) of patients who had positive CSF cultures and negative blood cultures.
BM carried a high mortality rate (22.7%). These include: One infant 6 m, one female patient 40 years old, one girl 16 years old, and all other cases were above 55 years old. Complications were found in 21.8% of BM patients who developed permanent sequelae such as hearing deficits, paralysis, cognitive slowness and memory trouble. Predictors of poor outcome of BM are CSF/serum glucose ratio >0.6, CSF protein >80 mg/dl and Tonsillitis (Figure 2 and Tables 3 and 4).
DISCUSSION
In this study, 350 patients admitted to Mansoura Fever, Egypt with clinical features suggestive of meningitis. They underwent lumbar puncture, BM was diagnosed in 110 patients (31.43%), viral meningitis in 130 (37.14%), meningism in 80 (22.86%) cases and encephalitis in 30 (8.57%). The ratio of bacterial to aseptic meningitis cases vary between different studies. Abdelkader et al. (2017), found that the majority of meningitis cases over the two years period were possibly viral meningitis (42.86%) and bacterial meningitis cases with positive culture growth were (5.08%). However, in the study conducted by Amarilyo et al. (2011), bacterial meningitis was diagnosed in 10.3% of meningitis patients and 89.7% were found aseptic. This difference in the percentage of bacterial versus aseptic meningitis can be attributed to differences in the place and time of studies done. In developed countries, bacterial meningitis became less common in relation to viral meningitis especially after the implementation of anti-capsular vaccines.
In our study males were affected (61.8%) more commonly than females (38%). Our results are in agreement with the study of Abdkader et al. (2017), males were 44 (64.7%), compared to 24 who were females (35.3%). Our data showed that BM occurred more in rural than urban areas. This is in agreement with the study done by Afify et al. (2007) who demonstrated that 74.5% of BM patients had antecedent illnesses. Pneumonia was the most common predisposing condition in (33.6%) of patients. This is in agreement with the study of Brouwer et al. (2012), who showed that ear, sinus, or lung infections precede pneumococcal meningitis in 40% of patients.
Clinical features give clue to the early diagnosis of BM by a physician. In the current study the classic clinical features of bacterial meningitis include fever (90.9%), headache (88%), stiff neck (90.9%). Similar rates were reported by several investigators (Afifi et al., 2007; Arda et al., 2008). Kernig and Brudziniski signs were present in this study among (72.7 and 69% for each sign respectively. Another study on adults reported sensitivity values for Kernig's sign (36%) and Brudziniski's sign, 39% (Elmore et al., 1996). Furthermore, in a considerable number of meningitis patients, the above clinical features were absent (Heydarian et al., 2014; Dyckhoff-Shen et al., 2021). Prognostic factors that were associated with poor outcomes include old age≥ 65 years, neurological complications and late administration of antibiotics for patients with bacterial meningitis. From the above discussion it became evident that none of the symptoms and signs could accurately discriminate between patients with meningitis from those without it. Patients with suspected meningitis on clinical grounds should be referred for lumbar puncture and CSF examination as gold standard diagnostic tests (Brouwer and van de Beek, 2015).
In this study, 110 patients who showed clinical features of meningitis, 20.9% of these patients had a CSF leukocyte count >1,000 cells/mm3, and, 79% had a leukocyte count > 100 - 1,000 /mm3 with 100% sensitivity. Also, this study demonstrated a neutrophil dominance of CSF leukocyte count > 50% in 81.8% of bacterial meningitis patients with sensitivity of 81.8%. These results were documented in other studies (Martinot et al., 2018). This large number of patients with CSF leukocyte count less than 1,000/mm3 79% may be a reflection of the high rates of antibiotic use before hospital admission that reached 60% resulting in partially treated meningitis.
This study showed that 81.8% of patients with BM had decreased CSF glucose concentration (glucose concentration < 50 mg/dl). Also, there was a significant increase of CSF protein in bacterial meningitis patients. Most studies reported the same findings (Martinot et al., 2018). The CSF-blood barrier disruption causes a high CSF protein level in patient with meningitis (Julián-Jiménez and Morales-Casado, 2019).
Studies in adults have indicated that adding CSF lactate to routine CSF examination is better for the diagnosis of BM in a very short time (Alfred et al., 2021). The mechanism of increased lactate concentration in the CSF of patients with BM meningitis is not clear, but it may be due to increased anaerobic glycolysis of brain tissue due to a decrease in cerebral blood flow and oxygen uptake (Shamliyan, 2018; Xiao et al., 2016). In the present study, 99 (90%) patients with BM had elevated CSF lactate level, and 11(10%) had normal lactate with sensitivity of 90%. Many other studies showed the same result (Nazir et al., 2018; Shamliyan, 2018; Viallon et al., 2011). A meta-analytic study that included 404 neurosurgical patients revealed that SCF lactate levels were highly sensitive and specific for diagnosing bacterial meningitis (Houri et al., 2017).
In the current study, out of 110 specimens; using Gram stain 55 (50%) were found to be Gram positive and 40 (36%) were found to be Gram negative. From 55 Gram positive isolate, 24 were S. pneumococca. 19 Staphylococcus aureus, 9 S. epidermidis, 5 GBS, and 5 L. monocytogenes. Different isolates were found, of 40 Gram negative isolates, 20 were N. meningitides., 15 Hib, 13 E. coli. Comparing our results with Abdelkader et al. (2017), 48 isolates (67.6%) were found to be Gram-positive and 23 isolates (32.4%) were Gram-negative. Among 48 Gram positive isolates the majority were S. pneumonia.
Another study by Afifi et al. (2007) that was also conducted in Egypt found that S. pneumoniae was the leading cause of bacterial meningitis. This reflects a change in disease epidemiology since N. meningitides was for a long time the most common pathogen causing bacterial meningitis. Other several studies have reported high prevalence of S. pneumonia around the world (Jones et al., 2004; Owusu et al., 2012; Nasiri et al., 2019). Meningococcus was considered as the second common cause of BM in this study. This is in agreement with study of Kerstin et al. (2018) and WHO report (2017).
Urine reagent strips that measure glucose, protein, leukocytes and other urinary biomarkers have been used to evaluate CSF in several previous studies. In this study, the number of regent strips results coincide with the laboratory findings in 95 out of 110 patients (with sensitivity 86.4%) for leukocytes, 90 out of 110 (with sensitivity 81.2%) for protein, 86 out of 110 (with sensitivity 78.2%) for glucose. A higher rate of agreement between regent strips and laboratory results was also reported by Mazumder et al. (2018), Kumar et al. (2015) and Phillips et al. (2019). Another study found that increased CSF protein was increased in study group as compared to control group while cell count was significantly insensitive but its specificity was 63.79%. Sa?glam et al. (2013).
Since bacterial meningitis can occur secondary to underlying bacteremia from other sources, so routine ordering of blood cultures in suspected casesis crucial for accurate diagnosis (McGill et al., 2016). In this study, blood cultures were done in all cases (110), it was reported that 73 (66%) were positive by the same bacteria present in CSF culture while 14 (12.7%) were positive by different isolates. However, 23 (20.9%) of blood cultures were negative. This is in agreement with results of Troendle (2019), demonstrated that blood cultures detect causative organism in 71% of cases. Blood cultures helped to identify the causative organism in 50-80% of pediatric and adult cases. The yield of blood cultures decreases by 20% if the patient has been pretreated by ntibiotics (Troendle and Pettigrew, 2019).
Bacterial meningitis is still a life-threatening disease that is associated with significant mortality and morbidity. In the current study the reported mortality rate of BM was 22.7% and about 21.8 % of BM patients developed permanent sequelae such as hearing deficit, paralysis, cognitive slowness in the form of delayed processing speed and memory troubles (Liu et al., 2012, Olbrich et al., 2018). This is in agreement with results of Troendle et al. (2019). The mortality rate was 31%. Without any treatment, the case-fatality rate can reach 70%, and one in five survivors of bacterial meningitis may be left with permanent disability including hearing loss, neurologic disability, and cognitive dysfunction (Gudina et al., 2018; Tsai et al., 2019).
CONCLUSIONS
The clinical symptoms and signs were inadequate to make a definite diagnosis of bacterial meningitis. Lumbar puncture and CSF examination is the gold standard method for diagnosing bacterial meningitis and is necessary to be done as soon as possible.
Reagent strip can be used reliably in CSF examination where laboratory facilities are limited or even routinely done in all hospitals for early diagnosis of bacterial meningitis.
The frequencies of the most common infectious agents causing BM in this study are S. pneumonia, N. meningitidis based on bacterial culture. Predictors of poor outcome of BM are CSF/serum glucose ratio >0.6, CSF protein >80 mg/dl and Tonsillitis respectively.
The use of traditional methods for diagnosing bacterial meningitis is time-consuming and has low sensitivity. So, the search for novel accurate and rapid molecular methods is necessary. The combination of both metagenomic Next Generation Sequencing mNGS and Whole Exome Sequencing WES may help to increase precision of diagnosis of bacterial meningitis particularly in neonates for guiding rapid effective therapeutic interventions. Progress in the field of predictive and personalized medicine is also promising for improving the predictive power and accuracy for personalized antiobiotics treatment of BM.
Study strength and limitations:
Several studies were carried out internationally on the etiological and prognostic factors of bacterial meningitis yet, there are few studies conducted in Egypt. Up to the best of our knowledge, this study is one of few important studies that is carried out on the occurrence of bacterial meningitis in our hospitalized patients. Therefore, the strength of the current research is the study of the potential etiological and prognostic factors in those patients. Our study has some limitations that need to be acknowledged. The sample of patients was only conducted from a single center (Mansoura fever hospital) at a single time point. A small study sample reduces the power of event-free survival analysis, and the results obtained thus cannot be generalized globally to all Egyptian patients. These data can only be used to generate hypotheses that can be used in the future for a confirmatory study, that is, a mega randomized clinical trial. Since this is an observational study, many different techniques that can be applied to prevent or control for confounding could not be used. Moreover, the study did not consider the socioeconomic variables that significantly influence bacterial meningitis.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abdelkader MM, Aboshanab KM, El-Ashry MA, Aboulwafa MM (2017). Prevalence of MDR pathogens of bacterial meningitis in Egypt and new synergistic antibiotic combinations. Plos One 12(2):e0171349. |
|
|
Afifi S, Wasfy MO, Azab MA, Youssef FG, Pimentel G, Graham TW (2007). Laboratory-based surveillance of patients with bacterial meningitis in Egypt (1998-2004). European Journal of Clinical Microbiology and Infectious Diseases 26(5):331-340. |
|
|
Amarilyo G, Alper A, Ben-Tov A, Grisaru-Soen G (2011). Diagnostic Accuracy of Clinical Symptoms and Signs in Children With Meningitis. Pediatric Emergency Care 27(3):196-199. |
|
|
Arda B, Sipahi OR, Atalay S, Ulusoy S (2008). Pooled Analysis of 2,408 Cases of Acute Adult Purulent Meningitis from Turkey. Medical Principles and Practice 17(1):76-79. |
|
|
Assegu FD, Lemma K, Tadele H, Tadesse BT, Derese B (2020). Antimicrobial sensitivity profile and bacterial isolates among suspected pyogenic meningitis patients attending at Hawassa University Hospital: Cross-sectional study. BMC Microbiology 20:1-10. |
|
|
Brouwer MC, Thwaites GE, Tunkel AR, van de Beek D (2012). Dilemmas in the diagnosis of acute community-acquired bacterial meningitis. The Lancet 380(9854):1684-1692. |
|
|
Brouwer MC, van de Beek D (2015). Earlier Treatment and Improved Outcome in Adult Bacterial Meningitis Following Guideline Revision Promoting Prompt Lumbar Puncture. Clinical Infectious Diseases 61(4):664-665. |
|
|
Centers for Disease Control (CDC) (2017). Bacterial Meningitis. Centers for Disease Control and Prevention. |
|
|
Centers for Disease Control (CDC) (2015). Meningococcal disease. Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th ed. Atlanta, GA: CDC pp. 231-46.32. |
|
|
Dian S, Hermawan R, van Laarhoven A, Immaculata S, Achmad TH, Ruslami R, van Crevel R (2020). Brain MRI findings in relation to clinical characteristics and outcome of tuberculous meningitis. PLoS One 15(11):e0241974. |
|
|
Dyckhoff-Shen S, Koedel U, Pfister HW, Klein M (2021). SOP: emergency workup in patients with suspected acute bacterial meningitis. Neurological Research and Practice 3(1):1-7. |
|
|
Elmore JG, Horwitz RI, Quagliarello VJ (1996). Acute meningitis with a negative gram's stain: Clinical and management outcomes in 171 episodes. The American Journal of Medicine 100(1):78-84. |
|
|
Giuliano C, Patel CR, Kale-Pradhan PB (2019). A Guide to Bacterial Culture Identification And Results Interpretation. Pharmacy and Therapeutics 44(4):192-200. |
|
|
Gray LD (1992). FEKORKO DP-Laboratory diagnosis of bacterial meningitis. Clinical Microbiology Reviews 5:130-145. |
|
|
Gudina EK, Tesfaye M, Wieser A, Pfister HW, Klein M (2018). Outcome of patients with acute bacterial meningitis in a teaching hospital in Ethiopia: A prospective study. Plos One 13(7):e0200067. |
|
|
Heydarian F, Ashrafzadeh F, Rostazadeh A (2014). Predicting factors and prevalence of meningitis in patients with first seizure and fever aged 6 to 18 months. Neurosciences 19(4):297-300. |
|
|
Houri H, Pormohammad A, Riahi SM, Nasiri MJ, Fallah F, Dabiri H, Pouriran R (2017). Acute bacterial meningitis in Iran: systematic review and meta-analysis. PLoS One 12(2):e0169617. |
|
|
Hrishi AP, Sethuraman M (2019). Cerebrospinal fluid (CSF) analysis and interpretation in neurocritical care for acute neurological conditions. Indian Journal of Critical Care Medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine 23(Suppl 2):S115. |
|
|
Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS (2010). Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infectious Diseases 10(1):67. |
|
|
Jones ME, Draghi DC, Karlowsky JA, Sahm DF, Bradley JS (2004). Prevalence of antimicrobial resistance in bacteria isolated from central nervous system specimens as reported by U.S. hospital laboratories from 2000 to 2002. Annals of Clinical Microbiology and Antimicrobials 3(1):3-10. |
|
|
Julián-Jiménez A, Morales-Casado MI (2019). Usefulness of blood and cerebrospinal fluid laboratory testing to predict bacterial meningitis in the emergency department. Neurología (English Edition) 34(2):105-113. |
|
|
Kumar A, Debata PK, Ranjan A, Gaind R (2015). The Role and Reliability of Rapid Bedside Diagnostic Test in Early Diagnosis and Treatment of Bacterial Meningitis. The Indian Journal of Pediatrics 82(4):311-314. |
|
|
Leazer R, Erickson N, Paulson J, Zipkin R, Stemmle M, Schroeder AR, Bendel-Stenzel M, Fine BR (2017). Epidemiology of Cerebrospinal Fluid Cultures and Time to Detection in Term Infants. Pediatrics. 139(5):e20163268. |
|
|
Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Child Health Epidemiology Reference Group of WHO and UNICEF (2012). Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The lancet 379(9832):2151-2161. |
|
|
Martinot M, Greigert V, Souply L, Rosolen B, De Briel D, Mohseni Zadeh M (2018). Cerebrospinal fluid monocytes in bacterial meningitis, viral meningitis, and neuroborreliosis. Médecine et Maladies Infectieuses 48(4):286-290. |
|
|
Mazumder S, Ramya BS, Biligi D (2018). Utility of urine reagent strips in cerebrospinal fluid analysis: An aid to bedside diagnosis of meningitis. Indian Journal of Pathology and Microbiology 61(3):356. |
|
|
McGill F, Heyderman RS, Panagiotou S, Tunkel AR, Solomon T (2016). Acute bacterial meningitis in adults. The Lancet 388(10063):3036-3047. |
|
|
Moosa AA, Quortum HA, Ibrahim MD (1995). Rapid diagnosis of bacterial meningitis with reagent strips. Lancet 345(8960):1290-1291. |
|
|
Nasiri M, Tabatabaei S, Shamshiri A, Weinberger D, Dadashi M, Karimi A (2019). Pneumococcal meningitis in Iran: a systematic review and meta-analysis. Journal of Acute Disease 8(3):99. |
|
|
Nath A (2016). Meningitis: bacterial, viral, and other. In: Goldman L, editor. Goldman-Cecil Medicine, 25th ed. Philadelphia, PA: Saunders pp. 2480-2495. |
|
|
Nazir M, Wani WA, Malik MA, Mir MR, Ashraf Y, Kawoosa K, Ali SW (2018). Cerebrospinal fluid lactate: a differential biomarker for bacterial and viral meningitis in children. Jornal de Pediatria (Versão em Português) 94(1):88-92. |
|
|
Olbrich KJ, Müller D, Schumacher S, Beck E, Meszaros K, Koerber F (2018). Systematic Review of Invasive Meningococcal Disease: Sequelae and Quality of Life Impact on Patients and Their Caregivers. Infectious Diseases and Therapy 7(4):421-438. |
|
|
Owusu M, Nguah SB, Boaitey YA, Badu-Boateng E, Abubakr AR, Lartey RA, Adu-Sarkodie Y (2012). Aetiological agents of cerebrospinal meningitis: a retrospective study from a teaching hospital in Ghana. Annals of Clinical Microbiology and Antimicrobials 11(1):1-8. |
|
|
Phillips RJ, Watanabe KM, Stowell JR, Akhter M (2019). Concordance between blood and cerebrospinal fluid cultures in meningitis. The American Journal of Emergency Medicine 37(10):1960-1962. |
|
|
Portnoy A, Jit M, Lauer J, Blommaert A, Ozawa S, Stack M, Murray J, Hutubessy R (2015). Estimating costs of care for meningitis infections in low- and middle-income countries. Vaccine 33(Suppl 1):A240-A247. |
|
|
Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM (2001). Meningococcal disease. The New England Journal of Medicine 344:1378-1388. |
|
|
Sa?glam M, Zer Y, Balci?I (2013). Causative agents of bacterial meningitis. African Journal of Microbiology Research 7(20):2221-2227. |
|
|
Shamliyan TA (2018). Evidence Review: Diagnostic Accuracy of Cerebrospinal Fluid Lactate for Differentiating Bacterial Meningitis from Aseptic (Viral) Meningitis. Elsevier Evidence-Based Medicine Center 28. |
|
|
Sharma N, Zahoor I, Sachdeva M (2021). Deciphering the role of nanoparticles for management of bacterial meningitis: an update on recent studies [published online ahead of print, 2021 Sep 20]. Environmental Science and Pollution Research (International) pp.1-18. |
|
|
Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL (2011). Bacterial Meningitis in the United States, 1998-2007. New England Journal of Medicine 364(21):2016-2025. |
|
|
Troendle M, Pettigrew A (2019). A systematic review of cases of meningitis in the absence of cerebrospinal fluid pleocytosis on lumbar puncture. BMC Infectious Diseases 19(1):692-703. |
|
|
Tsai WC, Lien CY, Lee JJ (2019). The clinical characteristics and therapeutic outcomes of cryptococcal meningitis in elderly patients: a hospital-based study. BMC Geriatrics 19(1):91. Published 2019 Mar 25. doi:10.1186/s12877-019-1108-0 |
|
|
van Veen KEB, Brouwer MC, van der Ende A, van de Beek D (2017). Bacterial meningitis in patients using immunosuppressive medication: A population-based prospective Nationwide study. Journal of Neuroimmune Pharmacology 12(2):213-218. |
|
|
Viallon A, Desseigne N, Marjollet O, Birynczyk A, Belin M, Guyomarch S, Zeni F (2011). Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Critical Care 15(3):1-9 |
|
|
World Health Organization (WHO) (2017). Meningococcal meningitis fact sheet. World Health Organization, Geneva. |
|
|
Xiao X, Zhang Y, Zhang L, Kang P, Ji N (2016). The diagnostic value of cerebrospinal fluid lactate for post-neurosurgical bacterial meningitis: a meta-analysis. BMC Infectious Diseases 16(1):483-495. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0