Full Length Research Paper
ABSTRACT
The broad genetic diversity of HIV requires regular reassessment of adopted HIV diagnostic tests. The objective of this study was to reassess the performances of Determine® HIV 1/2 and Uni-gold® HIV1/2, 2 HIV rapid diagnostic tests of the national algorithm, adopted since 2005 in Central African Republic. A reference prospective panel of 150 plasma samples were tested in duplicate with Determine® HIV 1/2 and Uni-gold® HIV, according to reference serological immune-enzymatic method. Analytical and virological characteristics were calculated. Sensitivity, specificity, positive and negative predictive values (PPV, NPV) of Determine® HIV 1/2 were 100%, as well as the specificity and NPV of Uni-gold® HIV 1/2. Uni-gold® HIV 1/2 sensitivity and PPV were 96 and 92%, respectively. The Cohen k coefficient was close to 1 for the 2 tests, 1 for Determine® HIV 1/2 and 0.97 for Uni-gold® HIV 1/2. Except for the sensitivity of Uni-gold® HIV 1/2, the performances of 2 tests were acceptable and in perfect agreement with the reference tests. These results require a revision of the algorithm. In addition, an exploration in molecular biology is indicated to determine the subtypes of the HIV strains of the 4 samples that tested false negative with Uni-gold® HIV 1/2.
Key words: HIV, rapid diagnosis test, sensitivity, specificity, Africa, Central African Republic.
INTRODUCTION
According to Joint United Nations Program on HIV/AIDS (UNAIDS) 2019 report, 38 million people were living with HIV/AIDS (PLHIV) and 32.7 million people have died from HIV/AIDS since the start of the epidemic worldwide. Sub-Saharan African countries are heavily impacted by HIV/AIDS, with two-thirds of PLHIV and HIV/AIDS-related deaths of all cases worldwide (ONUSIDA, 2020). To eliminate HIV infection by 2030, the UNAIDS ‘‘95-95-95’’ goals recommend that first, 95% of the HIV-infected population should know their HIV status; second, 95% of those who know their status should receive antiretroviral therapy (ART); and finally, 95% of patients receiving ART should have a suppressed viral load (undetectable HIV RNA <50 copies/mL) after 2020 (UNAIDS, 2015). Thus, to control the HIV disease and achieve its elimination, HIV diagnosis appears to be the first step. In high HIV epidemic burden and limited resource settings, such as sub-Saharan African countries, reliable and accurate diagnostic tests of HIV is therefore crucial for assessing HIV status and introducing patients to a continuum of cares. Indeed, misdiagnosis leads to inappropriate decisions which often delay the initiation of ARV treatment during this period of the ‘‘test and treat’’ strategy. Although WHO periodically evaluates HIV rapid diagnostic tests (RDTs) to indicate which ones are prequalified for use in the developing countries, the World Health Organization (WHO) still recommends evaluating the performance of tests under local conditions of use on the field before adopting them in national strategies and their widespread use (CDC, WHO, APL, 2001; WHO, 2016).
This is, for example, the case of the Central African Republic, a country of approximately 5 million inhabitants with a national seroprevalence among people aged 15 to 45 years of 3.6%, that is, a total of 120,000 people living with HIV (spectrum). The Determine® HIV 1/2 (Alere, Japan) and Unigold® HIV 1/2 (Trinity Biotech, Ireland), 2 rapid tests, were selected in 2005 after a study in a sequential HIV screening algorithm that had a sensitivity and specificity of 100% and over 98%, respectively (Ménard et al., 2005). Many studies have subsequently shown that the CAR is a country where HIV strains of wide genetic diversity circulate, which is also a dynamic phenomenon with more than 70% of CRFs and the appearance of subtypes that were absent there (Gody et al., 2008; Charpentier et al., 2012; Mossoro et al., 2017). This genetic variability could be the source of under-detection of certain strains (Mossoro et al., 2016). It then becomes crucial to recheck the performance of these tests 16 years later to guarantee the algorithm's effectiveness in detecting all HIV strains. The objective of this study was to assess the performance of Determine® (Alere, Japan) and Unigold® (Trinity Biotech, Ireland), 16 years after their adoption in national strategies.
METHODS
This is a cross-sectional study that took place in Laboratoire National de Biologie Clinique et de Santé Publique in Bangui, the capital city of the CAR, which became the national reference laboratory (LNR) of HIV, with the implementation of the molecular biology unit in 2011. Among other tasks, it is responsible for evaluating diagnostic tests. A reference panel of 150 samples, including 100 positive and 50 negative plasmas were prospectively collected, which had been tested according to reference national serological algorithm for HIV testing, using in parallel Genscreen® ULTRA HIV Ag-Ab HIV-1/2 Version 2 (Bio-Rad, Marnes-la-Coquette, France) and Murex® HIV 1.2.0 Ag/Ab Combination (Diasorin, Saluggia, Italy), as the gold standard. All plasma were frozen at -80°C until processing. Genscreen® ULTRA HIV Ag-Ab HIV-1/2 Version 2 (Bio-Rad, Marnes-la-Coquette, France) and Murex® HIV 1.2.0 Ag/Ab Combination (Diasorin, Saluggia, Italy) are 4th generation ELISA tests which contain recombinant HIV-1 and 2 capsid and surface proteins. The reference panel was further tested with Determine® VIH 1/2 (Abbott, Japan) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland), following the instructions of the manufacturers, by two clinical microbiologists blinded regarding the sample groups. Indeterminate readings were further read by a third microbiologist. Determine® VIH 1/2 (Abbott, Japan) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) are 2 rapid immunochromatographic diagnostic tests whose reagents contain like ELISA reference tests, recombinant surface capsid and surface proteins of HIV-1 and 2. This alternative algorithm uses Determine® VIH 1/2 (Abbott, Japan Ag) rapid immunochromatographic test as a screening test and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) as a confirmatory test. For statistical analysis, sensitivity (Se) was calculated as the number of real positives divided by the sum of real positives plus false negatives. Specificity (Sp) was calculated as the number of real negatives divided by the sum of real negatives plus false positives. HIV-1 seroprevalence of 3.5% in general adult population of the CAR in 2019 ([email protected]) was used in calculating positive predictive value (PPV) and negative predictive value (NPV), according to Bayes’ formulae, as follows (Collectif BioBayes., 2015):
PPV = sensitivity × prevalence/[sensitivity × prevalence + (1-specificity) × (1-prevalence)]
NPV= specificity × prevalence/[(1-sensitivity) × prevalence + specificity × (1-prevalence)]
The confidence intervals for each variable was calculated at 95% (95%CI) using a normal distribution. The 95% CI of the estimated sensitivities, specificities, PPV, and NPV were calculated using the formula: f ± 1.96 [f (1-f) /n]1/2, where f is the sensitivity, the specificity, PPV, or NPV and n is the number of specimens tested. The Cohen’s coefficient κ (Cohen, 1960) was interpreted according to the Landis and Koch scale (< 0 as indicating no agreement, 0-0.20 as slight, 0.21-0.40 as fair, 0.41-0.60 as moderate, 0.61-0.80 as substantial, and 0.81-1 as near perfect agreement) (Landis and Koch, 1977). The study was approved by the Ethical and Scientific Committee, Faculty of Health Sciences, University of Bangui constituting the Institutional Ethical Committee.
RESULTS
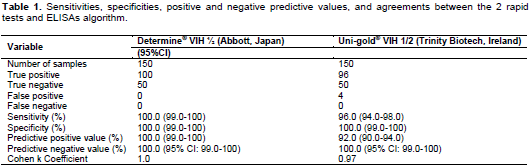
The results of the analytical performances of Determine® VIH 1/2 (Abbott, Japan) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) are depicted in Table 1. Among the 100 HIV samples known to be HIV-positive with ELISA algorithm, all were positive with Determine® VIH 1/2 (Abbott, Japan), 96 were positive and 4 were negative with Uni-gold® VIH 1/2 (Trinity Biotech, Ireland). All the 50 HIV samples known to be HIV-negative with ELISA algorithm were also negative with both rapid tests. Taken together, sensitivity and specificity of the Determine® VIH 1/2 (Abbott, Japan) were 100.0%, as well as the specificity of Uni-gold® VIH 1/2 (Trinity Biotech, Ireland).
But the sensitivity of Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) was 96.0% (95%CI: 94.0-98.0). The reliability of Determine® VIH 1/2 (Abbott, Japan) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) estimated by the Cohen’s k coefficient was 1 and 0.97, respectively, measuring the concordance between each rapid test and reference serological algorithm, demonstrating almost a perfect agreement (0.81-1) according to Landis and Koch scale. At HIV-1 seroprevalence of 3.5% in general adult population of the CAR in 2019 (UNAIDS, 2019), the PPV and NPV were 100% (95% CI: 99.0-100) for Determine® VIH 1/2 (Abbott, Japan), 92.0% (95% CI: 90.0-94.0) and 100.0% (95% CI: 90.0-100) for Uni-gold® VIH 1/2 (Trinity Biotech, Ireland), respectively.
DISCUSSION
The analytical performances of the sequential alternative algorithm which associates Determine® VIH 1/2 (Abbott, Japon) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) in the Central African Republic (CAR) were herein evaluated. The results of this assessment showed that both tests had the specificity, NPV of 100% such as sensitivity and positive predictive value of Determine. In contrast, Unigold's sensitivity and PPV were 96 and 92%, respectively. The virological performances of HIV rapid tests in the Central Africa Republic are yet poorly established. The evaluation of virological performances of Determine® VIH 1/2 (Abbott, Japon) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) was done in 2005 (Menard et al., 2005). In addition, Central Africa is characterized by the broad genetic diversity of HIV-1 strains (Gody et al., 2008; Charpentier et al., 2012; Mossoro et al., 2017) which can be associated with false negativity of HIV immunochromatographic rapid diagnostic tests (Aghokeng et al., 2009), and by a variety of factors which can be associated with false positivity or unspecific reactivities, including disturbances affecting the B cell-driven immunity during infectious diseases, such as marked immunological stimulation, strong nonspecific polyclonal B-cell activation, hypergammaglobulinemia, and production of circulating immune complexes (Klarkowski et al., 2014, 2013; Mbopi-Keou et al., 2014).
HIV rapid tests are more and more developed and used, particularly in resource-limited settings and may be of variable quality (UNITAID and WHO, 2018). In the present study, the analytical performances of Determine® VIH 1/2 (Abbott, Japan) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) in the CAR, were evaluated using a collection of 100 positive and 50 negative sera randomly selected through the CAR HIV seroprevalence surveillance survey. The results showed excellent analytical performances of Determine® VIH 1/2 (Abbott, Japan) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland), except for the sensitivity of Uni-gold® VIH 1/2 (Trinity Biotech, Ireland), despite the risk of false-positive results with frequent inconclusive sera in this area of Africa (Klarkowski et al., 2013; Mbopi-Keou et al., 2014). The sensitivity of Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) was 96.0% due to 4 positive samples with the reference tests which were negative with Uni-gold® VIH 1/2 (Trinity Biotech, Ireland). Finally, the analytical performances of Determine® VIH 1/2 (Abbott, Japan) were within the limits required by the WHO for HIV rapid tests (that is, sensitivity ≥99.0% and specificity ≥98.0% (WHO, 2016, 2017), likely allowing it to detect all HIV-1 strains circulating in the CAR. The sensitivity of the Uni-Gold HIV test (Trinity Biotech, Dublin, Ireland), which is low (96.0%) unlike the 2005 assessment (? 98.0%) (Menard et al., 2005) needs improvement. Previous studies showed similar results. In Tanzania, Determine® VIH 1/2 (Abbott, Japan) had high similar virological performances, sensitivity and specificity were 100% but specificity decreased (96.8%) when using whole blood (Kroidl et al., 2012). Likely, the sensitivity of Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) was 81.9% in Australia (Keen et al., 2017).
This study has some limitations. In particular, the number of blood samples is limited. Thus, the number of negative and positive specimens to be analyzed should be sufficiently high; a higher number of 200 in each case is recommended by the French accreditation committee (Comité Français d’accréditation, 2015). The determination of the subtypes of the 4 negative samples with Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) is indicated to understand the reasons for this negativity.
CONCLUSION
Taken together, the alternative rapid tests algorithm which associates Determine® VIH 1/2 (Abbott, Japan) and Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) in the CAR must be revised, 16 years after adoption. Thus, it may be suitable for routine use in the general population of the CAR. Further studies are necessary to understand the reasons for the negativity of Uni-gold® VIH 1/2 (Trinity Biotech, Ireland) in the 4 samples.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Aghokeng AF, Mpoudi-Ngole E, Dimodi H, Atem-Tambe A, Tongo M, Butel C, Delaporte E, Peeters M (2009). Inaccurate diagnosis of HIV-1 group M and O is a key challenge for ongoing universal access to antiretroviral treatment and HIV prevention in Cameroon. PLoS One 4(11):e7702. |
|
|
CDC, WHO, APL (2001). Guidelines for Appropriate Evaluations of HIV Testing Technologies in Africa. |
|
|
Charpentier C, Gody JC, Mbitikon O, Moussa S, Matta M, Péré H, Fournier J, Longo JDD, Bélec L (2012). Virological response and resistance profiles after 18 to 30 months of first- or second-/third-line antiretroviral treatment: a cross-sectional evaluation in HIV type 1-infected children living in the Central African Republic. AIDS Research and Human Retroviruses 28:87-94. |
|
|
Cohen J (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement 20:37-46. |
|
|
Collectif BioBayes (2015). Initiation à la statistique bayésienne: bases théoriques et qpplications en qlimentation, environnement, épidémiologie et génétique. Edition Ellipses Paris, 360P. |
|
|
Comité Français d'accréditation. (2015). Guide de vérification (portee A) /validation (portee B) des méthodes en Biologie Médicale: Document SH GTA 04 Révision 01. 191p. |
|
|
Gody JC, Charpentier C, Mbitikon O, Si-Mohamed A, LeGoff J, Gresenguet G, Bélec L (2008). High prevalence of antiretroviral drug resistance mutations in HIV-1 Non-B Subtype strains from african children receiving antiretroviral therapy regimen according to the 2006 revised WHO recommendations. Journal of Acquired Immune Deficiency Syndromes 49(5):566-569. |
|
|
Keen P, Conway DP, Cunningham P, McNulty A, Couldwell DL, Davies SC, Smith DE, Gray J, Holt M, O'Connor CC, Read P, Callander D, Prestage G, Guy R (2017). Multi-centre field evaluation of the performance of the Trinity Biotech Uni-Gold HIV 1/2 rapid test as a first-line screening assay for gay and bisexual men compared with 4th generation laboratory immunoassays. Journal of Clinical Virology 86:46-51. |
|
|
Klarkowski D, Glass K, O'Brien D, Lokuge K, Piriou E, Shanks L (2013). Variation in specificity of HIV rapid diagnostic tests over place and time: An analysis of discordancy data using a Bayesian approach. PLoS One 8(11):e81656. |
|
|
Klarkowski D, O'Brien DP, Shanks L, Singh KP (2014). Causes of false-positive HIV rapid diagnostic test results. Expert Review of Anti-infective Therapy 12:49-62. |
|
|
Kroidl I, Clowes P, Mwalongo W, Maganga L, Maboko L, Kroidl AL, Geldmacher C, Machibya H, Hoelscher M, Saathoff E (2012). Low specificity of determine HIV1/2 RDT using whole blood in south west Tanzania. PLoS One 7(6):e39529. |
|
|
Landis JR, Koch GG. (1977). The measurement of observer agreement for categorical data. Biometrics 33(1):159-174. |
|
|
Mbopi-Keou FX, Ndjoyi-Mbiguino A, Talla F, Péré H, Kebe K, Matta M, Sosso MA, Bélec L (2014). Association of inconclusive sera for human immunodeficiency virus infection with malaria and Epstein-Barr virus infection in Central Africa. Journal of Clinical Microbiology 52(2):660-662. |
|
|
Ménard D, Maïro A, Mandeng MJ, Doyemet P, Koyazegbe TA, Rochigneux C, Talarmin A (2005). Evaluation of rapid HIV testing strategies in under equipped laboratories in the Central African Republic. Journal of Virological Methods 126(1-2):75-80. |
|
|
Mossoro-Kpinde CD, Gody JC, Mboumba Bouassa RS, Mbitikon O, Jenabian MA, Robin L, Matta M, Zeitouni K, Longom JDD, Costiniuk C, Grésenguet G, Touré Kane NC, Bélec L (2017). High levels of virological failure with major genotypic resistance mutations in HIV-1-infected children after 5 years of care according to WHO-recommended 1st-line and 2nd-line antiretroviral regimens in the Central African Republic. Medicine (Baltimore) 96:10. |
|
|
Mossoro-Kpindé CD, Jenabian MA, Gody JC, Robin L, Talla P, Longo JDD, Grésenguet G, Belec L (2016). Evaluation of the Upgraded Version 2.0 of the Roche COBAS® AmpliPrep/COBAS® TaqMan HIV-1 Qualitative Assay in Central African Children. The Open AIDS Journal10:158-163. |
|
|
ONUSIDA (2020). Data sheet 2020-Latest statistics on the state of the AIDS epidemic. |
|
|
UNAIDS (2015). Understand fast track accelerating section to end the AIDS epidemic by 2030. |
|
|
UNAIDS (2019). Central African Republic Data sheet. |
|
|
UNITAID, WHO (2018). HIV rapid diagnostic tests for self-testing. 4th edition. Market and technology landscape. |
|
|
World Health Organization (WHO) (2016). Human immunodeficiency virus (HIV) rapid diagnostic tests for professional use and/or self-testing-TSS-1. |
|
|
World Health Organization (WHO) (2017). Technical Specifications Series for submission to WHO Prequalification-Diagnostic Assessment: Human immunodeficiency virus (HIV) rapid diagnostic tests for professional use and/or self-testing. Geneva: World Health Organization. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0