ABSTRACT
Cyclic lipopeptides (CLPs) with antibiotic, biosurfactant producing fluorescent pseudomonads were isolated from sugar beet-maize intercropped in sandy loam soils at Maize Research Station, Vagarai, TNAU. Approximately 20 fluorescent pseudomonads from sandy loam soils were isolated by using two different growth media. The strains were distinguished based on their growth, CFU/g, fluorescence, and pigment production. Growth inhibition of maize pathogens by CLP producing fluorescent pseudomonads strains were studied by dual culture experiments. The impact of CLP producing flurorescent pseudomonads strain on the zoospores of Downy mildew pathogen of maize was studied by direct microscopy and encysted zoospores were observed. Invitro, biochemical experiments confirmed the presence of Viscosinamide producing strain among the fluorescent pseudomonads isolates in terms of utilization of C and N sources. The particular strain was tested for its growth promoting activity by treating the maize seeds for their germination, and seedling vigour performance. Fluorescent pseudomonads can be affiliated to group under CLP producing biotypes/biovars. Purification of CLP (Viscosinamide) and characterization by HPLC analysis was carried out. Pot culture experiments were conducted to test the performance of CLP producing Pf strains in maize crop for testing their disease resistance. These biovars with antibiotic properties are the potential targets for the disease management in maize. CLPs in general receive considerable attention as potent antimicrobial drugs.
Key words: Cyclic lipopeptides, fluorescent pseudomonads, viscosinamide, zoospores, antifungal.
Biosurfactants are found to be structurally diverse in nature and are commonly synthesized by micro-organisms.The structure of biosurfactants comprise of a hydrophilic moiety of amino acids or peptide, anionsor cations, mono-or polysacharides, and a hydrophobic moiety consisting of fatty acids. Biosurfactants have been commonly classified as: (i) Low molecular-weight molecules, which decrease surface tension efficiently; and (ii) High-molecular weight polymers which bind to surfaces (Rosenberg and Ron, 1997). Low-molecular weight biosurfactants belongs to the classes of glycolipids or lipopeptides. Basically, biosurfactants have a number of advantages over chemical surfactants such as lower toxicity, higher biodegradability, environmental conditions (for instance temperature, pH and salinity). Large group of microbes are capable of producing biosur-factants, which includes Pseudomonas spp. strains producing rhamnolipids (Lang and Wullbrandt, 1999; Providenti et al., 1995; Shreve et al., 1995) and Bacillus sp. strains, producing surfactins (Fuma et al., 1993; Yakimov et al., 1995). Within the group of biosurfactant producing microbes, fluorescent pseudomonads received more atten-tion for the past two decades (Hotte and Altier, 2010; Raaijmakers and Mazzola, 2012; Olorunleke et al., 2015).
The role and applications of biosurfactants (mainly glycolipids and lipopeptides) have been investigated from medicinal and therapeutic properties. Cameotra and Makkar (2004) reviewed properties of biosurfactants as antimicrobial agents, immunoregulators, adhesives and desorptive agents in surgical procedures. Various Pseudomonas biocontrol strains produce CLP type biosurfactants (Olorunleke et al., 2015). CLPs are amphiphilic molecules composed of a cyclic oligopeptide lactone ring coupled to a fatty acid tail (Raaijmakers et al., 2010). CLPs possess broad spectrum of antibiosis against bacteria, fungi, protozoa and human tumor cell lines (Raaijmakers et al., 2010; Roongsawang et al., 2010). They are potential pharmaceutical candidates for the biological control of plant pathogens (Banat et al., 2010, Sachdev and Cameotra, 2013). Many cyclic lipopeptides are antimicrobial agents, among them Viscosinamide produced by Pseudomonas spp. isolated from sugarbeet rhizosphere has antibiotic properties towards root-pathogenic fungi (Nielsen et al., 2003). Screening of Pseudomonas spp. for their capability to produce cyclic lipopeptides is an important criterion for the selection of biological control agents, as it may be used as single strain/consortium of strains to improve multiple antagonistic traits.
Isolation of surfactant producing Pseudomonas spp. strains
Soil samples were collected from loamy sand, where maize crop was intercropped with sugarbeet and kept at 5°C until use. The samples were weighed for 50 g in polythene vials with the bulk density 1.1 g cm-3. Maize seeds were sown in vials (3 seeds/vial) and kept in 15°C under 16 h light and 8 h dark cycle. The seedlings were uprooted along with adhering soils and transferred to 10 ml
sterile 0.9% Nacl. The sample was vortexed for 1 min and sonicated for 0.5 min and plated in solid media.
High density population of Pseudomonas spp. was obtained in two different media: (i) On King’s B medium fluorescent Pseudomonas spp. were detected by exposing the agar plates with UV light (254 nm) and the fluorescent colonies were randomly picked. (ii) Gould’s S1 medium, containing 10 g sucrose, 10 ml of glycerol, 5 g of casamino acids, 1 g of NaHCO3, 1 g of MgSO4.7H2O, 2.3 g of K2HPO4, 1.2 g of sodium lauryl sulphate and 15 g of agar per liter was autoclaved, and then 5 ml of 100 mg of trimethoprim, 8.5 ml of methanol, and 16.5 ml of Milli-Q water was added to the medium. The colonies appearing in Gould´S1 selective medium were eligible for random picking.
Isolates from the two media were further streaked onto Gould’ S1 agar and checked for fluorescence before culturing in 3 ml of Luria-Bertani medium per liter containing 10 g of tryptone, 5 g of yeast extract, 10 g of Nacl, and 1 g of glucose pH 7.2 for subsequent preservation at -80°C.
Swarming and biofilm assays
Bacterial cells grown for 24 h on GS1 (Gould’s S1) medium agar plates were dissolved in sterile distilled water to a final density of 109 CFU ml-1 (OD600 = 1), pelleted by centrifugation and washed once with sterile distilled water. Swarming assays were performed on soft agar plates (KB medium with 0.6% (W/V) agar, five microlitres of the cell suspension were placed in the centre of a soft agar plate. The ability of the bacterial colony to spread was evaluated after 24, 48 and 72 h of incubation at 25°C (Neilson et al., 1999).
The biofilm assays were performed in flat-bottom non-detachable 96 wells plates (Nunc.ImmunoTMMicroWellTM, SIGMA-ALDRICH, USA) according to the methods described by O’Toole et al. (1999) and Bruine de Bruin et al. (2007). The 96 wells were filled with 180 µl of Gould’s S1 medium and 20 µl bacterial suspension (1×109 cells ml-1) and 20 µl bacterial suspension (1×109 cells ml-1) and incubated for 24 h at 25°C. Biofilms were stained with crystal violet and visualized at 600 nm (Bruine de Bruin et al., 2007). The biofilms were observed in side walls of the 96 well plates and the OD was measured at 600 nm.
Zoosporicidal and antifungal activity
Bacterial cell suspensions (109CFU ml-1) were prepared from colonies grown on GS1 plates for 48 h at 25°C. A 10 µl aliquot of the bacterial cell suspension was mixed on a glass slide with downy mildew zoospores (104 ml-1) in a 1:1 ratio (v:v). Zoospore lysis was observed microscopically at 100X magnification for up to 2 min. Dual culture inhibition assays were performed by spot inoculating fluorescent Pseudomonas to the edge of an agar plate and incubation for 3 days at 25°C followed by placing a fungal agar plug (5 mm diameter) to the centre of the plate and incubation at diverse temperatures for up to 14 days.
Growth analysis
Seeds were surface-sterilized for 5 min in 1% (w/v) sodium hypochlorite, rinsed in sterile distilled water, and allowed for uniform coating in talc formulations of Pf strains overnight at 25°C. Seeds were then sown on a layer of brown germination towel of thin,wet paper and rolled. Seedlings were grown for 15 day at 25°C, and were harvested when the shoots were 35 to 40 cm tall.
Structural diversity of Pseudomonas spp. surfactants
The surfactants of the Pseudomonas spp. were characterized by high-pressure liquid chromatography (HPLC). Analysis was performed after culturing of all isolates at 20°C for 2 days in 25 ml glass tubes with 3 ml of King’s B broth. Samples were obtained by extraction for 1 h with 5 ml of ethyl acetate containing 1% formic acid. The surfactant compounds wereanalyzed by HPLC using a Hypersil BDS C18 column (100 by 4.6 mm; 3 µM particle diameter) held at 40°C, and UV detection (200-400 nM) was performed on a Hewlett-Packard model 1100 HPLC diode array detector. The samples were analyzed in a gradient of 85% eluent B to 100% after 40 min. Eluent flow rate was 1 ml per min. Chromatograms were analyzed using the Hewlett-Packard Chemstation Software package. The identical surfactants were considered when retention times in HPLC chromatograms varied by less than 0.1 min with retention times of one/two major peak.
Statistical analysis
Data were subjected to statistical analysis by following CRD using standard procedure (Steel et al., 1997). The differences among treatment means were compared by applying the Duncan’s multiple range tests (DMR) (Duncan, 1955).
CLP producing pseudomonads
The abundance of fluorescent Pseudomonas spp. was
approximately 5×106 colonies per gram of rhizosphere soil sample when tested in two different media. Among the 20 strains, three were selected for their growth performance, CFU/g, fluorescence pigment production (Table 1). When a total of 20 fluorescent pseudomonads were tested for their frequencies of swarming, biofilm assays, 5 isolates were highly variable. Biosurfactant-producing Pseudomonas spp. strains were initially screened by drop collapse assay (Table 2).
HPLC analysis
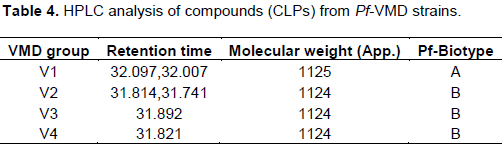
CLP producing Pf strains were subsequently verified by HPLC analysis. Peaks (retention time between 27 and 36 min) with the absorbtion spectra at approximately 200 nm (endpoint absorption) were identified as CLP producing Pf strains and they were found to be antifungal against major diseases of maize. Three strains were selected based on their color reactions in Hiassorted Rapid Biochemical Identification-Test kit (Table 3) based on their sugar utilization and subsequently used for the extraction of VMD for testing their antifungal potential, antiserum production and formulation.
Pf-VMD1 strain belongs to group 1 was colonized well in dual antibiotic selection pressure (Trimethoprim, Streptomycin) and tested against maize pathogens under field conditions.
Antifungal assay
Among the biosurfactant producing Pf strains from Maize
Sugar Beet Rhizosphere, three strains with maximum CFU/g were selected for testing for their antifungal potential against maize pathogens. The strain Pf-VMD1 exhibited highest antifungal activity against maize diseases under in vitro conditions (Figure 1). Production of metabolites, change in color of the media was observed in the Pf-VMD1 strain, when they were grown in dual culture against the pathogens (Data not shown). Downy mildew zoospores treated with Pf-VMD1 strain were lysed within 90 s at concentrations of 104 zoospores/ml (Figure 3).
Growth promotion
The Pf VMD1 strain improved the seedling growth, when applied as seed treatment. Increased root lengths with numerous lateral roots were observed (Figure 2). These isolates inhabitants of loamy sand soil were able to produce CLPs. The data further indicating that the soil type may be important for the frequency of CLP-producing strains, since they were isolated from sandy loam soil at Maize Research Station, Vagarai based on the findings of Nielsen and Sorensen (2003).
Biosurfactants are amphipathic molecules with a hydrophilic and a hydrophobic moiety, that localize preferentially at the interface between fluid phases with different degrees of polarity such as oil/water or air/water interfaces. Swarming and biofilm assays in the present study proved the presence of such compounds in Pf-VMD strains isolated from sugar beet/maize rhizosphere soil. Lipopeptides form an important group of biosurfactants which are produced by a large variety of bacteria from different genera such as Bacillus, Lactobacillus, Streptococcus, Serratia, Burkholderia, and Pseudomonas (Velraeds et al., 2000; Mireles et al., 2001; Huberet et al., 2002). Several chemical and biological aspects of CLP production in fluorescent pseudomonads has been discussed by Nybroe and Sorensen (2004). In a recent review, Raaijmakers et al. (2010) have highlighted the structural diversity and activity of CLPs produced by plant-associated Pseudomonas spp. Many of the CLPs have 9 or 11 amino acids in the peptide ring with a C10 fatty acid at one of the amino acids (Nielsen et al., 2002). HPLC analysis of purified compounds confirmed the presence of Viscosinamide (Based on the retention time between 27 and 36 min) in this present study.
The “V” group are assigned under Viscosinamide-like compound producers, will produce CLPs with MW value of approximately 1120 to 1125 with the retention time between 31 and 33 min. We have observed 5 out of 20 surfactant- producing isolates form one single group V1, since one major surfactant peak was present in all the isolates. The V1 group surfactant viscosinamid was produced by 25% of the strains (Table 4). We found an interesting result of CLP producing fluorescent Pseudomonads inhabiting maize/sugar beet intercropping in sandy loam soils. Latour et al. (1996) reported that the diversity of CLP producing microbes was mainly influenced by soil type and less by crop type. Similar findings were observed by Bachmann and Kinzel (2001). Apart from these factors, Hoper et al. (1995) suggested that basic soil characteristics such as pH and texture may influence the density of fluorescent pseudomonads. Based on the length and composition of the fatty acid chain as well as the peptide chain, CLPs of Pseudomonas species were classified into four major groups, that is, the Viscosin, amphisin, tolaasin, and syringomycin groups (Raaijmakers et al., 2010). The Viscosin class harbours CLPs with 9 amino acids and Pseudomonas sp. producing this class of CLPs originate from diverse environmental niches including soil, rhizosphere, phyllosphere, as well as marine environments (Raaijmakers et al., 2010). Pseudomonas-derived CLPs are currently divided in eight different structural groups that differ in length and composition of the oligopeptide and fatty acid tail (Olorunleke et al., 2015). The CLPs from the syringomycin class show structural similarity with Viscosin group but contain unused amino acids including Dhb, or 2,4-diamino butyric acid and the lactose ring is formed between the N-terminal and the C-terminal amino acids whereas the ring is formed between the C-terminal amino acid and the 3rd amino acid in the peptide moiety for Viscosin.
In this study, CLP production in Pseudomonas spp. isolates from the maize rhizosphere, the exclusive assignment of Viscosinamide production (group V1) P. fluorescens biovar I was reported. Membrane interaction and pore formation are often assumed to lie behind the antimicrobial activities of these molecules (Lo Cantore et al., 2006). Pore formation has also been suggested as the mechanism responsible for the adverse effect of Viscosinamide on zoospores of maize downy mildew pathogen (NeilsGeudens et al., 2014).
In a search for the fungal inhibition action of Viscosinamide, Thrane et al. (1991) found that the compound inhibits growth by formation of ion-channels in the fungal membrane. This phenomenon has sub-sequently been confirmed by challenging an Aspergillus awamori transformant expressing the Ca2+ -sensitive protein aequorin with viscosinamide. The fungus responded to the viscosinamide by a large and immediate increase in cytoplasmic Ca2+ -level. Warburton and Deacon (1998) have shown that the permeability of zoospores of Phytopthora parasitica increased due to intake of Ca2+ just before encystment, resulting in higher intracellular Ca2+ levels could thus explain why viscosinamide triggered instant encystment of the fungal zoospores on non-plant surfaces in this study. Compounds with surfactant properties have been successfully deployed in hydroponic systems to control zoospore-producing fungal pathogens (Stanghellini et al., 1997). Apart from the antifungal action of Viscosinamide, it is also involved in the primary metabolism, cell proliferation and strongly binds to the producing cells of the strain DR54 (Nielsen et al., 2000). The findings of the present research also emphasize the above said informations on Viscosinamide-like compounds.
Since, the CLP producers are having synergistic effect of surface motility and the synthesis of antifungal compounds; they could efficiently check and terminate growth of pathogen and could prevent the plants from infection by the pathogen (Koch et al., 2002; Alsohim et al., 2014).
Antimicrobial biosurfactant producing fluorescent Pseudomonads biovar (Pf-VMD1) was isolated from maize/sugar beet rhizosphere in sandy loam soil and tested for the presence of viscosinamide by HPLC analysis. The strain performed its antifungal activity against major fungal disease of maize with zoosporicidal activity against downy mildew pathogen of maize. The strain is grouped under V1 (Viscosinamide-like compounds producers).
The authors have not declared any conflict of interests.
REFERENCES
|
Alsohim AS,Taylor TB,Barrett GA, Gallie J, Zhang XX, Altamirano-Junqueira AE, Johnson LJ, Rainey PB and Jackson RW (2014). The biosurfactant viscosin produced by Pseudomonas fluorescens SBW25 aids spreading motility and plant growth promotion. Environ.Microbiol. 16:2267-2281.
Crossref
|
|
|
|
Bachmann G, and Kinzel H (2001). Physiological and ecological aspects of the interactions between plant roots and rhizosphere. Biochem. 24:543-552.
|
|
|
|
|
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti M.G, Fracchia L (2010).Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 87:427-444.
Crossref
|
|
|
|
|
Bruine de Bruin W, Parker AM, Fischhoff B (2007). Individual differences in adult decision-making competence. J. Person. Soc. Psychol. 92(5):938-956.
Crossref
|
|
|
|
|
Cameotra S, Makkar RS (2004). Recent application of biosurfactants as a biological and immunological molecules. Curr. Opin. Microbiol. 7:262-66.
Crossref
|
|
|
|
|
Duncan DB (1955). Multiple range and multiple F-test. Biometrics 11:1-42.
Crossref
|
|
|
|
|
Fuma S, Fujishima Y, Corbell N, D'Souza C, Nakano MM, Zuber P & Yamane K (1993). Nucleotide sequence of 5' portion of srfA that contains the region required for competence establishment in Bacillus subtilis. Nucleic Acids Res. 21:93-97.
Crossref
|
|
|
|
|
Höfte M, Altier N (2010).Fluorescent pseudomonads as biocontrol agents for sustainable agricultural systems. Res. Microbiol. 161:464-471.
Crossref
|
|
|
|
|
Hoper H, Steinberg C, Alabouvette C (1995). Involvement of clay type and pH in the mechanisms of soil suppressiveness to Fusarium wilt of flax. Soil Biol. Biochem. 27:955-967.
Crossref
|
|
|
|
|
Koch B, Nielsen T H, Sorensen D, Andersen JB, Christophersen C, Molin S, Givskov M, Sorensen J and Nybroe O (2002). Lipopeptide production in Pseudomonas sp. strain DSS73 is regulated by components of sugar beet exudates via the Gactwocomponent regulatory system. Appl. Environ. Microbiol. 68:4509-4516
Crossref
|
|
|
|
|
Lang S, Wullbrandt D (1999). Rhamnose lipids-biosynthesis, microbial production and application potential. Appl. Microbiol. Biotechnol. 51(1):22-32.
Crossref
|
|
|
|
|
Latour X, Corberand T, Laguerre G, Allard F, Lemanceau P (1996). The composition of fluorescent pseudomonad populations associated with roots is influenced by plant and soil type. Appl. Environ. Microbiol. 62:2449-2456.
|
|
|
|
|
Laycock MV, Hildebrand PD, Thibault P, Walter JA, Wright JLC (1991). Viscosin, a potent peptidolipidbiosurfactant and phytopathogenic mediator produced by a pectolytic strain of Pseudomonas fluorescense. J. Agric. Food Chem. 39:483-489.
Crossref
|
|
|
|
|
Mireles II JR, Toguchi A, Harshey RM (2001). Salmonella entericserovartyphimurium swarming mutants with altered biofilm-forming abilities: Surfacin inhibits biofilm formation. J. Bacteriol. 183: 5848-5854.
Crossref
|
|
|
|
|
Niels Geudens, Matthias de Vleeschouwer, Krisztina Feher, Hassan Rokni-Zadeh, Maarten GK. Ghequire, AnnemiekeMadder, Rene de Mot, Jose C. Martins, Davy Sinnaeve (2014). Impact of a stereocentre inversion in cyclic lipodepsipeptides from the Viscosin group: A comparative study of the Viscosinamide and Pseudodesmin conformation and self assembly. Chem. Bio-Chem.15:2736-2746.
Crossref
|
|
|
|
|
Nielsen R, Tarpy DR, Reeve HK (2003). Estimating effective paternity number in social insects and the effective number of alle-les in a population. Molec. Ecol. 12:3157-3164.
Crossref
|
|
|
|
|
Nielsen TH and Sorensen J (2003). Production of Cyclic Lipopeptides by Pseudomonas fluorescens Strains in Bulk Soil and in the Sugar Beet Rhizosphere. Appl. Environ. Microbiol.69. 861–868.
Crossref
|
|
|
|
|
Nielsen TH, Sorensen D, Tobiasen C, Andersen JB, Christophersen C, Givskov M and Sorensen J (2002). Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl. Environ. Microbiol. 68: 3416-3423.
Crossref
|
|
|
|
|
Nielsen TH, Thrane C, Christophersen C, Anthoni U and Sorensen J (2000). Structure, production characteristics and fungal antagonism of tensin—a new antifungal cyclic lipopeptide from Pseudomonas fluorescens strain 96.578. J. Appl. Microbiol. 89:992-1001.
Crossref
|
|
|
|
|
NielsonTH,Christopherson C,Anthoni U and SorensenJ (1999). Viscosinamide, a new cyclic depsipeptide with surfactant and antifungal properties produced by Pseudomonas fluorescens DR54. J. Appl. Microbiol. 87(1):80-90.
Crossref
|
|
|
|
|
O'Toole GA, Pratt L A, Watnick P I, Newman D K, Weaver VB. & Kolter R (1999). Genetic approaches to the study of biofilms. Methods Enzymol. 310:91-109.
Crossref
|
|
|
|
|
Olorunleke FE, KieuNP and Höfte M (2015). Recent advances in Pseudomonas biocontrol,"in Bacterial-Plant Interactions: Advance Research and Future Trends, eds Murillo J, Vinatzer BA,Jackson RW,and Arnold Norfolk DL : Caister Academic Press), Pp.167-198.
|
|
|
|
|
Providenti MA, FlemmingCA, Lee H, Trevors JT (1995). Effect of addition of rhamnolipidbiosurfactants or rhamnolipid producing Pseudomonas aeruginosa on phenanthrene mineralization in soil slurries. FEMS Microbiol. Ecol. 17:15-26.
Crossref
|
|
|
|
|
Raaijmakers JM, deBruijn I, deKock MJ (2006). Cyclic lipopeptide production by plant-associated Pseudomonas spp.:diversity, activity,biosynthesis,andregulation. Mol. Plant Microbe Interact. 19:699-710.
Crossref
|
|
|
|
|
Raaijmakers JM, Mazzola M (2012).Diversityandnaturalfunctionsof antibiotics produced by beneficial and plant pathogenic bacteria. Annu.Rev. Phytopathol. 50:403-424.
Crossref
|
|
|
|
|
Raaijmakers JM, deBruijn I, Nybroe O and Ongena M (2010). Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol. Rev. 34:1037-1062.
Crossref
|
|
|
|
|
Roongsawang N, Washio K, Morikawa M (2010). Diversityof non ribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int. J. Mol. Sci. 12:141-172.
Crossref
|
|
|
|
|
Rosenberg E, Ron EZ (1999). High- and low-molecular-mass microbial surfactants. Appl. Microbiol. Biotechnol. 52:154-162.
Crossref
|
|
|
|
|
Sachdev DP, Cameotra SS (2013). Biosurfactants in agriculture. Appl.Microbiol.Biotechnol. 97:1005-1016.
Crossref
|
|
|
|
|
Shreve BR, Moore JPA, Daniel TC, Edwards DR and Miller DM (1995).Reduction of phosphorous in runoff from field applied poultry litter using chemical amendments. J.Environ.Qual.24:106-111.
Crossref
|
|
|
|
|
Stanghellini ME, Miller RM (1997). Biosurfactants: Their identity and potential efficacy in the biological control of zoosporic plant pathogens. Plant Dis. 81:4-12.
Crossref
|
|
|
|
|
Steel RGD, Torrie JH, Dicky DA (1997). Principles and Procedures of Statistics-A Biometrical Approach (3rd Ed.) McGraw-Hill Book International Co., Singapore.
|
|
|
|
|
Thrane C, Olsson S, Nielsen TH, Sorenen J (1999). Vital fluorescent strains for detection of stress in Pythium ultimum and Rhizoctoniasolani challenged with viscosinamide from Pseudomonas fluorescens DR 54. FEMS Microbiol. Ecol. 30:11-23.
Crossref
|
|
|
|
|
Velraeds MM, van de Belt-Gritter B, Busscher HJ, Reid G, van der Mei HC (2000). Inhibition of uropathogenic biofilm growth on silicone rubber in human urine by lactobacilli-a teleologic approach. World J. Urol. 18:422-426.
Crossref
|
|
|
|
|
Warburton AJ, Deacon JW (1998). Transmembrane Ca2+ fluxes associated with zoospore encystment and cyst germination by the phyto pathogen. Phytopthora parasitica. Fungal Genet. Biol. 25:54-62.
Crossref
|
|