ABSTRACT
Three sugarcane varieties were used in this experiment to optimize specific plant hormones. The present work was conducted in 2014 at Nuclear Institute of Agriculture (NIA), Tando jam. The experiment was designated with three sugarcane varieties (NIA-2012, NIA-105 and GULABI-95) obtained from Nuclear Institute of Agriculture (NIA) Tando jam. Regeneration of plantlets was compared under different concentration of auxins and cytokinin (IAA, IBA and kinetin (2.0, 3.0 mg1-1), highly significant (p<0.05) variations were observed for all parameters of regeneration and root formation. Interactive effect of variety x treatment x concentration was non-significant for number of regenerated plantlets. Auxins and cytokinins at 3.0 + 3.0 mg1-1 concentration were most optimized and effective for regenerated plantlets and number of shoots. These concentrations should be used in the future for in vitro culture of sugarcane.
Key words: In vitro, regeneration, cytokinins, sugarcane, growth regulators.
Sugarcane is the most important agro-industrial crop, belonging to the Poaceae family with chromosome number = 80 (Khan et al., 2005). This crop provides many byproducts such as gur, sugar, biofuel and energy (Garacia et al., 2007). The yield of sugarcane is low in Pakistan as compared to other countries of the world (Chengalrayan and Gallo-Meagher, 2001). The main reason for this low yield is genetic improvement which takes place through conventional hybridization in Pakistan. Plant in vitro regeneration technique offers successful sugarcane propagation (Franklin et al., 2006; Roy et al., 2007). This procedure is most helpful in controlling bundle of problem which is faced during conventional breeding practices. These techniques ensure disease free multiplication of elite varieties (Khan et al., 2004) and minimizes time span required for mass production. There are many researchers from different countries who have used tissue culture for genetic improvement of sugarcane (Dibax et al., 2011;, Takahshi and Takamizo, 2013). It was also observed that callus derived from different auxins have different regeneration potential (Solangi et al., 2016).
Plant regeneration from regenrable callus obtained from meristem have been exploited by number of researchers (Blanco et al., 1997 and Vickers et al., 2005) using (MS) medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D) and cytokinins (Sughra et al., 2014). Regeneration from callus culture creates genetic as well as epigenetic variations induced by enforced hormonal stimuli (Nawaz et al., 2013; Karim et al., 2015). To fulfill sugar need of the increasing population, this study was planned to induce soma clonal variation which is a necessary component of any conventional crop breeding program (Mathur, 2013). The typical crop improvement cycle takes 8 to 16 years to complete and includes germplasm manipulations, variety selection and stabilization. Regeneration of plantlets through callus culture provides genetic variability known as somaclonal variation. Genotype increases, testing variety protection of crop and production stages. Plant tissue culture is an enabling technology from which many novel tools have been developed to assist plant breeders with improved in vitro regeneration ability of these three sugarcane varieties influenced by auxins, cytokinins and sucrose.
Three varieties selected for this experiment are early maturing NIA-2012, mid maturing NIA-105 and late maturing Gulabi-95. Selected callus was shifted to the fresh media for regeneration of the plantlets. Different tissues were selected as explants sources like roots, leaves, and stems which produce more variations than explants with pre-existing meristems such as shoot tips and axillary buds. The mass of regenerable calli produced with the help of five callus induction media were transferred to 5 types of regeneration media. MS modified with various concentration of cytokinins and auxins include indole-3- acetic acid IAA, indole-3- butyric acetic acid IBA 2,4-D, picloram and NAA. Shoot regeneration started with the appearance of green dots on callus within two weeks after regeneration medium and generally produced normal micro shooting. Regeneration results were not obtained in control or hormone free MS medium. After two subcultures (4 weeks of each), calli were transferred to bottle containing the medium of regeneration (MS modified 2,4-D 2, 4-dichloro-phenoxyacetic acid, Picloram, NAA naphthalene acetic acid, indole-3-acetic acid IAA, indole-3-butyric acid IBA and kinetin 2.00, 2.50 and 3.0 mg l-1) of each growth regulators. Cultures were incubated in growth room with 2000-3000 lux under 16th photo period at 25±2°C.
The effects of callus age on regeneration were predictable by transferring the calli to regeneration media after 15, 20, 26, and 37 days of culture. The plantlets obtained were aseptically transferred to the same regeneration medium for 5 other weeks. Shoot emerging takes place from green calli. Multiplication of shoots was continuously till healthy plantlets obtained for this purpose trimmed off shoots and transferred into fresh media. Data analysis was done by ANOVA on collected number of plantlets, micro shoot, and length of shoot using computer software Statistics version 8.1(Table 1). Experiment design was complete randomized design (CRD with three treatments and five different concentrations through two factorial designs, and the regenerated shoots from callus were counted for calculation of the shoot organogenesis. The regenerated shoots were scored for chlorophyll mutations. When the regenerated plantlets reached 7 to 8 cm height, these were subjected to rooting by culturing on rooting media.
An efficient protocol supporting grooming of callusing and regeneration potential is essential for successful genetic transformation of the commercial clones with somaclonal variation (Asad et al., 2009; Solangi et al., 2016). Sugarcane plantlets were regenerated successfully from all the tested clones.
Number of regenerated plantlets/callus
The 3 sugarcane varieties were cultured on MS modified media. Cultivars under exploration showed highly significant differences for various variables of in vitro callus of three different genotypes of sugarcane, NIA-2012, NIA-105 and Gulabi-95. Data for number of regenerated plantlets are presented in Tables 2, Figure 1a, b and c. Significant variations (P< 0.05) for regenerated plantlets were observed for all genotypes. Highest result was obtained in NIA- 2012 (6.46), and minimum in Gulabi- 95. According to the treatment variance, picloram with ABK gave best result for grooming of regeneration of plantlets while NAA and ABK gave no positive result for the sugarcane varieties. Mostly, NAA phytohormone preferable for root induction is not suggested for improvement of regeneration of sugarcane plantlets (Table 2, Figure 1a, b and c). The maximum proliferation of regeneration of plantlets was observed in the concentration of 3.0 mg /l 2,4-D +3.0 mg/l ABK. In the present investigation, shoot apical regeneration of different sizes increased with improved dose of all the treatment of auxins and cytokinins. As shown in Table 2, time for shoot formation was increased by enhancing the dose of phytohormone. Maximum rate of survival was achieved when concentration of 3.0 mg/l was used. This size exhibited 100% survival with 90% regeneration potential within 12 days of inoculation. These results are similar to that of Ali et al. (2010) and Ijaz et al. (2012). The present results vary as compared to that of the previous worker (Khan et al., 2008; Seema et al., 2001). They obtained good results of regeneration of plantlets at lower concentration of auxins and higher concentration of cytokinins.



Maximum number of shoots/plant
About 5-6 weeks shoot, vigorously growing regenerated plantlets were transferred to fresh medium in bottles for further growth and proliferation. Both stages of regeneration of plantlets were tested. Highest result was obtained in NIA-2012. Better results for shoot burgeoning were obtained in MS medium at the concentration of 3.0 mg/l. Proliferation of shoot started and during secondary proliferation stage, lateral shoots developed from the base of newly initiated shoot. As a result, a dense mass of shoots was developed in NIA-2012 followed by Gulabi- 95 and minimum in NIA-105. After about 20 days, these shoots were further sub-divided in small shoots containing 4-5 shoots and were transferred into fresh medium in bottles (Table 3). Best treatment was optimized (Picloram) for the variety, NIA-2012 followed by NIA- 105 and minimum by Gulabi-95. The present result of shoot formation and proliferation were obtained with concentration of 3.0 mg/l. Recent study also demonstrates the outcome of phytohormone for shoot formation and multiplication described positve correlation (Rocha, 2012). All the results are consistent with that of Torque et al. (2010) Sughra et al. (2014) and Solangi et al. (2016). The current study is quite different due to change in the variety or concentration of hormones (Roy and Kabir, 2007; Duminil and Di Michele, 2009).
Albino mutant plant production
The present study also describes the role of cytokinins, particularly kinetin in shoot formation and number of albino mutant plant. The main mode of action of plant growth hormone involves binding of active substances to a specific receptor molecule which bind either on cell surface or within the cytoplasm. Different growth regulators responsible for albino mutation, maximum albino mutant are obtained in the concentration of 3.0 mg/l (Table 4 and Figure 2). Highest albino plant was obtained in NIA-2012 and minimum in NIA-105. The results also suggest that shoot multiplication and albino formation in sugarcane depends on the genotype and media concentration. However, the best albino mutant plant was achieved when cultured on MS medium supplemented with 3.0 mg/L 2,4-D (Khan et al., 2004; Roy et al., 2010; Begum et al., 2011) and decreased concentration reduced shootlets induction and albino plantlets (Biradar et al., 2009; Ali et al., 2010).
Length of shoots/day
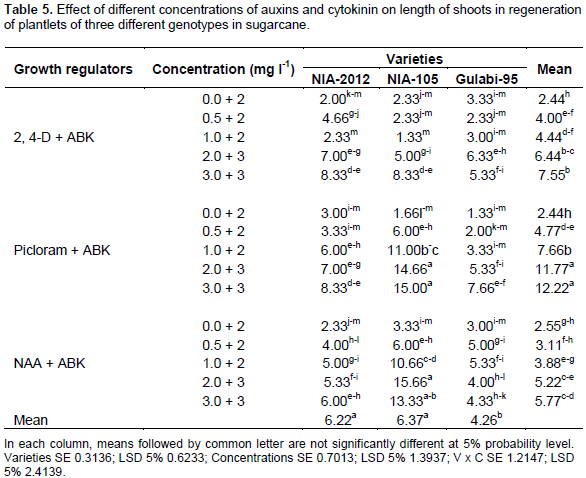
Among the three varieties of sugarcane, on average, NIA-105 requires minimum days (21) for the induction of the shoot, while the maximum days were recorded in NIA-2012 (19) and Gulabi-95 (18). However, more vigorous shoot length development was achieved when the plantlets were separated and cultured on MS medium supplemented shoot induction with 3 mg/L of Picloram. The overall highest length of shoots for NIA-105 (15 cm) was seen with medium containing Picloram + ABK.Among the variability parameters studied, there was significant variation at the probability level (p<0.05) in all the genotypes. First factor indicated the magnitude of variations exclusively due to the gene action which occurred due to the change in the concentration of hormones (Table 5), whereas, the latter indicated the total variations generated and was attributed to the conditions provided during environmental component together with the genotypic variations. The results indicated resemblance with the results of Zamir et al. (2012) and varied from that of Sahoo et al. (2011).
The basic phytohormones influence and enhance cell division, cell elongation and cell differentiation, and integrates the overall development of shooting properly. Efficient regeneration potential of callus is helpful for transformation of genetic variation in commercial elite sugarcane replicates. Albino mutant occur due to genetic variation which mainly depends on type and concentration of auxins and cytokinins. In vitro regeneration of plantlets showed increasing ability of regeneration when additive concentration of plant hormones is applied on the callus.
The authors have not declared any conflict of interests.
REFERENCES
|
Ali S, Iqbal J, Khan MS (2010). Genotype independent in vitro regeneration system in elite varieties of sugarcane. Pak. J. Bot. 42(6):3783-3790.
|
|
|
|
Begum MK, Islam MO, Miah MAS, Hosain MA, Islam N (2011). Production of somaclone in vitro for drought stress tolerent plantlet selection in sugarcane. Agriculturists 9(1-2):18-28.
|
|
|
|
Chengalrayan K, Gallo-Meagher M (2001). Effect of various growth regulators on shoot regeneration of sugarcane. In Vitro Cell. Dev. Biol. Plant 37:434-439
Crossref
|
|
|
|
Dibax R, de Alcântara GB, Bespalhok Filho JC, Machado MP, de Oliveira Y, da Silva AL (2011). Plant regenration of sugarcane sugarcane cv. RB931003 and RB 98710 from somatic embryos and acclimatization. J. Biotechnol. Biodivers. 2:3.
|
|
|
|
Duminil J, Di Michele M (2009). Plant species delimitation: a comparison of morphological and molecular markers. Plant Biosyst. 143:528-542.
Crossref
|
|
|
|
Franklin G, Arvinth S, Sheeba CJ, Kanchana M (2006). Subramonian N Auxin pretreatment promotes regeneration of sugarcane (Saccharumspp. Hybrids) midrib segment explants. Plant Growth Regul. 50:111-119.
Crossref
|
|
|
|
Garacia P, Leon O, Fernandez E (2007). Genetic diversity of genus Saccharum revealed with DNA markers. Rev. Prot. Veg. 12:73-77.
|
|
|
|
Ijaz S, Rana IA, Khan IA, Saleem M (2012). Establishment of an in vitrc regeneration system for genetic transformation of selected sugarcane genotypes. Genet. Mol. Res. 1(1):512-530.
Crossref
|
|
|
|
Karim MZ, Alam R, Baksha R, Paul SK, Hossain MA, Rahman BMM (2015). In vitro clonal propogation of sugarcane (Saccharum officinarum) variety Isd 31. Pak. J. Biol. Sci. 5:659-661.
|
|
|
|
Khan IA, Dahot MU, Seema N, Yasmin S, Bibi S, Raza S, Khatri A (2008). Genetic variability inplantlets derived from callus culture in sugarcane. Pak. J. Bot. 40(2):547-564.
|
|
|
|
Khan IA, Khatri A, Nizamani GS, Siddiqui MA, Khanzada MH, Dahar NA, Seema N, Naqvi MH (2004). In vitro culture studies in sugarcane. Pak. J. Biotechnol. 1(1):6-10.
|
|
|
|
Khan IA, Khatri AB, Nizamani GS, Siddiqui MA, Raza S, Dahar NA (2005). Effect of NPK fertilizers on the growth of sugarcane clone AEC86-347 developed at NIA, Tando Jam. Pak. J. Bot. 37:355-360.
|
|
|
|
Mathur S (2013). Conservation of biodiversity through tissue culture. Res. Rev. J. Microbiol. Biotechnol. 2:1-6.
|
|
|
|
Nawaz M, Ullah I, Iqbal N, IQBAL MZ, Javed MA (2013). Improving in vitro leaf disk regeneration system of sugarcane (Saccharum officinarum L.) with concurrent shoot/root induction from somatic embryos. Turk. J. Biol. 37(6):726-732.
Crossref
|
|
|
|
Rocha MVP (2010). Produção de Bioetanol a partir de Pedúnculo de Caju (Anacardium occidentale L.)por Fermentação Submersa. Ph.D. Thesis, Universidade Federal do Rio Grande do Norte. (In Portuguese).
|
|
|
|
Roy PK, Kabir MH (2007). In vitro mass propagation of sugarcane var.1sd 32 through shoot tip and folded leaves and culture. Biotechnology 6(4):588-592.
Crossref
|
|
|
|
Sahoo M, Hosokawa M, Doi M (2011). Somaclonal variation is induced de novo via the tissue culture process: a study quantifying mutated cells in Saintpaulia. PLoS One 6:e23541.
Crossref
|
|
|
|
Sandeep B, Biradar DP, Patil VC, Patil SS, Kambar NS (2009). In vitro plant regeneration using shoot tip culture in commercial cultivar of sugarcane. Karnataka J. Agric. Sci. 22(1):21-24.
|
|
|
|
Seema G, Pande HP, Lal J, Madan VK (2001). Plantlet regeneration of sugarcane varieties and transient GUS expression in calli by electroporation. Sugar Technol. 3(1-2):27-33.
Crossref
|
|
|
|
Solangi SK, Qureshi ST, Khan IA, Raza S (2016). Establishment of invitro callus in sugarcane (saccharum officinarum l.) Afr. J. Biotechnol. 15(29):1541-1550.
Crossref
|
|
|
|
Sughra MG, Altaf SA, Rafique RM, Muhammad MS, Balouch SN, Umar DM (2014). In vitro regenerability of different sugarcane MU (saccharum officinarum l.) varieties through shoot tip culture Pak. J. Biotechnol. 11(1):13-23.
|
|
|
|
Takahshi W, Takamizo T (2013). Plant Regeneration from Embryogenic Calli of the Wild Sugarcane (Saccharum spontaneum L.) Clone Glagah Kloet. Bull. NARO Inst. Livest. Grassl. Sci. 13:23-32.
|
|
|
|
Torque MMM, Mannan MA, Bhuiyan MSR, Rahaman MM (2010). Micropropagation of sugarcane through leaf sheath culture. Int. J. Sustain. Crop Prod. 5(2):13-15.
|
|
|
|
Vickers JE, Grof CP, Bonnett GD, Jackson PA, Morgan TE (2005). Effects of tissue culture, biolistic transformation, and introduction of PPO and SPS gene constructs on performance of sugarcane clones in the field. Aust. J. Agric. Res. 56(1):57-68.
Crossref
|
|
|
|
Zamir R, Khalil SA, Shah ST, Khan MS, Ahmad K, Shahenshah, Ahmad N (2012). Efficient in vitro regeneration of sugarcane (Saccharum officinarum L.) from bud explants. Biotechnol. Biotechnol. Equip. 26(4):3094-3099.
Crossref
|