ABSTRACT
Bacterial chemical reactions, such as urea hydrolysis can induce calcium carbonate precipitation. The induced production of calcium carbonate formed by microorganisms has been widely used in environmental and engineering applications. The present study aimed to isolate, identify and optimize growth conditions of urease positive bacteria from urea rich soil in Gaza Strip. Bacterial isolates, which tolerated ≥10% urea concentration, were selected for the investigation. Eight isolates recovered and identified to be spore forming, urease positive, alkaliphile, halotolerant, and presumptively belonged to Bacillus species. All isolates showed best growth at temperature 37°C, and pH 9-9.5. After exposure to UV irradiation, most isolates showed improved tolerance to urea concentration, however, other strains showed a decline in their adaption to urea concentrations. The mutant form of isolate in soil sample #3 showed the highest tolerance to urea concentrations at all exposure intervals, when compared with wild type. Moreover, all isolates precipitated calcium carbonate. The locally recovered isolates are promising contributors in the process of calcite Biomineralizaion and may be utilized in the remediation of concrete cracks, increase of compressive strength of concrete, decrease water permeability, and solve the problems of soil erosions.
Key words: Calcite bio-mineralization, microbial induced calcium carbonate precipitation (MICP), urease, Bacillus spp., Gaza strip.
Biological precipitation of minerals (Bio-mineralization) is a widespread phenomenon in the microorganism's world, and is mediated by bacteria, fungi, protists, and even by plants. Calcium carbonate (Calcite) is one of those minerals that naturally precipitate as a by-product of microbial metabolic activities (Seifan and Berenjian, 2019).
Microbial metabolic activities facilitate calcium carbonate (calcite) precipitation, in a well-studied process called microbial induced calcium carbonate precipitation (MICP) (Zambare et al., 2019). MICP usually occurs due to the chemical alteration of the environment induced by the microbial activity (Sarikaya, 1999; Stocks-Fischer et al., 1999; Warren et al., 2001; De Muynck et al., 2010a). Bacteria can be invested as a major player in the MICP phenomenon through various mechanisms. The most significant mechanism is the bacterial ureolytic activity (Stocks-Fischer et al., 1999; Warren et al., 2001; Krajewska, 2018). Urea hydrolysis can be facilitated by bacteria that can produce urease (urea amidohydrolase) enzyme and thus are able to induce CaCO3 precipitation (Stocks-Fischer et al., 1999; Hammes and Verstraete, 2002; Phillips et al., 2013; Bhaduri et al., 2016). Calcium carbonate precipitation is a chemical process controlled mainly by four key factors: (1) calcium ions concentration, (2) dissolved inorganic carbon (DIC) levels, (3) the pH, and (4) the availability of nucleation sites (Hammes and Verstraete, 2002; Seifan and Berenjian, 2018).
Over recent years, MICP has received considerable attention and has been proposed as a potent solution to address many environmental and engineering issues (Seifan and Berenjian, 2019). It has been intensely investigated in bulk systems, sand columns (Dhami et al., 2013a; Seifan et al., 2016; Tziviloglou et al., 2016), and bio-cementation processes (Seifan et al., 2016). It has been found that MICP may drive many potential applications in civil engineering such as enhancing stability of slopes and dams, reducing the liquefaction potential of soil, road construction, prevention of soil erosion, increase durability and compressive strength of concrete, as well as the repair of the cracks in concrete (De Muynck et al., 2010a; Stabnikov et al., 2011, Shashank et al., 2016).
Many bacterial species have been studied to exploit their abilities in the biomineralizing of calcite (MICP). One of the most robust ureolytic bacteria is Sporosarcina pasteurii (formerly known as Bacillus pasteurii). S. pasteurii is facultative anaerobes, spore forming, bacilli bacteria. It utilizes urea as an energy source and eliminates ammonia which increases the pH in the environment and generates carbonate, causing Ca2+ and CO32- to be precipitated as CaCO3 (Clive, 1990, Stocks-Fischer et al., 1999; Chahal et al., 2011). Other studies showed the role of bacteria that are mostly related to Bacillus spp. in the process of MICP (Stocks-Fischer et al., 1999; Elmanama and Alhour, 2013; Ali et al., 2020). The aim of this study is to isolate, identify, and optimize growth conditions of locally isolated urease-producing bacteria that are able to produce calcite crystals.
Sample sources and characteristics
This study utilized soil samples varying in urea content from Gaza Strip. About 100 g of each sample were collected during June 2020 from the following sources: (1) Sea coastal sand from Rafah city beach, (2) outlet sewage water (treated sewage), (3) inlet sewage water (untreated sewage), (4) soil sample with cat`s urine from Gaza city, (5) coastal sand with dog`s urine from Rafah city beach, (6) sand with dog`s urine from Rafah city, (7) agricultural soil with dog`s urine from Gaza city, (8) agricultural soil with dog`s urine from Rafah city, (9) urea rich soil from greenhouse in Gaza city, and (10) ammonia rich soil from a greenhouse in Gaza city.
Sample processing and bacterial isolation
Five grams of soil samples were mixed with 20 ml sterile saline (stock suspension) and dilutions 10-1 and 10-2 were made. A volume of 0.1 ml of the stock suspension as well as the two dilutions were cultured onto 2 and 3% urea containing Nutrient Agar (NA) plates (HiMedia, India). The media were prepared according to HiMedia manufacturer recommendations. Extra pure urea suspensions (Honeywell Riedel-de Haen, Germany) were filtered, then added to media after autoclaving and cooling to 50°C. Cultures were incubated at 37°C, and plates were examined after 24 and again after 48 h.
Bacterial tolerance to high urea concentration
Bacterial isolates were obtained as a pure culture and then cultured on 5, 8, 10, 12, and 15% urea enriched NA media, incubated at 37°C for 48-72 h, and after the incubation period bacteria were harvested to be cultivated in nutrient broth and agar plates. Bacterial isolates tolerated ≥ 10% urea concentration were selected for further testing.
Bacterial identification and biochemical characterization
The selected isolates were Gram stained (Liofilchemm Italy), spore position was identified, growth at basic media under normal conditions and at 45°C has been evaluated, and tested for oxidase, catalase, urease, O-Nitrophenyl-β-D-Galactopyranoside (ONPG), arginine dihydrolase (ADH), lysine decarboxylase (LDC), ornithine decarboxylase (ODC). Isolates were also inoculated into Triple sugar iron agar (TSIA), Citrate agar, Sulfide-Indole-motility media (S.I.M), and starch agar (HiMedia, India). Isolates were also tested for gelatin liquefaction, Voges proskauer (VP), and sugar fermentation tests adopted from API-20E.
pH profile
The isolates were inoculated into 3 ml of Nutrient Broth tubes with pH scale of 1 to 14. A bacterial suspension was made and the turbidity was adjusted to 0.5% McFarland standard, incubated for 24 h at 37°C, and growth has been measured as turbidity at O.D 600 nm using CT-2200 spectrophotometer (Chrom Tech, Taiwan). Results were recorded against a blank of bacterial suspension. 1N HCl (HiMedia, India) and 1N NaOH (Frutarom, Palestine) were used to adjust the pH. An additional nutrient broth tubes at pH 7 were inoculated with the bacterial isolates, incubated at 37°C, and the change in pH was monitored during growth, using a pH meter (Jenway pH Meter 3510 /mV, USA ) results were recorded after 30 min, 1 , 2, 4, 8, 24, and 32 h of inoculation.
Effect of sodium chloride concentration on growth
Bacterial isolates were inoculated onto Yeast Extract agar (HiMedia, India), that was supplemented with 0.2, 0.5, 0.8, 1, 1.5, 2, 3 and 5% NaCl (HiMedia, India). Plates were observed for growth after 24 and after 48 h of incubation at 37°C.
Effect of temperature on growth
Bacterial isolates were inoculated into nutrient broth tubes (HiMedia, India), adjusted to 0.5% McFarland standard, incubated for 24 h at 0, 4, 25, 37, 45 and 60°C, and the turbidity was measured using spectrophotometer at O.D 600 nm.
Ultraviolet (UV) induced mutagenesis for bacterial isolates
The selected isolates were grown overnight in NB + 2% urea in a shaking incubator (Boeco, Germany) at 37°C. The isolates were washed three times with sterile phosphate-buffered saline, re-suspended in urea free and sterile NB. The turbidity of cell suspensions was adjusted to a 0.5% McFarland reagent and exposed to UV light using a Philips 20 W germicidal lamp for 2-20 min with 2 min intervals. From each exposure interval, a loopful of the exposed bacteria was cultured onto urea-based agar (HiMedia, India). After incubation of 24 h at 37°C, a single, well-defined colony was chosen, cultivated on NA plates, and then inoculated onto NA with varying urea concentrations; 5, 8, 10, 12 and 15% respectively. After incubation, bacterial growth was observed and compared to wild type growth on the different urea concentrations.
Mini-scale of calcium carbonate precipitation
Bacterial isolates were subjected to calcium carbonate production test as described previously (Ghosh et al., 2019). Alive bacterial isolates were inoculated into nutrient broth (NB) containing both urea and calcium chloride (NBUC), NB with only urea (NBU), and NB with only calcium chloride (NBC). The same procedures were repeated with autoclave killed bacterial suspension (pellet and supernatant filtrate). To all tubes, a concentration of 0.012 g/L phenol red was used as a pH indicator. NBUC and NBU were prepared to contain 2% of urea. NBUC and NBC were prepared to contain 2.8 g/L of calcium chloride. Urea and calcium chloride solutions were filter sterilized and separately added to phenol red containing NB before bacterial inoculation. A urease enzyme reagent obtained from Blood urea nitrogen kit (Biosystems, Spain) was used as a positive control, while Escherichia coli ATCC 25922 (urease negative) was used as a negative control. Non-inoculated tubes were used as a validity control. All tubes were incubated at 37°C for 24 h. The trial was performed in triplicate.
Bacterial isolates characteristics
After the incubation of soil samples cultures, one to four colonies of isolated bacteria were selected from those plates containing few and well isolated colonies. Colonies were creamy white or pale yellow to bright orange colored and slightly convex with an entire margin.
Selection of the suitable urease producing isolates
By culturing all isolates on 5, 8, 10, 12, and 15% urea agar media, those that tolerated ≥ 10% urea concentration has been chosen to proceed with. Table 1 shows the highest concentration of urea that each isolate tolerated. Thus, eight isolates: 3, 7, 8.1, 8.2, 8.3, 8.4, 9, and 10.1 were selected for further testing.
Bacterial Identification and biochemical characterization
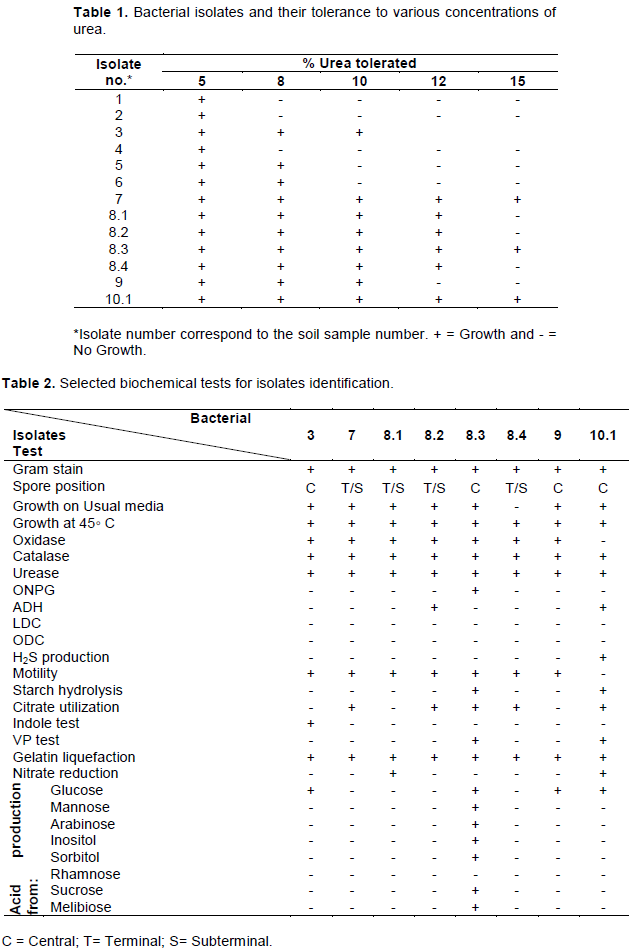
All selected isolates were spore-forming, Gram-positive bacilli, catalase and urease positive. Table 2 shows the phenotypic characteristics of the eight isolates and indicates the biochemical tests that have been used in the identification process. ABIS online Software has been used in bacterial identification (Costin and Lonut, 2017). Table 3 shows the presumptive identification of the selected isolates according to the ABIS online Software.
Growth conditions
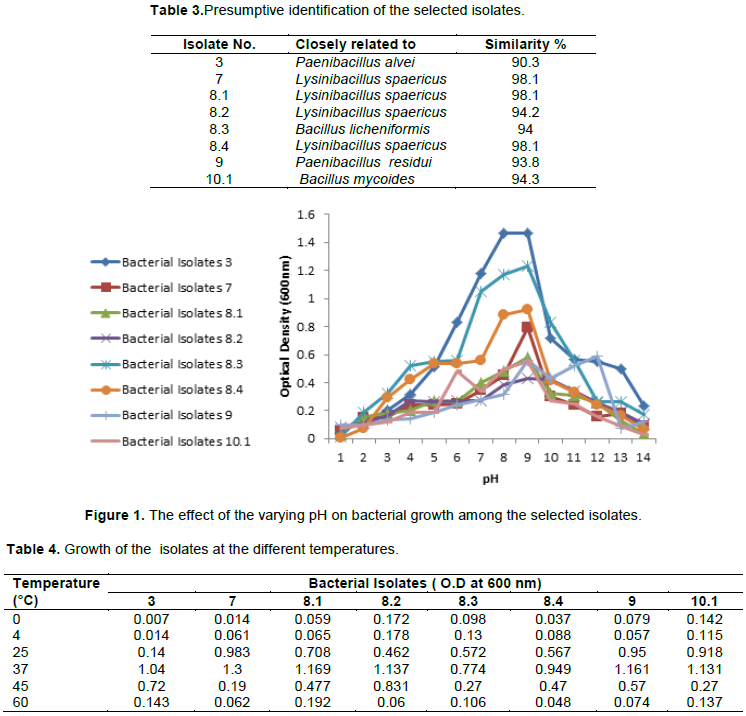
The optimal pH at which all selected isolates showed the highest turbidity and rapid growth ranged from 7 to 10, with a preference to the pH 9 (Figure 1), thus all tested isolates are moderate alkaliphiles. For most isolates, the pH of media has increased during growth to reach the maximum of 9 to 9.5. All isolates showed significant growth at temperature 37°C (Table 4). Most isolates showed halophilic characteristic as they grew at NaCl concentration up to 5%.
Urea tolerance for bacterial isolates after UV exposure
In general, most isolates showed improved tolerance to urea concentration after exposure to UV light when compared with the wild type (Table 5). The mutant form of isolate #3 showed the higher tolerance to urea concentrations at all exposure intervals, when compared with wild type. At certain time intervals, some isolates showed decrease tolerance to high urea concentrations in compared to their wild type. Isolate # 8.1 showed no difference of tolerance to urea between wild type and mutant form.
Mini-scale calcium carbonate precipitation experiment
NBUC tubes for all live isolates, and the pure urease enzyme showed change in pH from neutral to alkaline (yellow to pink), and a precipitate of calcium carbonate were noticed at the bottom of the tubes. NBU tubes for all live isolates and urease enzyme showed only change in pH. NBC tubes for all live isolates and urease enzyme showed neither a change in pH nor calcium carbonate precipitation. Changes in color and pH indicate ureolytic activity (Table 6).
A comparison of NBUC of live bacteria versus killed isolates (both killed cells and supernatant) and E. coli, all of them were unable to change pH, so there was no urea hydrolytic activity due to the absence of the enzyme. Consequently, there was no calcium carbonate precipitation. Unchanged non-inoculated tubes suggests that results obtained are reproducible and representative (Table 6). Precipitate containing and non-containing tubes were examined under light microscope to confirm the presence of calcium carbonate crystal (Figure 2).
The present study was conducted to isolate, characterize, and optimize locally adapted urease-releasing bacteria that inhabits urea rich soils. Microbial activity that involves the cleavage of urea into ammonia and carbon dioxide by the urease enzyme, leading to the precipitation of carbonate ions as calcium carbonates. This potentially useful application explains the need to enhance urease production by various methods among candidate microorganisms (Vempada et al., 2011). The biochemical profile of the selected isolates showed that all isolates belong to the genus Bacillus (Table 3). This is similar to a previous study that isolated and characterized urease positive bacteria from urea rich soils, in which several isolates were mostly related to the Bacillus group (Ali et al., 2020).
Despite the differences in their characteristics, the obtained isolates showed similar behavior in their ureolytic capability. This is in agreement with the findings of Stocks-Fischer et al. (1999); Hammes et al. (2003) and Stabnikov et al. (2011) that reported the same ureolytic Bacillus strains that can be isolated and cultivated using the same followed protocols of isolation and cultivation. Phenotypic and biochemical profiles of the isolates were matched to those Bacillus species reported previously that proved active in MICP process (Stocks-Fischer et al., 1999; Elmanama and Alhour, 2013).
Ureolysis-driven MICP is a phenomenon that has many applications for biochemical and engineering purposes (Omoregie et al., 2020). It has been widely investigated for soil stabilization, healing of concrete cracks, restoration of limestone surfaces, preventing soil erosions, and treatment of industrial wastewater and removing heavy metals (Whiffin et al., 2007; Sarda et al., 2009; Van paassen, 2009; De Muynck et al., 2010a; De Muynck et al., 2010b; Wu et al., 2019).
All obtained isolates showed ureolytic activity, tolerance to high urea concentrations, as well as calcium carbonate production. This suggests that isolates are potential candidates for the applications of MICP. Isolates 10.1 that was identified as B. mycoides, has been previously isolated and showed an efficient role of increased sand consolidation and compressive strength of cement (Elmanama and Alhour, 2013). Isolate 8.3 has been identified as B. licheniformis, has been reported in a previous study that it was able to precipitate calcium carbonate by ureolysis (Helmi et al., 2016).
Other studies mentioned the use of Sporosarcina pasteurii (the bacteria used in most studies) (Achal et al., 2009, 2010, 2011). Bacillus sphaericus, Bacillus subtilis (Atkinson and Fisher, 1991; Dhami et al., 2013b; Stabnikov et al., 2013), and Bacillus cereus in MICP applications (Wu et al., 2019; Dhami et al., 2013b).
Bacteria are previously known to breakdown urea in order to: (1) elevate the ambient pH (Burne and Marquis, 2000), (2) consume it as a nitrogen source (Burne and Chen, 2001), and (3) use it as a source of energy (Mobley and Hausinger, 1989). The amount and rate of urea that can be cleaved were influenced by the urea and calcium source (Wang et al., 2017). In this system, urea is the source of the carbonate. The more urea is supplied, the more CaCO3 can be produced, if a sufficient amount of calcium ions is available (Wu et al., 2019).
In this study, isolates that were selected tolerated and grew in the presence of 10 - 15% urea concentration. This because urease activity, as well as, calcium carbonate production rate depend on urea concentration. A previous study utilized S. pasteurii and emphasized the role of urea containing cultural medium in the proliferation of bacteria. Moreover, it reported that bacteria cultivated with urea displayed a healthier cell surface and more negative surface charge for calcium ion binding than the bacteria have been cultivated without urea (Ma et al., 2020).
Increasingly, it has been reported that the bacterial concentration and ureolytic activity are important contributors in the efficiency of MICP process. The urea hydrolysis is an extremely slow process, whereas the presence of urease enzyme can substantially increase the hydrolysis of urea (De Belie et al., 2018). Therefore, the selection of the bacterial isolates with higher ureolytic activity is desirable for the higher production of calcium carbonate.
However, it has been shown that when the content of urea is excessive, bacterial growth and ureolytic activity are inhibited. For instance, when the urea concentration was greater than 0.75 mol/L, the amount of urea breakdown was decreased and thus appears as an inhibitory component. The reason could be due to too high urea molecule transportation over the cell membrane into the cell, at elevated urea concentrations, inhibiting other cellular processes. Therefore, a certain amount of bacteria can only metabolize a certain amount of urea hydrolysis (Wu et al., 2019).
In our study, the local isolates were halo-tolerant, and corroborate with the findings of previous studies (Stabnikov et al., 2013). The observation of the pH tolerance profile of bacterial isolates showed a common moderate alkaliphile property. The best growth was at pH range 7-10 with a preference to pH 9. This is in agreement with a previous study that showed the good alkali tolerance of B. cereus which was successfully used to heal concrete cracks (Stabnikov et al., 2013; Wu et al., 2019).
Generally, the optimal pH range for bacterial growth is 7 to 8. Under higher alkaline conditions (pH 9- 12) bacteria can still grow but at a much-declined rate. Although the pH is relatively high in fresh concrete, the pH at cracks may drop to 8-11 due to carbonation, exposure, and humidity (De Muynck et al., 2010a). Above pH 11, the bacteria have a limited capacity to precipitate CaCO3, thus limited ability to heal cracks. This implies that bacterial spores will keep dormant after being embedded in the concrete matrix (pH > 12), and only start to become active after cracks appear and crack surface pH drops (Wang et al., 2017; Wu et al., 2019). Therefore, alkaline pH is the primary factor by which bacteria promote calcite precipitation (Castanier et al., 2000; Fujita et al., 2000). Another study showed that the calcium carbonate yield (mg calcium carbonate/CFU) in the presence of Bacillus species increases when bacteria grown at a relatively high pH in compared with those bacteria that grown at uncontrolled pH solution (Seifan et al., 2017). Another study investigated the factors affecting the S. pasteurii induced biomineralization process, reported that the rise in medium pH to 9.5 accelerate bacterial growth (Ma et al., 2020). This may be promising that Bacillus isolates in this study can be used to heal concrete cracks. Especially, in the pH range of 7-11, bacteria will have a remarkable ureolytic activity, which ensures the decomposition of urea and the precipitation of CaCO3. This meets also with (Phang et al., 2018) findings, It has been reported that some bacterial ureases exhibited high activity in alkaline conditions at pH of 9.
In the present study, the effect of temperature on isolates growth showed a temperatures range from 25 to 40°C. Bacterial mediated urea hydrolysis is an enzymatic reaction controlled by many factors including temperature. It has been reported in the literature that temperature affects bacterial activity, urease activity, and therefore reaction rate. Hence, the rate of formation of biogenic CaCO3 and crack healing efficiency will be affected as well.
Urease activity is stable between 15 and 25°C, and an increase in temperature (until 60°C) results in increased urease activity (Whiffin, 2004; Peng and Liu, 2019).
Isolates that were exposed to UV irradiation were compared with their corresponding wild type isolates for the ability to tolerate higher urea concentrations. Most isolates showed improved tolerance to urea concentration. However, other strains showed a decline in their adaption to urea concentrations. This suggests that the mutagenesis process is random and did not correlate to the time of exposure to UV light. This is similar to the findings of a previous study, which used UV irradiation on S. pasteurii in order to improve urease activity (Wu et al., 2019).
The established calcium carbonate precipitation process showed that all NBUC tubes containing the viable isolates showed accompanied ureolytic and calcite precipitation activity. On the other hand, NBUC tubes containing the autoclave-killed isolates (pellet or supernatant) showed neither ureolytic nor calcite precipitation activity. This suggests that bacteria activity and urease positivity is a principal contributing to pH change due to urea cleavage, as well as calcium carbonate precipitation. In all NBC tubes inoculated with the viable isolates there was no calcium carbonate precipitation observed. This suggests that calcium carbonate production is enhanced by the change of pH. In NBC tubes (without urea), there was no difference in color change or calcium carbonate precipitation between live bacteria, killed bacteria, or E. coli. All NBU tubes inoculated with the viable isolates showed a change in pH as a proof for the ureolytic activity they possess. Negative control (E. coli) showed no change in pH or calcite production. These findings matched a previous study that reported the ability of urease producing bacteria S. pasteurii to produce calcium carbonate crystals under the same conditions (Ghosh et al., 2019). This is in agreement with the previous studies that reported that Bacillus sp. is with high respect in compared with other genus and that this might be due to their physiological ability to adapt to stressed conditions (Helmi et al., 2016).
In conclusion, this study successfully and easily isolated several Bacillus species from locally collected soil samples. These strains are alkaliphile, grow well at pH 7-10, and tolerate high urea concentrations. They showed calcite biomineralizing properties and may be employed in bacterial remediation of concrete cracks, increasing the compressive strength of concrete, decreasing water permeability, and solve the problems of soil erosions. Further studies on a larger scale are recommended to confirm the findings.
The authors have not declared any conflict of interests.
This study was financed in a part by a publication grant from the UCAS Technology Incubator. Authors of this article would like to acknowledge the UCASTI for supporting this research and their contribution in the ease of knowledge spread.
REFERENCES
|
Achal V, Mukherjee A, Basu P (2009). Lactose mother liquor as an alternative nutrient source for microbial concrete production by Sporosarcina pasteurii. Journal of Industrial Microbiology and Biotechnology 36(3):433-438.
Crossref
|
|
|
|
Achal V, Mukherjee A, Reddy MS (2010). Biocalcification by Sporosarcina pasteurii using Corn steep liquor as nutrient source. Industrial Biotechnology 6(3):170-174.
Crossref
|
|
|
|
|
Achal V, Mukherjee A, Reddy MS (2011). Microbial Concrete: Way to Enhance the Durability of Building Structures. Journal of Materials in Civil Engineering 23(6):730-734.
Crossref
|
|
|
|
|
Ali NA, Karkush MO, Al Haideri HH (2020). Isolation and Identification of Local Bactria Produced from Soil-Borne Urease. IOP Conference Series: Materials Science and Engineering 901:012035.
Crossref
|
|
|
|
|
Atkinson MR, Fisher SH (1991). Identification of genes and gene products whose expression is activated during nitrogen-limited growth in Bacillus subtilis. Journal of Bacteriology 173:23-27.
Crossref
|
|
|
|
|
Bhaduri S, Debnath N, Mitra S, Liu Y, Kumar A (2016). Microbiologically induced calcite precipitation mediated by Sporosarcina pasteurii. Journal of visualized experiments 110:1-7.
Crossref
|
|
|
|
|
Burne RA, Chen RE (2001). Bacterial ureases in infectious diseases. Microbes and Infection 2(5):533-542.
Crossref
|
|
|
|
|
Burne RA, Marquis RE (2000). Alkali production by oral bacteria and protection against dental caries. FEMS Microbiology Letters 193(1):1-6.
Crossref
|
|
|
|
|
Castanier S, Metayer-Levrel GL, Perthuisot JP (2000). Bacterial roles in the precipitation of carbonate minerals. Microbial Sediments pp. 32-39.
Crossref
|
|
|
|
|
Chahal N, Rajor A, Siddique R (2011). Calcium carbonate precipitation by different bacterial strains. African Journal of Biotechnology 10(42):8359-8372.
Crossref
|
|
|
|
|
Clive E (1990). Microbiology of extreme environments. First ed. McGraw-Hill, New York.
|
|
|
|
|
Costin S, Ionut S (2017). ABIS online - Advanced bacterial identification Software, an original tool for phenotypic bacterial identification.
View (Oct. 1, 2020).
|
|
|
|
|
De Belie N, Gruyaert E, Alâ€Tabbaa A, Antonaci P, Baera C, Bajare D, Darquennes A, Davies R, Ferrara L, Jefferson T, Litina C, Miljevic B, Otlewska A, Ranogajec J, Roigâ€Flores M, Paine K, Lukowski P, Serna P, Tulliani Jâ€M, Vucetic S, Wang J, Jonkers HM (2018). A review of self-healing concrete for damage management of structures. Advanced Material Interfaces 5(17):1-28.
Crossref
|
|
|
|
|
De Muynck W, De Belie N, Verstraete W (2010a). Microbial carbonate precipitation in construction materials: a review. Ecological Engineering 36(2):118-136.
Crossref
|
|
|
|
|
De Muynck W, Verbeken K, De Belie N, Verstraete W (2010b). Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecological Engineering 36(2):99-111.
Crossref
|
|
|
|
|
Dhami NK, Reddy MS, Mukherjee A (2013a). Biomineralization of calcium carbonates and their engineered applications: a review. Frontiers in Microbiology 4:1-13.
Crossref
|
|
|
|
|
Dhami NK, Reddy MS, Mukherjee A (2013b). Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites. Journal of Microbiology and Biotechnology 23(5):707-714.
Crossref
|
|
|
|
|
Elmanama AA, Alhour MT (2013). Isolation, Characterization and Application of Calcite Producing Bacteria from Urea Rich Soils. Advanced Science and Engineering Research 3(4):388-399.
|
|
|
|
|
Fujita Y, Ferris FG, Lawson RD, Colwell FS, Smith RW (2000). Calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiology Journal 17(4):305-318.
Crossref
|
|
|
|
|
Ghosh T, Bhaduri S, Montemagno C, Kumar A (2019). Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface. PLoS ONE 14(1):1-15.
Crossref
|
|
|
|
|
Hammes F, Boon N, Clement G, de Villiers J, Siciliano SD, Verstraete W (2003). Molecular, biochemical and ecological characterisation of a bio-catalytic calcification reactor. Applied Microbiology and Biotechnology 62(2-3):191-201.
Crossref
|
|
|
|
|
Hammes F, Verstraete W (2002). Key roles of pH and calcium metabolism in microbial carbonate precipitation. Reviews in Environmental Science and Biotechnology 1(1):3-7.
Crossref
|
|
|
|
|
Helmi FM, Elmitwalli HR, Elnagdy SM, El-Hagrassy AF (2016). Calcium carbonate precipitation induced by ureolytic bacteria Bacillus licheniformis. Ecological Engineering 90:367-371.
Crossref
|
|
|
|
|
Krajewska B (2018). Urease-aided calcium carbonate mineralization for engineering applications: a review. Journal of Advanced Research 13:59-67.
Crossref
|
|
|
|
|
Ma L, Pang A, Luo Y, Lu X, Lin F (2020). Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii. Microbial Cell Factories 19(1):1-12.
Crossref
|
|
|
|
|
Mobley HLT, Hausinger RP (1989). Microbial Ureases: Significance, regulation and molecular characterisation. Microbiological Review 53:85-108.
Crossref
|
|
|
|
|
Omoregie A, Ngu L, Ong D, Nissom P (2020). Low-Cost Cultivation of Sporosarcina Pasteurii Strain In Food-Grade Yeast Extract Medium For Microbially Induced Carbonate Precipitation (MICP) Application. Biocatalysis and Agricultural Biotechnology 17:247-255.
Crossref
|
|
|
|
|
Peng J, Liu Z (2019). Influence of temperature on microbially induced calcium carbonate precipitation for soil treatment. PLoS ONE 14(6):1-17.
Crossref
|
|
|
|
|
Phang IRK, San Chan Y, Wong KS, Lau SY (2018). Isolation and characterization of urease-producing bacteria from tropical peat. Biocatalysis and Agricultural Biotechnology 13:168-175.
Crossref
|
|
|
|
|
Phillips AJ, Gerlach R, Lauchnor E, Mitchell AC, Cunningham AB, Spangler L (2013). Engineered applications of ureolytic biomineralization: a review. Journal of Bioadhesion and Biofilm Research 29(6):715-733.
Crossref
|
|
|
|
|
Sarda D, Choonia H, Sarode D, Lele S (2009). Biocalcification by Bacillus pasteurii urease: a novel application. Journal of Industrial Microbiology and Biotechnology 36(8):1111-1115.
Crossref
|
|
|
|
|
Sarikaya M (1999). Biomimetics: materials fabrication through biology. Proceedings of the National Academy of Sciences 96(25):14183-14185.
Crossref
|
|
|
|
|
Seifan M, Berenjian A (2018). Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete. World Journal of Microbiology and Biotechnology 34(11):168.
Crossref
|
|
|
|
|
Seifan M, Berenjian A (2019). Microbially induced calcium carbonate precipitation: a widespread phenomenon in the biological world. Applied microbiology and biotechnology 103:4693-4708.
Crossref
|
|
|
|
|
Seifan M, Samani AK, Berenjian A (2016). Bioconcrete: next generation of self-healing concrete. Applied microbiology and biotechnology 100(6):2591-2602.
Crossref
|
|
|
|
|
Seifan M, Samani AK, Berenjian A (2017). New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Applied Microbiology and Biotechnology 101:3131-3142.
Crossref
|
|
|
|
|
Shashank BS, Sharma S, Sowmya S, Latha RA, Meenu PS, Singh DN (2016). State-of-the-art on geotechnical engineering perspective on bio-mediated processes. Environmental Earth Sciences 75(3):1-16.
Crossref
|
|
|
|
|
Stabnikov V, Jian C, Ivanov V, Li Y (2013). Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World Journal of Microbiology and Biotechnology 29(8):1453-1460.
Crossref
|
|
|
|
|
Stabnikov V, Naeimi M, Ivanov V, Chu J (2011). Formation of water-impermeable crust on sand surface using biocement. Cement and Concrete Research 41(11):1143-1149.
Crossref
|
|
|
|
|
Stocks-Fischer S, Galinat JK, Bang SS (1999). Microbiological precipitation of CaCO3. Soil Biology and Biochemistry 31(11):1563-1571.
Crossref
|
|
|
|
|
Tziviloglou E, Van Tittelboom K, Palin D, Wang J, Sierra-Beltran MG, ErÅŸan YC, De Belie N (2016). Bio-based self-healing concrete: from research to field application. Self-healing Materials 1:345-385.
Crossref
|
|
|
|
|
Van Paassen L (2009). Biogrout- Ground improvement by microbially induced carbonate precipitation. Ph.D. thesis, The Delft University of Technology, Delft, Netherlands.
|
|
|
|
|
Vempada S, Reddy SS, Rao MV, Sasikala C (2011). Strength enhancement of cement mortar using microorganisms-An Experimental Study. International Journal of Earth Sciences and Engineering 4(6):933-936.
|
|
|
|
|
Wang JY, Jonkers HM, Boon N, Belie DB (2017). Bacillus sphaericus LMG 22257 is physiologically suitable for self-healing concrete. Applied Microbiology and Biotechnology 101(12):5101-5114.
Crossref
|
|
|
|
|
Warren LA, Maurice PA, Parmar N, Ferris FG (2001). Microbially mediated calcium carbonate precipitation: implications for interpreting calcite precipitation and for solid-phase capture of inorganic contaminants. Geomicrobiology Journal 18(1):93-115.
Crossref
|
|
|
|
|
Whiffin VS (2004). Microbial CaCO3 precipitation for the production of biocement Ph.D thesis, Murdoch University, Western Australia.
|
|
|
|
|
Whiffin VS, van Paassen L, Harkes M (2007). Microbial carbonate precipitation as a Soil improvement Technique. Geomicrobiology Journal 24(5):417-423.
Crossref
|
|
|
|
|
Wu M, Hu X, Zhang Q, Xue D, Zhao Y (2019). Growth environment optimization for inducing bacterial mineralization and its application in concrete healing. Construction and Building Materials 209:631-643.
Crossref
|
|
|
|
|
Zambare NM, Lauchnor EG, Gerlach R (2019). Controlling the Distribution of Microbially Precipitated Calcium Carbonate in Radial Flow Environments. Environmental Science and Technology 53:5916-5925.
Crossref
|
|