ABSTRACT
Olea europeae (L.) has been reported to have antibacterial, antifungal and antiviral activities. The aim of the present study is to evaluate antiviral activity of olive leaves extract (OLE) against herpes simplex viruses (HSV) type-1 virus. Screening of antiviral activity was assessed by measuring inhibition of viral-induced cytopathic effect of in vero cells of different OLE fractions that have been successively extracted using solvents of increasing polarities, against HSV type-1 virus. Negligible antiviral activity has been shown of different fractions, except for ethyl acetate and n-butanol fractions, showing strong and moderate anti-HSV type -1 activity, respectively. High performance liquid chromatography (HPLC) chromatographic analysis of both fractions revealed high oleuropein content in ethyl acetate fraction in addition to other phenolic and flavonoid contents, whereas n-butanol fraction showed only high content of other phenolic and flavonoid compounds. Cytotoxicity of ethyl acetate fraction was assessed in vero cell line, the mean cytotoxic concentration CC50, was reported to be 610 µg/ml. On the other hand, the 50% inhibitory concentration (IC50), against HSV-1, was of value as low as 40 µg/ml (SI = 15.2). This concentration could be more reduced to 33 µg/ml (SI = 16.9); that is, 17% reduction in dose, by formulating a microemulsion dosage form, with particle size of 13 to 19 nm, being assessed by Malvern Zetasizer Ver. 6.2 and electron microscopy. Acyclovir, a recommended anti-HSV agent, was used as a positive control. Oleuropein pure standard and the main phenolic component of OLE, was also assessed for its anti-HSV type-1 virus. As conclusion, microemulsion formulation enhanced antiviral activity of crude OLE.
Key words: Olive leaves extract, OLE, acyclovir, anti-HSV activity, microemulsion, oleuropein.
Medicinal plants, including the olive tree (Olea europaea L.) have been used for treatment of different types of diseases (Vijayan et al., 2004; Salah et al., 2012). Olive tree leaves have been widely used in European and Mediterranean countries as a traditional remedy in the form of extracts, herbal teas and powder (EI and Karakaya, 2009). Olive leaves extract has been recommended as a potential nutraceutical; its phytochemical content as polyphenols and flavonoids (Micol et al., 2005) were known to have diverse pharmacological activities (Lee et al., 2009). Olive leaves extract showed antibacterial and antifungal actions at low concentrations (Markin et al., 2003) as well as antiviral activity such as flu and colds. Olive leaves extract (OLE) is also effective against various diseases such as coronary artery disease, hypertension, high cholesterol level, arrhythmia, cancer, diabetes, overweight and osteoporosis (Erdohan and Turhan, 2011). Oleuropein was reported as the most abundant biophenol in olive leaves (Ilias et al., 2011), and it has been claimed in a U.S. patent to have potent antiviral activities against herpes mononucleosis, hepatitis virus, rotavirus, bovine rhinovirus, canine parvovirus and feline leukemia virus (Fredrickson, WR, F & S group, Inc., 2000). Oleuropein and the phenolic compounds of OLE have been reported to show antimicrobial activities against viruses, retroviruses, bacteria, yeasts, fungi and other parasites (Korukluoglu et al., 2010). Other clinical effects of oleuropein and hydroxytyrosol are the potentiation of cellular and organismal protection through the macrophage-mediated response, and the inhibition of platelet aggregation and eicosanoid production, respectively (Petroni et al., 1995; Benavente-Garcia et al., 2000). The aim of the present study is to investigate the in vitro antiviral activity of olive leaves extract against HSV type-1 virus, in its crude form and after incorporation in a microemulsion formulation.
Plant material, reagents and standard
1. Olive leaves were kindly provided from the Experimental station of Medicinal and Aromatic Plants, Faculty of Pharmacy, Cairo University, Giza, Egypt.
2. N-hexane, ethyl acetate, n-butanol and chloroform were purchased from Adwic, El-Nasr pharmaceutical chemicals company.
3. Pure oleuropein standard (HPLC grade), acyclovir, isopropyl myristate (IPM), tween 80, and transcutol were purchased from Sigma-Aldrich Chemical Company, USA. All chemicals were of analytical grade.
Cell line and virus
African Green Monkey kidney cells (Vero cell line) were obtained from the Egyptian Organization for Biological Products and Vaccines (VACSERA). Hepatitis A virus (HAV), Coxsackievirus group B (Cox B virus), and Herpes simplex virus type 1 (HSV-1) were kindly provided from the antimicrobial activity unit in The Regional Center for Mycology and Biotechnology, Al Azhar University.
Model virus selection
Screening of OLE antiviral activity was performed against Hepatitis A virus (HAV), Coxsackievirus group B (Cox B virus), and Herpes simplex virus type 1 (HSV-1). Significant antiviral activity was observed only against HSV-1 virus, which was selected as a model virus in the present study. It was propagated in Vero cells and stored at -80°C until use. Virus titre was determined by a plaque assay on Vero cell monolayers.
Preparation of olive leaves extract
The olive leaves were air dried in shade, powdered, and kept in tightly closed dark glass container till extraction. The powdered leaves were extracted with 70% ethanol at room temperature, then the extract was concentrated under vacuum in a rotary evaporator, further dissolved in distilled water and subjected to liquid-liquid fractionation in a glass separating funnel by successive extraction with solvents of increasing polarities (n-hexane, chloroform, ethyl acetate and n-butanol). The obtained four extracted fractions were then examined for their antiviral activity against HSV type-1 virus.
High performance liquid chromatography (HPLC) analysis and identification of olive leaves extract components
HPLC analysis of the fractions that showed anti-HSV type-1 activity was performed using HPLC Hewlett Packard (series 1050) equipped with UV detector to identify their components. Phenolic compounds of the active extract fractions, ethyl acetate and n-butanol extractives, were determined at λ280 nm by reverse phase HPLC (RP-HPLC) using Alltima C18 (5 µm, 150 mm×1.6 mm id) packed column. Gradient separation was carried out with methanol and acetonitrile as a mobile phase at a flow rate of 1 ml/min. Retention time and peak area were used to calculate the concentration of phenolic compounds by the data analysis of Hewlett Packard software (Goupy et al., 1999) and (Mattila et al., 2000).
Preparation of microemulsion formulation
The most active fraction (ethyl acetate) of olive leaves extract was formulated into a topical microemulsion dosage form. Pseudoternary phase diagrams were constructed using the water titration method. Three phase diagrams were prepared, consisting of the surfactant/co-surfactant (S-Cos) mixture Tween80/Transcutol with mass ratios 3:1, 1:1 and 1:3, mixed with the oil phase (isopropyl myristate) at the weight ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2 and 9:1. These mixtures were diluted dropwise with distilled water mixed with DMSO (10%), under moderate agitation. Clear transparent liquid was classified as microemulsion. The chosen microemulsion is illustrated in Table 3. The ethyl acetate extract was dissolved in the distilled water with 10% DMSO, then the aqueous phase was added dropwise to the mixture of oil/S-Cos and the microemulsion with concentration of 1 mg/ml was obtained by stirring the mixtures at ambient temperature by vortex (Zheng et al., 2010; Moghimipour et al., 2013).
Particle size measurement and electron microscopy scanning of microemulsion
The mean particle size and zeta potential of OLE microemulsion were measured using Malvern Zetasizer Ver. 6.2 (Malvern Instruments Ltd., UK). Samples were placed in clear disposable zeta cells and results were recorded. The microstructure of formulated microemulsion was investigated by electron microscope (JOEL, JEM-1400 TEM) at a voltage of 80 kV (Munin and Edwards-Levy, 2011).
Evaluation of cytotoxicity of olive leaves crude extract, formulated microemulsion, pure oleuropein standard and acyclovir
Cell toxicity was monitored by observing the change in cell morphology and viability. Vero cells were grown and propagated in growth medium (Dulbecco’s modified Eagle medium, DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 0.22% sodium bicarbonate (Sigma) and 50 µg/ml gentamicin (Gibco). The cells were maintained at 37°C in a humidified incubator with 5% CO2 for 24 h. Serial twofold dilutions of the prepared extracts and formulated microemulsion (31 - 1000 µg/ml) were added to confluent Vero cell monolayers in a 96-well microtitre plate (Falcon, NJ, USA), and incubated at 37°C in a humidified incubator with 5% CO2 for 48 h. Control cells were incubated without the test samples. At the end of incubation period, media was aspirated and crystal violet staining solution (0.1%) was added to each well for at least 15 min. Afterwards, the stain was aspirated and the plate was rinsed using distilled water, then the absorbance of the plate was measured with a microplate reader (Bio-Rad 550) at wavelength of 490 nm. Absorbance values of treated cell samples were compared to the control cells cultured without the test sample (Lee-Huang et al., 2003; Shoeib et al., 2011). The concentration reducing the cell viability by 50% (CC50), was calculated by regression analysis using the dose-response curve (Figure 3), generated from the experimental data.
Evaluation and comparison of antiviral activity of olive leaves extracted fractions, formulated microemulsion, pure oleuropein standard and acyclovir using cytopathic effect inhibition assay
Different non-toxic concentrations of the extracted fractions of OLE and formulated microemulsion (1.5 to 200 µg/ml) were checked for their antiviral activity, using cytopathic effect (CPE) inhibition assay, against HSV type-1 virus. In brief, confluent monolayers of Vero cells were challenged with 104 PFU/ml herpes simplex type-1 virus doses, and simultaneously the cultures were treated with equal volumes of two-fold serial dilutions of the crude extracts, microemulsion, acyclovir as a positive control and pure oleuropein standard; then incubated at 37°C in a humidified incubator with 5% CO2 for 48 h. Also, an infection control as well as untreated Vero cells growth control, without the tested compounds, was made. Every 24 h, observation under the inverted microscope was made until the virus in the control wells showed complete viral-induced cytopathic effect (CPE). The cell monolayer sheet was stained with 0.1% crystal violet staining solution for at least 15 min, then removed by aspiration and rinsing with distilled water, then the absorbance was measured with microplate reader (Bio-Rad 550) at wavelength of 490 nm. The viral inhibition rate was calculated according to the study of Bag et al. (2012) and Dargan (1998) as follows:
(ODtv-ODcv) / (ODcd-ODcv) x 100%
Where, ODtv indicates the absorbance of the test sample with virus infected cells. ODcv indicates the absorbance of the virus infection control. ODcd indicates the absorbance of the cell growth control.
The concentration reducing the viral-induced CPE by 50% (IC50), was calculated by regression analysis using the dose-response curve (Figure 4), generated from the experimental data. A selectivity index (SI) was calculated for each test sample by dividing its CC50 by the corresponding IC50 value.
Statistics
Data were analyzed using GraphPad Prism for Windows version 6. Reduction of viral-induced CPE for each compound was expressed as mean value + standard deviation. Comparison between different compounds within each concentration was done using analysis of variance (ANOVA), followed by multiple comparison tests (post-Hoc tests), that were used for pair-wise comparison with multiplicity adjusted (exact) p values. All tests were two-tailed. A p-value <0.05 was considered significant.
HPLC analysis and identification of olive leaves extract components
The phenolic compounds of the ethyl acetate extract that showed strong anti-HSV type-1 virus activity were determined by reverse phase HPLC (RP-HPLC) at λ280 nm. As shown in Figure 1, oleuropein content was the major component of phenolic compounds (1.8% w/w) of ethyl acetate extract, compared to pure oleuropein standard; whereas, the content percent in butanol extract was only 0.06% w/w (Figure 2).
Evaluation of cytotoxicity of olive leaves extract and its microemulsion form relative to acyclovir and pure oleuropein standard on vero cells
The cytotoxicity of crude olive leaves extract and its microemulsion form was assessed, compared to Acyclovir and pure Oleuropein standard; the results are presented in Table 1. As shown in Table 1 and Figure 3, the cytotoxicity of the tested samples on Vero cell line was concentration-dependent. The concentration reducing cell viability by 50% (mean cytotoxic concentration, CC50) of the tested samples was calculated by GraphPad prism for Windows Version 6 statistical program. Pure Oleuropein standard showed highest cytotoxic effect on vero cells followed by formulated microemulsion. The crude olive leaves extract showed lower cytotoxic effects. Acyclovir had no significant cytotoxicity on Vero cells.
Comparison of anti-HSV type-1 activity of olive leaves extract and its microemulsion form with acyclovir and pure oleuropein standard using cytopathic effect inhibition assay
The antiviral activity of the tested samples was assessed by measuring their protective effects on infected vero cells (Cann, 1999; Del Barrio and Parra, 2000).
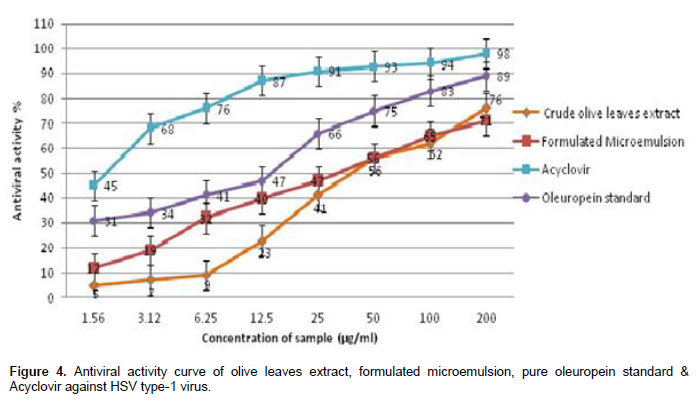
Anti-HSV type-1 activity of crude olive leaves extract and its formulated microemulsion dosage form was determined by the inhibition of virus-induced cytopathic effect, compared to positive (Acyclovir) and negative controls (Table 2). As shown in Figure 4, formulated microemulsion exhibited significant higher virus inhibitory activity than the crude form of the olive leaves extract within the concentration limit range from 1.56 to 12.5 µg/ml. Pure oleuropein standard exhibited significant higher antiviral activity relative to the tested samples. Whereas, Acyclovir (positive control) showed the highest anti-HSV type-1 activity compared to all samples. All samples exhibited concentration-dependent in-vitro antivi-ral activity against HSV type-1 virus.
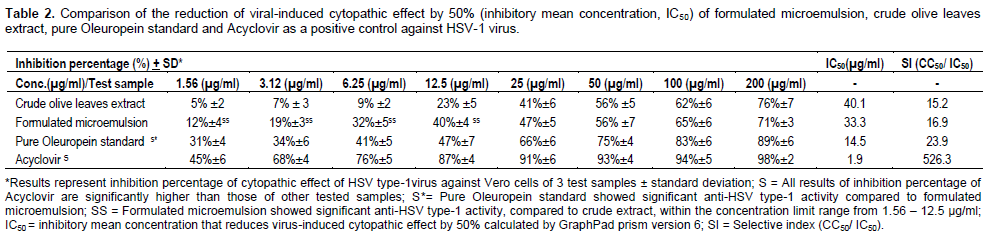
Also the selective index of olive leaves crude extract has increased after incorporation in the microemulsion dosage form, indicating increased selectivity against HSV type-1 virus. Oleuropein pure standard has higher selective index value relative to the tested samples. Acyclovir has the highest selective index value. All the tested samples have higher selective index values than 10, showing potential promising and selective anti-HSV type-1 virus candidates (De Clercq, 1993; Dargan, 1998).
Phase studies of formulated microemulsion
The aim of construction of pseudoternary phase diagrams was to find out the existence region of micro- emulsions.
The pseudoternary phase diagrams of isopropyl myristate / Tween80: Transcutol (3:1) / Water system is presented in Figure 5 (a to c). It was found that o/w microemulsion created with the three systems (Figure 5 a, b and c) was thermodynamically stable, optically transparent and single phase of liquid solution.
The effect of the weight ratio of surfactant and co-surfactant (Km) on the area of o/w microemulsion region was compared. It can be seen from the figures that the area of o/w microemulsion increased with increasing the ratio of Km to be 3:1 (tween 80: transcutol), respectively. The maximum area of o/w microemulsion region was observed at Km 3:1.
Characterization of the selected formulation
According to the constructed pseudoternary phase diagrams, one formulation of the Km ratio 3:1 (tween 80: transcutol) was selected and crude olive leaves extract was incorporated in it as shown in Table 3.
Measurement of particle size and zeta potential
Polydispersity is a measure of particle homogeneity and it
varies from zero to 1. The nearer the value of polydispersity to zero, the higher is the homology between the particles. The polydispersity index of microemulsion was 0.429 ± 0.09 (Table 4), indicating wide particle size distributions (Jia et al., 2010). These results suggest that the mixture favoured to form nanoparticles with more homogenous size distributions due to size reduction. The zeta potential evaluation is an indication of long-term stability due to electrostatic repulsion that prevents particle aggregation; the higher the value, the more stability.
Scanning of medicated microemulsion by electron microscopy
Scanning electron micrograph demonstrated spherical droplets in the nanometer range in a multi-disperse system (Figure 6) which is in agreement with the particle size data determined by Zetasizer.
The earlier mentioned results show that incorporation of the olive leaves crude extract in a microemulsion formulation improved its therapeutic index as an anti-HSV type-1 virus, where the inhibitory mean concentration (IC50) of the crude extract was reduced from 40 to 33 µg/ml, with increased selective index (SI) from 15.2 to 16.9.
Accordingly, the antiviral activity and selective index values of the extract was enhanced by micronization. It has also been reported by Kulkarni (2011) and Soliman et al. (2010) that a number of plant constituents have shown enhanced therapeutic effect at similar or lower doses when being incorporated into novel drug delivery systems, as a result of the micronized particle size of the extract relative to its conventional crude form, which has led to better bioavailability and improved drug uptake due to better adherence to membranes and transport of bioactive molecules in a more controlled pattern (Soliman et al., 2010; Kogan and Garti (2006). Also, it was reported that the lower the viscosity of the vehicle, the faster is the release (Kogan and Garti, 2006).
Moreover, the higher extract solubility in the external water phase of o/w microemulsion has caused enhanced release of the solubilized extract and consequent improvement in the antiviral activity than the crude form itself at the concentration limit of 1.56 to 12.5 µg/ml as shown in Figure 4, followed by slower release of the extract from the internal oil phase of microemulsion. It has been also reported by Kogan and Garti (2006) that incorporation of the drug in the external phase of o/w microemulsion increases the diffusion rate, leading to release of larger amounts of the drug.
Also, the antiviral activity of pure oleuropein standard, which is the main biophenol constituent of olive leaf extract, is relatively high (IC50 of 14.5 µg/ml) with selective index (SI= 23.9), compared to the formulated ME and the crude extract. This can indicate that this active constituent could be responsible for the antiviral activity of olive leaves extract (Ritchason, 1999).
In previous study, it has been reported that the antiviral activity of OLE might be attributed to the prevention of attachment and adsorption of virus particles to the cell; thereby blocking their entry into the cells. Oleuropein interacts with the viral envelope by interacting with the surface of phospholipid bilayer present in the virus envelope, promoting drastic changes on the membrane surface to interfere with the binding of proteins to phospholipid domains, enriched in negatively charged phospholipids, such as phosphatidylglycerol or phosphatidylserine, leading to reduced membrane-fusion capacity. Anionic phospholipids have been shown to play a crucial role on the viral entry process (Micol et al., 2005).
Also micronized topical dosage forms of herbal extracts increase their solubilization and bioavailability at deeper epidermal layers, leading to more specifity and efficiency with decreased risk of toxicity due to reduction in the effective dose and improved abso rption of active ingredient at site of action, with reduced systemic side effects (Kesarwani and Gupta, 2013; Gupta et al., 2013).
Consequently, it can be observed that microemulsion formulation of OLE can be a promising technique of enhancing their pharmacological action and reducing their side effects.
The authors have not declared any conflict of interests.
REFERENCES
|
Bag P, Chattopadhya D, Mukherjee H, Ojha D, Mandal N, Sarkar MC, Chatterjee T, Das G, Chakraborti S (2012). Anti-herpes virus activities of bioactive fraction and isolated pure constituent of Mallotuspeltatus: an ethnomedicine from Andaman Islands. Virol. J. 9:98.
Crossref
|
|
|
|
Benavente-Garcia O, Castillo J, Lorente J, Ortuno A, Del Rio JA (2000). Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 68:457-462.
Crossref
|
|
|
|
Cann AJ (1999). Antiviral testing. In Cann A (Ed.) Virus Culture: A practical approach, New York: Oxford University Press: pp. 201-219.
|
|
|
|
Dargan DJ (1998). Investigation of the anti-HSV activity of candidate antiviral agents. Methods in Molecular Medicine, Herpes Simplex Virus Protocols. (Edited by: Brown, SM, MacLean AR) Humana Press Inc. Totowa, NJ. pp.10:387-405.
|
|
|
|
De Clercq E (1993). Antivirals for the treatment of herpesvirus infections. J. Antimicrobial Chemother. 32(Suppl A):121-132.
Crossref
|
|
|
|
Del Barrio G, Parra F (2000). Evaluation of the antiviral activity of an aqueous extract from Phyllanthus orbicularis. J. Ethnopharmacol. 72:317-322.
Crossref
|
|
|
|
EI SN, Karakaya S (2009). Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 67(11):632-638.
Crossref
|
|
|
|
Erdohan ZO, Turhan KN (2011). Olive leaf extract and usage for development of antimicrobial food packaging. Science against microbial pathogens: communicating current research and technological advances, microbiology book series, published by Formatex. 2:1094-1101.
|
|
|
|
Fredrickson WR, F, S group Inc. (2000). Method and composition for antiviral therapy with olive leaves. U.S. Patent 6117844: Appl. No. 668324.
|
|
|
|
Goupy P, Hugues M, Boivin P, Amiot MJ (1999). Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agric. 79(12):1625-1634.
Crossref
|
|
|
|
Gupta R, Ramteke PW, Pandey H, Pandey AC (2013). Nano-structured herbal antimicrobials. Int. J. Pharm. Sci. Res. 4(6):2028-2034.
|
|
|
|
Ilias F, Kholkhal W, Gaouar N, Bekhechi C, Bekkara FA (2011). Antibacterial and antifungal activities of olive (Olea europaea L.) from Algeria. J. Microbiol. Biotech. Res. 1(2):69-73.
|
|
|
|
Jia L, Zhang D, Li Zi, Feng F, Wang Y, Dai W, Duan C, Zhang Q (2010). Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Delivery 17(1):11-18.
Crossref
|
|
|
|
Kesarwani K, Gupta R (2013). Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 3(4):253-266.
Crossref
|
|
|
|
Kogan A, Garti N (2006). Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 123-126:369-385.
Crossref
|
|
|
|
Korukluoglu M, Sahan Y, Yigit A, Ozer ET, Gucer S (2010). Antibacterial activity and chemical constitutions of Olea europaea L. leaf extracts. J. Food Processing Preservation 34:383-396.
Crossref
|
|
|
|
Kulkarni GT (2011). Herbal drug delivery systems: An emerging area in herbal drug research. J. Chronotherapy Drug Deliv. 2(3):113-119.
|
|
|
|
Lee-Huang S, Zhang L, Huang PL, Chang YT, Huang PL (2003). Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 307:1029-1037.
Crossref
|
|
|
|
Lee OH, Lee BY, Lee J, Lee HB, Son JY, Park CS, Shetty K, Kim YC (2009). Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 100:6107-6113.
Crossref
|
|
|
|
Markin D, Duek L, Berdicevsky I (2003). In vitro antimicrobial activity of olive leaves. Mycoses 46:132-136.
Crossref
|
|
|
|
Mattila P, Astola J, Kumpulainen J (2000). Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J. Agriculture Food Chem. 48(12): 5834-5841.
Crossref
|
|
|
|
Micol V, Caturla N, Pérez-Fons L, Más V, Pérez L, Estepa A (2005). The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV). Antivir. Res. 66:129-136.
Crossref
|
|
|
|
Moghimipour E, Salimi A, Karami M, Isazadeh S (2013). Preparation and characterization of Dexamethasone microemulsion based on pseudoternary phase diagram. Jundishapur J. Nat. Pharm. Prod. 8(3):105-112.
Crossref
|
|
|
|
Munin A, Edwards-Levy F (2011). Encapsulation of natural polyphenolic compounds; A review. Pharmaceutics 3:793-829.
Crossref
|
|
|
|
Petroni A, Blasevich M, Salami M, Papini N, Montedoro GF, Galli C (1995). Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thrombosis Res. 78(2):151-160.
Crossref
|
|
|
|
Ritchason J (1999). Olive leaf extract: Potent antibacterial, antiviral and antifungal agent. Woodland Publishing pp. 5-10.
|
|
|
|
Salah MB, Abdelmelek H, Abderraba M (2012). Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Med. Chem. 2(5):107-111.
|
|
|
|
Shoeib ARS, Zarouk AW, El-Esnawy NA (2011). Screening of antiviral activity of some terrestrial leaf plants against acyclovir-resistant HSV type-1 in cell culture. Aust. J. Basic Appl. Sci. 5(10):75-92.
|
|
|
|
Soliman SM, Abdel Malak NS, El-Gazayerly ON, Abdel Rehim AA (2010). Formulation of microemulsion gel systems for transdermal delivery of celecoxib: In vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Discov. Ther. 4(6):459-471.
|
|
|
|
Vijayan P, Raghu C, Ashok G, Dhanaraj SA, Suresh B (2004). Antiviral activity of medicinal plants of Nilgiris. Indian J. Med. Res. 120:24-29.
|
|
|
|
Zheng WW, Zhao L, Wei Y, Ye Y, Xiao S (2010). Preparation and the in vitro evaluation of nanoemulsion system for the transdermal delivery of Granisetron hydrochloride. Chem. Pharm. Bull. 58(8):1015-1019.
Crossref
|