ABSTRACT
This study aimed to investigate the in vitro antioxidant activity of the novel anticonvulsant levetiracetam and benzodiazepine clonazepam. To do this, the mice brain homogenates were incubated with levetiracetam (50, 100 or 200 ïg/ml) or clonazepam (50, 100 or 200 ïg/ml), and then, submitted to heating at 37°C for 1 h. Ascorbic acid (vitamin C, 200 ïg/ml) was used as reference antioxidant drug. The markers of oxidative stress, such as lipid peroxidation, nitrite-nitrate content, catalase activity, and reduced glutathione (GSH) levels, were measured in brain homogenates. The group submitted to the heating-induced oxidative stress showed an increase in lipid peroxidation, nitrite-nitrate content, and catalase activity. Previous incubation with levetiracetam and clonazepam, mainly at lower doses (50 and 100 ïg/ml), and similarly to vitamin C, prevented these pro-oxidative changes, reducing the lipid peroxidation, nitrite-nitrate contents and catalase activity, and increasing GSH levels. These findings demonstrate antioxidant properties of levetiracetam and clonazepam, and help to elucidate the role of protection against oxidative stress in the neuroprotective mechanism of antiepileptic drugs.
Key words: Levetiracetam, clonazepam, oxidative stress, vitamin C, antiepileptic drugs.
The role of free radical-mediated reactions in human neuropathology continues to attract interest. The production of free radicals is associated with injury to cell structures and the pathogenesis of many neurological disorders, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and epilepsy (Li et al., 2013; Kong and Lin, 2010). Over-accumulation of reactive oxygen species (ROS) caused by imbalance between the generation and elimination of these entities frequently results in severe harmful effects to cells, a phenomenon known as oxidative stress (OS) (Gutteridge and Halliwel, 2010; Kong and Li, 2010). Furthermore, in sufficiently high concentrations, nitrogenous compounds, mainly nitric oxide (NO), can also damage cellular structures in a process known as nitrosative stress (NS). This occurs through nitrosation of amines and thiols, which also results in an excess of oxidative species and subsequent molecular damage (Aguiar et al., 2012).
In this context, OS and NS are possible mechanisms implicated in the pathogenesis of epilepsy (Chang and Yu, 2010). Studies have already verified that status epilepticus changes redox potential and decreases the level of ATP, which can lead to a collapse in brain energy production and supply (Aguiar et al., 2012; Wasterlain et al., 1993). Moreover, it was demonstrated that an increase in mitochondrial OS and NS occurs after persistent seizures and promotes subsequent dysfunction of this organelle and cell death (Freitas et al., 2010; Ueda et al., 1997). Therefore, since mitochondrion is the main source of ATP for neurons and has an important role in the homeostasis of intracellular calcium, injury to it may strongly affect neuronal excitability and synaptic transmission (Chang and Yu, 2010). Further support for the role of free radicals in seizures comes from the successful use of exogenously administered antioxidants, as vitamin C and vitamin E, to protect the brain against seizure-induced damage (Barros et al., 2007; Murashima 1998; Santos et al., 2009; Ueda et al., 1997).
The second-generation antiepileptic drug levetiracetam (LEV) is a new molecule that is clearly differentiated from conventional antiepileptic drugs by its pharmacologic properties and mechanisms of action (Gibbs et al., 2006; Lyseng-Williamson 2011; Ueda et al., 2009). Although the exact molecular mechanism of action of LEV remains uncharacterized, it is known that this drug binds to synaptic vesicle protein 2A. By binding to this vesicle, this drug appears to act as a modulator of synaptic vesicle exocytosis, leading to direct inhibition of pre-synaptic neurotransmitter release (Rigo et al., 2002). Moreover, LEV also has been previously demonstrated to protect against oxidative stress-induced neurotoxicity in several models of seizures (Oliveira et al., 2007; Ueda et al., 2009; Zona et al., 2001).
Benzodiazepines, such as clonazepam (CNZ), are established and important agents for the treatment of seizures and epilepsy (Talarek and Fidecka, 2003). Studies have suggested the antioxidant properties of some benzodiazepines. CNZ has been shown to have a protective effect against free radical mediated brain damage in models of diabetic and depressive-like behavior in mice, which involve an oxidative stress process (Haeser et al., 2007; Wayhs et al., 2013a; Wayhs et al., 2013b). Moreover, nitric oxide synthase (NOS) inhibitors can potentiate the anticonvulsant action of benzodiazepines, which suggests the participation of NO system, and possibly of the NS, in the neuroprotective effect of these drugs (Borowicz et al., 2000; Talarek and Fidecka, 2003); However, although the literature suggests that the anticonvulsant effects of antiepileptic drugs (AEDs) could be related to their antioxidant properties, the basis of these properties is still unclear. Therefore, this work aimed to investigate the antioxidant activity of the novel anticonvulsant levetiracetam and benzodiazepine anticonvulsant clonazepam. The study also aimed to investigate how these AEDs could counteract oxidative stress in brain tissues. The investigation of the antioxidant activity of these AEDs and their possible neuroprotective effect may provide stronger evidences for the hypothesis of the involvement of oxidative damage in the pathophysiology of epilepsy.
Drugs
Levetiracetam (Keppra®) was obtained from UCB Pharmaceutical Sector (Chemin du Foriest, Belgium); clonazepam (Rivotril®) was purchased from A F Hoffmann-La Roche AG (Brazil); ascorbic acid (vitamin C) was obtained from Sigma-Aldrich® (St. Louis, MO, USA). All other chemicals were purchased from Sigma-Aldrich® (St. Louis, MO, USA).
Animals
Experimentally naive, male Swiss mice Mus musculus, from the Animal House of the Federal University of Ceará, weighing 25 to 30 g, were used. The animals were maintained at a controlled temperature (24±2°C) with a 12-h dark/light cycle and food and water ad libitum. Mice were caged in groups of 8 in a 41 × 34 × 16 cm cage.
The animals were used according to the NIH Guide for the Care and Use of Laboratory Animals. The experiments were performed after approval of the protocol by the Ethics Committee on Animal Research of the Federal University of Ceará (with protocol number 59/07), in accordance with the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA). Moreover, the experiments were performed to minimize the number of rats and their suffering, following the ethical doctrine of the three “R”s: Reduction, Refinement and Replacement.
Assessment of antioxidant capacity in vitro
Antioxidant activity of the compounds was evaluated by measuring the production of thiobarbituric acid (TBARS), as an indicator of lipid peroxidation, reduced glutathione (GSH) levels and catalase activity. In order to assess the effects of these drugs on nitric oxide production, nitrite-nitrate levels were determined. Mice were decapitated and the brains were removed rapidly under standard conditions at 4°C. The whole brain, apart from the cerebellum, was homogenized in 50 mM potassium phosphate buffer (pH 7.4) and the concentration adjusted to 1 g wet weight of brain per 60 ml. Then, 250 ml of the brain homogenates was maintained in the absence or presence of LEV (50, 100 or 200 mg/ml), CNZ (50, 100 or 200 mg/ml) or vitamin C (VIT C) (200 mg/ml) for 24 h at 10°C. Following this, oxidative stress was induced by incubation of the brain homogenates for 1 h at 37°C with samples without oxidative stress acting as controls (Auddy et al., 2003; Mattei et al., 1998; Stocks et al., 1974).
Measurement of TBARS
The homogenates were incubated in a water bath for 1 h at 37°C. After incubation, assessment of the levels of thiobarbituric acid concentration was performed according to the method described by Houng et al. (1998), with absorbance measured at a wavelength of 532 nm and expressed as micromol of malondialdehyde (MDA)/mg of protein. The protein concentration was determined by using Lowry assay (Lowry et al., 1951).
Nitrite-nitrate determination
For the assessment of nitrite-nitrate, derived from nitric oxide (NO), 100 ml of Griess reagent (1% sulfanilamide dissolved in 5% H3PO4, 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride and distilled water, 1:1:1:1) was added to 100 ml of brain homogenate or to 100 ml of NaNO2 at concentrations ranging from 0.75 to 100 mM (standard curve) or for the blanks to 100 ml of the buffer used in the homogenates. The absorbance was measured with a reader plate at 560 nm (Green et al., 1981).
Measurement of catalase activity
This was measured by using hydrogen peroxide to generate H2O and O2. The substrate mixture contained 0.3 ml of hydrogen peroxide in 50 ml of 0.05 M phosphate buffer, pH 7.0. The sample aliquot (20 ml) diluted with phosphate buffer was added to 980 ml of the substrate mixture. Absorbances were read after 1 and 6 min at 230 nm. A standard curve was established using purified catalase (Sigma MO, USA) and the results were expressed as mM/min/mg protein (Chance and Maehly, 1955). Protein measurement was performed with the Lowry assay (Lowry et al., 1951).
Determination of glutathione (GSH) levels
GSH levels were evaluated to estimate endogenous defenses against oxidative stress. The method was based on the reaction of Ellman’s reagent (DTNB) with free thiol groups. The measurement of this parameter was performed according to the test steps of Sedlak and Lindsay (1998). GSH concentration was determined by the absorbance at 412 nm and was expressed as mg of GSH/mg of protein. The protein concentration was determined with the Lowry assay (Lowry et al., 1951).
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). Analysis of variance (ANOVA) was used for the statistical analysis followed by Student–Newman–Keuls test to identify differences between experimental groups. Values of p < 0.05 were considered significant. The results of VIT C group was compared with internal groups for each antiepileptic drug, LEV or CNZ, studied in this work.
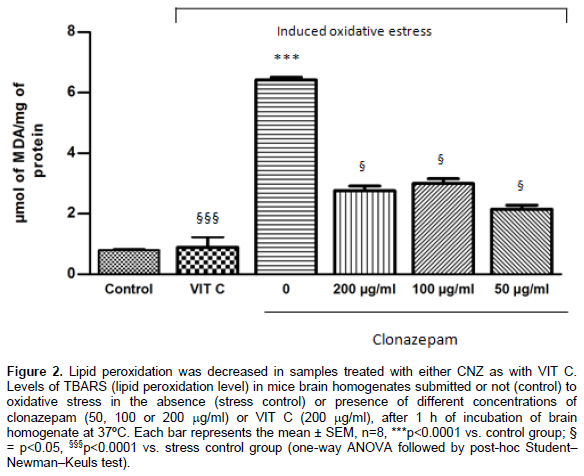
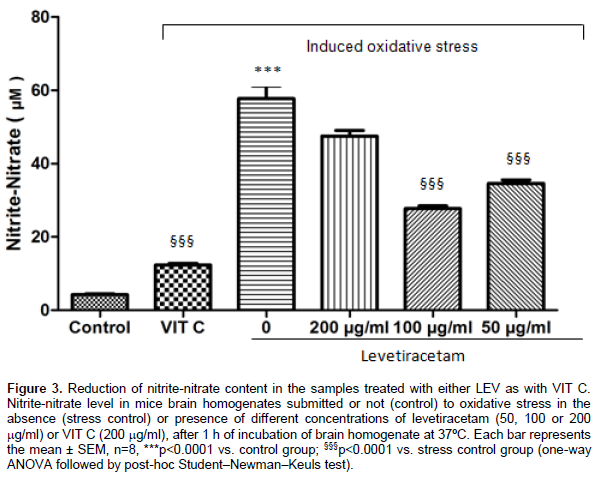
According to in vitro antioxidant assays, brain homogenates exposed to oxidative stress, without pre-incubation with CNZ, LEV or VIT C, (stress control) showed an increase in the lipid peroxidation and nitrite-nitrate contents, when compared with brains not exposed to oxidative stress (control) (p<0.0001). In comparison, previous incubation with LEV (p<0.001) or CNZ (p<0.05), at all tested concentrations, and with VIT C (p<0.0001) diminished the TBARS levels when compared with stress control. Regarding nitrite-nitrate concentrations, LEV, only at concentrations of 50 and 100 mg/ml (p<0.0001), CNZ, at all tested concentrations (p<0.05), and VIT C (p<0.0001) were able to significantly reduce nitrite-nitrate concentration when compared with stress control. Results of TBARS measurements: LEV (Control: 0.87 + 0.08; Stress control: 5.01 + 0.44; LEV200: 1.21 + 0.11; LEV100: 1.17 + 0.15; LEV50: 0.94 + 0.09; VIT C: 0.89 + 0.6) (Figure 1); CNZ (Control: 0.79 + 0.05; Stress control: 6.57 + 0.15; CNZ200: 2.76 + 0.27; CNZ100: 3.00 + 0.27; CNZ50: 2.15 + 0.23; VIT C: 0.89 + 0.6) (Figure 2), results were expressed in mmol of malondialdehyde (MDA)/mg of protein. Results of nitrite-nitrate levels: LEV (Control: 4.25 + 0.42; Stress control: 57.73 + 5.96; LEV200: 47.53 + 2.59; LEV100: 27.76 + 1.22; LEV50: 34.61 + 1.69; VIT C:12.35 + 0.8) (Figure 3); CNZ (Control: 4.81 + 0.39; Stress control: 58.56 + 5.26; CNZ200: 33.18 + 1.38; CNZ100: 27.76 + 1.22; CNZ50: 27.57 + 1.21; VIT C: 12.35 + 0.8) (Figure 4), results were expressed in μM.


A significant increase in catalase activity was observed in the group of homogenates submitted to oxidative stress (stress control) as compared to the control group (p<0.0001). In the samples pre-incubated with both test drugs, LEV (p<0.0001) and CNZ (p<0.05), at all concentrations, the catalase activity was reduced compared to the stress control, showing that LEV and CNZ pre-incubation was able to keep catalase activity at normal levels. Pre-incubation with VIT C also demonstrated a significant reduction of catalase activity compared to the stress control (p<0.0001). Results of catalase activity: LEV (Control: 8.24 + 0.80; Stress control: 42.37 + 4.05; LEV200: 4.99 + 1.31; LEV100: 7.09 + 1.61; LEV50: 7.34 + 1.35; VIT C: 6.68 + 0.38) (Figure 5); CNZ (Control: 7.40 + 0.60; Stress control: 38.15 + 4.15; CNZ200: 1059 + 1.03; CNZ100: 13.42 + 1.93; CNZ50: 10.06 + 0.96; VIT C: 6.68 + 0.38) (Figure 6), results were expressed in μM/min/mg protein.
.png)

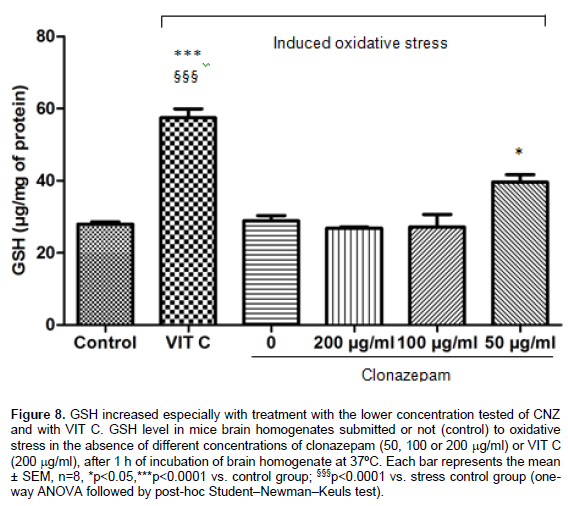
No alteration in GSH levels was observed in the samples after the in vitro induced oxidative stress. An increase in GSH level was shown at the lowest concentration of CNZ (50 mg/ml) compared to the control group (p < 0.05). An increase in GSH was also demonstrated with the two lowest tested concentrations of LEV (50 and 100 mg/ml) as compared to the control (p < 0.0001) and to the stress control (p<0.001). An increase in GSH levels also occurred in samples with VIT C as compared to the control (p < 0.001) and to the stress control (p<0.001). Results of GSH levels: LEV (Control: 34.23+ 3.14; Stress control: 38.76+ 3.89; LEV200: 50.26 + 5.17; LEV100: 79.59 + 7.63; LEV50: 69.03 + 5.89; VIT C:57.4 + 4.31) (Figure 7); CNZ (Control: 27.96 + 1.12; Stress control: 28.87 + 2.54; CNZ200: 26.86 + 0.49; CNZ100: 28.44 + 0.06; CNZ50: 33.89+ 1.54; VIT C: 57.4 + 4.31) (Figure 8), results were expressed in μg/μg of protein.
In this study, to further investigate the antioxidant properties of levetiracetam and clonazepam, these drugs were tested in an established and specific in vitro model of oxidative stress, proposed by Stocks et al. (1974) and modified by Mattei et al. (1998) and Huong et al. (1998). This model is based on the fact that brain homogenates autoxidize spontaneously and reproducibly when heated to 37°C for 1 h, and this preparation may be used to assay the antioxidant effect of several agents in neuronal substrate (Huong et al., 1998; Mattei et al., 1998; Stocks et al., 1974). Moreover, to verify the potential antioxidant capacity of these AEDs in this model, the results obtained were compared with these drugs with the powerful water-soluble antioxidant vitamin C (VIT C). Previous studies have demonstrated not only the well-recognized antioxidant effects of VIT C, but also its anticonvulsant action, suggesting that this drug behaves as a potential neuronal protective agent, capable of attenuating the behavioral and pro-oxidative changes involved in development of seizures (Santos et al., 2009; Xavier et al., 2007).
Our results showed, as expected, that samples submitted to this in vitro stress model showed an increase in MDA and nitrite-nitrate concentrations and higher levels of catalase activity when compared with control samples not submitted to heating. These findings clearly indicate that an oxidative process occurred in brain homogenates submitted to this model. Furthermore, the elevation in free radical formation can be accompanied by a rapid compensatory increase in the activity of free radical scavenging enzymes (Ferreira and Matsubara, 1997; Bellissimo et al., 2001). The high level of catalase activity observed in our results suggests the development of an adaptive mechanism against oxidative stress and further supports that this process occurs in this model.
In a similar fashion to VIT C, treatment with LEV and CNZ, at all doses tested, was able to prevent the elevation of MDA levels induced by in vitro stress model, with values of MDA similar to those observed in the control group. Regarding nitrite-nitrate concentrations, both AEDs demonstrated the capacity to reduce the changes induced by heating, mainly at the lowest doses. VIT C was also able to reduce considerably the nitrite-nitrate concentrations in brain homogenates. Catalase activity was restored to control levels with pre-incubation with LEV and CNZ, and the same effect was observed in samples incubated with VIT C. Finally, the GSH amount was increased by the incubation with both AEDs, especially in lowest doses, and also by incubation with VIT C, when compared with groups submitted to stress procedure.
The degree of lipid peroxidation in tissues is commonly measured by determining the amount of mean product of this process, MDA, and this analysis may relate directly to the level of ROS-mediated injury to a tissue (Kunz et al., 2008; Schihiri, 2014). Free radical-mediated lipid peroxidation proceeds by a chain mechanism, that is, once it was initiated, free radicals can oxidize both lipid molecules in biological membranes and low density lipoproteins. This phenomenon has been shown to induce disturbance to membrane organization and functional loss/modification of proteins and DNA (Frantseva et al., 2000; Schihiri, 2014).
The generation of oxidative damage appears to result from excitotoxicity mechanisms already recognized for participating in several pathological conditions that lead to the development of seizures (Dubinsky et al., 1995; Duchen, 2000). In this context, overactivation of the glutamate receptors, especially the NMDA receptors, would lead to an increase in intracellular calcium, resulting to mitochondrial dysfunction and generation of ROS and pro-apoptotic factors, fundamental for the damage and cell death (Patel, 2004).
In this context, it has been found that NO is produced in response to N-methyl-D-aspartate (NMDA) receptor stimulation and may be involved in the modulation of neuronal damage (Ferrer et al., 2000; Mülsch et al., 1994). Moreover, the literature has suggested that seizures induce alterations in NO metabolism, increasing the production of its reactive metabolites (nitrite and nitrate). These metabolites, in turn, can interact with NMDA glutamatergic receptors and potentiate its excitotoxicity action on the CNS (Santos et al., 2009). Therefore, elevated nitrite-nitrate levels may be implicated in lipid peroxidation and others oxidative damages on brain tissues submitted to injury situations, as seizures.
Interestingly, according to our findings, LEV and CNZ, similarly to VIT C, was able to reduce the oxidative stress in brain homogenates by decreases of both TBARS and nitrite-nitrate concentrations. Together, these results reinforces the participation of nitrergic pathway, mainly through NO, in oxidative damage in brain tissues and the ability of these antiepileptic drugs, at least in part, through their antioxidant properties to protect the neurons of oxidative damage.
Furthermore, there is an endogenous ROS scavenging enzyme system, involving the cooperative action of superoxide dismutase (SOD), catalase and glutathione peroxidase (GSH-Px) enzymes. The oxidative stress involved, for example, in status epilepticus and seizures can alter the activity of these enzymes, such catalase, promoting an adaptive cellular response to increased free radicals milieu (Ferreira and Matsubara, 1997; Freitas et al., 2004; Bellissimo et al., 2001). In this context, pre-incubation with LEV and CNZ, preserved the catalase activity in control levels. VIT C was also able to preserve the catalase activity at levels similar to controls samples. These results indicated a possible reduction in toxic reactive substrates in the samples incubated with both AEDs and suggest that these drugs, similarly to a powerful exogenous antioxidant, could help the brain cells to counteract the stress-induced reactive oxygen species overproduction and the oxidative damage.
In the brain, GSH is thought to play a central role in defense against reactive oxygen species. This substance can either directly detoxify reactive oxygen species as can act as a substrate for several peroxidases (Dean et al., 2011). Under conditions of overproduction of free radicals or a deficiency of antioxidant systems, GSH is consumed and its levels fall (Wang and Cynader, 2000). In the present work, no alterations in GSH concentrations were observed in the group of samples submitted to oxidative stress compared to control. However, an increase in this biomarker was induced by pre-incubation with the two lowest tested concentrations of LEV and by the lowest tested concentration of CNZ. An important augment in GSH amount also occurred in samples treated with VIT C. This effect may be probably attributed the capacity of these compounds to avoid the depletion of endogenous antioxidants reserves by the reactive species. Additionally, some of these drugs may potentiate the biosynthesis of brain antioxidants, such as GSH, increasing their amounts. In this context, according to Al-Shorbagy et al. (2013), LEV would be able to up-regulate the action of cystine/glutamate exchanger and to increase the cysteine concentrations in glial cells, the limiting substrate to production of GSH (Al-Shorbagy et al., 2013; Arakawa and Ito, 2007; Kau et al., 2008).
Our findings demonstrate no concentration dependent effects of both LEV and CNZ on the parameters investigated. In fact, in general, the lowest concentrations tested present the highest antioxidant capacity and this property became distinctly smaller with the higher concentration of LEV and CNZ. These results suggest that high concentrations of these drugs could inhibit part of the endogenous antioxidant defense. In accordance with this, previous reports pointed to pro-oxidative effects related to long-term therapy or to administration of high doses of AEDs. In this context, increase in TBARS and decrease in the levels of thiols were detected in blood samples from patients under long-term AED therapy (Alshafei et al., 2013; Devi et al., 2008; Tan et al., 2009).
Therefore, this study reinforces that the pro-oxidant/antioxidant balance in brain is modulated by antiepileptic drugs. In addition, the ability of these compounds to reduce brain damage caused by seizures and their biochemical changes (that is., markers of oxidative stress) further supports the involvement of free radicals in seizure generation and highlights the possible role for antioxidants as adjuncts to antiepileptic drugs for better seizure control (Devi et al., 2008).
In the present work, it was demonstrated, by an in vitro model, the antioxidant ability of two antiepileptic drugs, with different mechanism of action, and that this property is mediated, at least in part, by reduction of lipid peroxidation and nitrite-nitrate contents, preservation of catalase activity at control parameters and increase of GSH levels. These results allow us to infer the participation of this effect in the neuroprotective mechanism of these drugs, and reinforce, even indirectly, the hypothesis of oxidative damage in the pathophysiology of epilepsy.
Additionally, our findings also suggest a potential therapeutic use of antioxidant compounds as alternative or complementary tools to the conventional treatment for this disease. However, more studies need to be performed to elucidate the relation between antioxidant activity and protection against seizure development and epilepsy.
This work was supported by research grants from the Brazilian National Research Council (CNPq). The authors thank David J. Woods (University of Otago, New Zealand) for his valuable comments and review of this work.
The authors have not declared any conflict of interests.
REFERENCES
|
Aguiar CCT, Almeida AB, Arajo PVP, Abreu RNDC De, Chaves EMC, Vale OCD, Macêdo DS, Woods DJ, Fonteles MMDF, Vasconcelos SMM (2012). Oxidative stress and epilepsy: Literature review. Oxid. Med. Cell. Longev.
Crossref
|
|
|
|
Alshafei MM, Kassem SS, Abdel MM (2013). Effect of Long Term Treatment with Antiepileptic Drugs on Oxidant Status, Zinc and Magnesium in Epileptic Patients. World Appl. Sci. J. 28:316-323
|
|
|
|
Al-Shorbagy MY, El Sayeh BM, Abdallah DM (2013). Additional Antiepileptic Mechanisms of Levetiracetam in Lithium-Pilocarpine Treated Rats. PLoS ONE 8:10.
Crossref
|
|
|
|
Arakawa M, Ito Y (2007). N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum 6:308-314.
Crossref
|
|
|
|
Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T, Mukherjee B (2003). Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 84:131-138.
Crossref
|
|
|
|
Barros DO, Xavier SML, Barbosa CO, Silva RF, Freitas RLM, Maia FD, Oliveira, AA, Freitas RM, Takahashi RN (2007). Effects of the vitamin E in catalase activities in hippocampus after status epilepticus induced by pilocarpine in Wistar rats. Neurosci. Lett. 416:227-230.
Crossref
|
|
|
|
Bellissimo MI, Amado D, Abdalla DS, Ferreira EC, Cavalheiro EA, Naffah-Mazzacoratti MG (2001). Superoxide dismutase, glutathione peroxidase activities and the hydroperoxide concentration are modified in the hippocampus of epileptic rats. Epilepsy Res. 46:121-128.
Crossref
|
|
|
|
Borowicz KK, Luszczki J, Kleinrok Z, Czuczwar SJ (2000). 7-Nitroindazole, a nitric oxide synthase inhibitor, enhances the anticonvulsive action of ethosuximide and clonazepam against pentylenetetrazol-induced convulsions. J. Neural Transm. 107:1117-1126.
Crossref
|
|
|
|
Chance B, Maehly AC (1995). Assay catalases and peroxidases. Meth. Enzymol. 2:764.
Crossref
|
|
|
|
Chang SJ, Yu BC (2010). Mitochondrialmatters of the brain: Mitochondrial dysfunction and oxidative status in epilepsy. J. Bioenerg. Biomembr. 42:457.
Crossref
|
|
|
|
Dean O, Giorlando F, Berk M (2011). N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J. Psychiatr. Neurosci. 36:78-86.
Crossref
|
|
|
|
Devi PU, Manocha A, Vohora D (2008). Seizures, antiepileptics, antioxidants and oxidative stress: An insight for researchers. Expert Opin. Pharmacother. 9:3169-3177.
Crossref
|
|
|
|
Dubinsky JM, Kristal BS, Elizondo-Fournier M (1995). An obligate role for oxygen in the early stages of glutamate-induced, delayed neuronal death. J. Neurosci. 15(11):7071-7078.
|
|
|
|
Duchen MR (2000). Mitochondria and calcium: From cell signalling to cell death. J. Physiol. Lond. 529:57-68
Crossref
|
|
|
|
Ferreira ALA, Matsubara LS (1997). Free radicals: Concepts, related diseases, defense system and oxidative stress. Rev. Ass. Med. Brazil. 43:61-68.
|
|
|
|
Ferrer I, López E, Blanco R, Rivera R, Krupinski J, Martí E (2000). Differential c-Fos and caspase expression following kainic acid excitotoxicity. Acta Neuropathol. 99:245-256.
Crossref
|
|
|
|
Frantseva MV, PerezVelazquez JL, Hwang PA, Carlen PL (2000). Free radical production correlates with cell death in an in vitro model of epilepsy. Eur. J. Neurosci. 12:1431-1439.
Crossref
|
|
|
|
Freitas RM, de Sousa FCF, Vasconcelos SMM, Viana GSB, Fonteles MMF (2003). Acute alterations of neurotransmitters levels in striatum of young rat after pilocarpine-induced status epilepticus. Arq. Neuropsiquiatr. 61:430-433.
Crossref
|
|
|
|
Freitas RM, do Nascimento KG, Ferreira PMP, Jordán J (2010). Neurochemical changes on oxidative stress in rat hippocampus during acute phase of pilocarpine-induced seizures. Pharmacol. Biochem. Behav. 94:341-345.
Crossref
|
|
|
|
Freitas RM, Nascimento VS, Vasconcelos SMM, Sousa FCF, Viana GSB, Fonteles MMF (2004). Catalase activity in cerebellum, hippocampus, frontal cortex and striatum after status epilepticus induced by pilocarpine in Wistar rats. Neurosci. Lett. 22(365):102-105.
Crossref
|
|
|
|
Gibbs JE, Walker MC, Cock HR (2006). Levetiracetam: Antiepileptic properties and protective effects on mitochondrial dysfunction in experimental status epilepticus. Epilepsia 47(3):469-478.
Crossref
|
|
|
|
Green LC, Tannenbaum SR, Goldman P (1981). Nitrate synthesis in the germfree and conventional rat. Science 212:56-58.
Crossref
|
|
|
|
Gutteridge JM, Halliwell B (2010). Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 393(4):561-564.
Crossref
|
|
|
|
da Silva Haeser AS, Sitta A, Barschak AG, Deon M, Barden AT, Schmitt GO, Landgraff S, Gomez R, Barros HM, Vargas CR (2007). Oxidative stress parameters in diabetic rats submitted to forced swimming test: the clonazepam effect. Brain Res. 1154:137-143.
Crossref
|
|
|
|
Huong NT, Matsumoto K, Kasai R, Yamasaki K, Watanabe H (1998). In vitro antioxidant activity of Vietnamese ginseng saponin and its components. Biol. Pharm. Bull. 21:978-981.
Crossref
|
|
|
|
Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA (2008). Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience 13(155):530-537.
Crossref
|
|
|
|
Kong QM, Lin CLG (2010). Oxidative damage to RNA: Mechanisms, consequences, and diseases. Cell. Mol. Life Sci. 67:1817.
Crossref
|
|
|
|
Kunz M, Gama CS, Andreazza AC, Salvador M, Ceresér KM, Gomes FA, Belmonte-de-Abreu PS, Berk M, Kapczinski F (2008). Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 32(7):1677-1681.
Crossref
|
|
|
|
Li J, OW, Li W, Jiang ZG, Ghanbari HA (2013). Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 14: 24438-24475.
Crossref
|
|
|
|
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-75.
|
|
|
|
Lyseng-Williamson K (2011). Levetiracetam: A Review of its Use in Epilepsy. Drugs 71(4):489-514.
|
|
|
|
Mattei R, Dias RF, Espínola EB, Carlini EA, Barros SB (1998). Guarana (Paullinia cupana): Toxic behavioral effects in laboratory animals and antioxidants activity in vitro. J. Ethnopharmacol. 60:111-116.
Crossref
|
|
|
|
Mülsch A, Busse R, Mordvintcev PI, Vanin AF, Nielsen EO, Scheel-Krüger J, Olesen SP (1994). Nitric oxide promotes seizure activity in kainate-treated rats. Neuroreport. 5(17):2325-2328.
Crossref
|
|
|
|
Murashima YL (1998). Antiepileptic effects of allopurinol on EL mice are associated with changes in SOD isoenzyme activities. Epilepsy Res. 32:254.
Crossref
|
|
|
|
Oliveira AA, Almeida JPC, Freitas RM, Nascimento VS, Aguiar LMV, Júnior HVN, Fonseca FN, Viana GSB, Sousa FCF, Fonteles MMF (2007). Effects of levetiracetam in lipid peroxidation level, nitrite-nitrate formation and antioxidant enzymatic activity in mice brain after pilocarpine-induced seizures. Cell Mol. Neurobiol. 27(3):395-406.
Crossref
|
|
|
|
Patel M (2004). Mitochondrial dysfunction and oxidative stress: Cause and consequence of epileptic seizures. Free Radic. Biol. Med. 37:1951.
Crossref
|
|
|
|
Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, Leprince P, Moonen G, Selak I, Matagne A, Klitgaard H (2002). The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br. J. Pharmacol. 136(5):659-672.
Crossref
|
|
|
|
Santos IM, Tomé Ada R, Saldanha GB, Ferreira PM, Militão GC, Freitas RM (2009). Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid. Med. Cell Longev. 2(4):214-221.
Crossref
|
|
|
|
Sedlak J, Lindsay RH (1998). Estimation of total protein bound and non-protein sulfhydril groups in tissues with Ellman reagents. Anal. Biochem. 25:192.
Crossref
|
|
|
|
Shichiri M (2014). The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 54(3):151-160.
Crossref
|
|
|
|
Stocks J, Gutteridge JM, Sharp RJ, Dormandy TL (1974). Assay using brain homogenate for measuring the antioxidant activity of biological fluids. Clin. Sci. Mol. Med. 47(3):215-222.
Crossref
|
|
|
|
Talarek S, Fidecka S (2003). Role of nitric oxide in anticonvulsant effects of benzodiazepines in mice. Pol. J. Pharmacol. 55:181.
|
|
|
|
Tan TY, Lu C-H, Chuang HY, Lin TK, Liou CW, Chang WN, Chuang YC (2009). Long-term antiepileptic drug therapy contributes to the acceleration of atherosclerosis. Epilepsia 50(2):1579-1586.
Crossref
|
|
|
|
Ueda Y, Doi T, Takaki M, Nagatomo K, Nakajima A, Willmore LJ (2009). Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: In vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res. 1266:1-7.
Crossref
|
|
|
|
Ueda Y, Yokoyama H, Niwa R, Konaka R, Ohya-Nishiguchi H, Kamada H (1997). Generation of lipid radicals in the hippocampal extracellular space during kainic acid-induced seizures in rats. Epilepsy Res. 26:329-333.
Crossref
|
|
|
|
Wang XF, Cynader MS (2000). Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 74:1434.
Crossref
|
|
|
|
Wasterlain CG, Fujikawa DG, Penix L, Sankar R (1993). Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia 34(1):37-53.
Crossref
|
|
|
|
Wayhs CA, Mescka CP, Vanzin CS, Ribas GS, Guerreiro G, Nin MS, Manfredini V, Barros HM, Vargas CR (2013a). Brain effect of insulin and clonazepam in diabetic rats under depressive-like behavior. Metab. Brain. Dis. 28(4):563-570.
Crossref
|
|
|
|
Wayhs CA, Tortato C, Mescka CP, Pasquali MA, Schnorr CE, Nin MS, Barros HM, Moreira JC, Vargas CR (2013b).The association effect of insulin and clonazepam on oxidative stress in liver of an experimental animal model of diabetes and depression. Pharm. Biol. 51(5):533-538.
Crossref
|
|
|
|
Xavier SML, Barbosa CO, Barros DO, Silva RF, Oliveira AA, Freitas RM (2007). Vitamin C antioxidant in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci. Lett. 420(1):76-79.
Crossref
|
|
|
|
Zona C, Niespodziany I, Marchetti C, Klitgaard H, Bernardi G, Margineanu DG (2001). Levetiracetam does not modulate neuronal voltage-gated Na+ and T-type Ca2+ currents. Seizure 10:279-286.
Crossref
|