Full Length Research Paper
ABSTRACT
Pistachio hull contains anacardic acids. It is a salicylic acid with a side chain either saturated or unsaturated. This work focused on the efficient extraction of anacardic acids using solvent. Six aliquots were weighed and extracted with hexane, ethyl acetate, and mixture of H2O/MeOH/HCl. Hexane was the most efficient solvent due to its ability to dissolve the anacardic acids. Only one anacardic acid standard was used to quantify all of them since the side chain does not affect the wavelength to a greater extent. HPLC-DAD was used for quantification purposes and HPLC-MS and retention time were used for qualitative analysis.
Key words: Anacardic acid, maceration, salicylic acid, pistachio hulls.
INTRODUCTION
Pistachio is a member of the cashew family found mostly in Central Asia and Middle East. The tree produces seeds that are consumed as food (https://en.wikipedia.org/wiki/Pistachio). Pistachio hull (external soft shell of pistachio) is the major part of pistachio by-product that has a high content of bio-active compounds (such as polyphenols, tocopherols, dietary fibers, essential oils, and unsaturated fatty acid) with antioxidant properties and health-promoting effects. Pistachio hulls were previously shown to contain a large diversity of phenolic compounds, including gallotannins, flavonoids, and anacardic acids. Among these, gallic acid, β-glucogallin, penta-O-galloyl-β-D glucose, quercetin 3-O-galactoside, quercetin 3-O-glucoside, and anacardic acids represent the most abundant constituents (Sevcan et al., 2017). Anacardic acids are phenolic lipids, chemical compounds and a mixture of several closely related organic compounds. Each consists of a salicylic acid substituted with an alkyl chain that has 15 or 17 carbon atoms. The alkyl group may be saturated or unsaturated. The non-linear structure, unsaturation in the alkyl chain and substitution to phenolic group open up new vistas in the innumerable applications in dyestuffs, foods, flavors, ion exchange resins, paints, plasticizers and polymers (Syed et al., 2020). Figure 1 is an image of anacardic acid.

“Anacardic acid is a hydroxybenzoic acid that is salicylic acid substituted by a side group at position 6. It is a major component of cashew nut shell liquid and exhibits an extensive range of bioactivities (Figures 2 and 3). It has a role as an EC 2.3.1.48 (histone acetyltransferase) inhibitor, an apoptosis inducer, a neuroprotective agent, an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor, an anticoronaviral agent, an antibacterial agent, an anti-inflammatory agent and a plant metabolite. It is a hydroxybenzoic acid and a hydroxy monocarboxylic acid. It derives from a salicylic acid (National Center for Biotechnology Information, 2022).” It is a yellow liquid partially miscible in alcohol and ether but nearly immiscible in water (Kumar et al., 2002). Cardanol and cardol are some derivatives of Anacardic acid; they are phenolic components and less effective in biological activity. Figure 4 is anacardic acid and its derivatives. This work focused on the extraction of anacardic acid from pistachio hulls using three solvents: hexane, ethyl acetate and mixture of H2O/MeOH/HCl in order to know the efficient solvent.
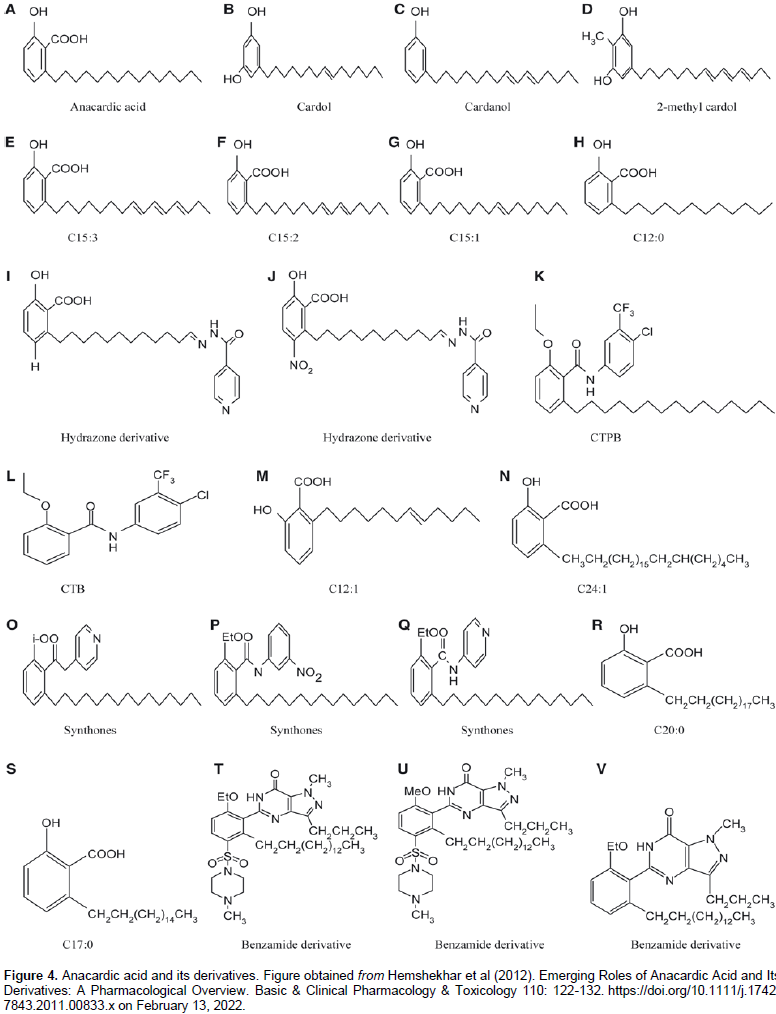
MATERIALS AND METHODS
Sample extraction procedure
The pistachio hull was grinded and homogenized. The sample was divided into six aliquots and the mass weighed by balance and recorded in Table 1. Three solvents were used for the extraction procedure namely: hexane, MeOH/H2O/HCl, and ethyl acetate. The solvents were chosen considering the polarity of the Anacardic acids. The greater the length of the side chain of the Anacardic acids, the less polar. The three solvents were used to obtain the more efficient extraction solvent. Anacardic acids are less soluble in more polar solvent. The hexane was used for extracting aliquots 1 and 2. MeOH/H2O/HCl was used for the extracting of aliquots 5 and 6 and ethyl acetate mixture was used for extracting aliquots 3 and 4 with each composition having a volume of 50ml, and a sample to solvent ratio of 1:10. An ultrasound-assisted extraction, both in bath and probe was used for the extraction in order to compare the effectiveness of the two methods. Ultrasound bath was used for extracting aliquots 1, 2 and 5 and 6. The probe was used for extracting aliquots 3 and 4. The extraction time allowed for each aliquot was 5 min. Using ultrasound probe, sound waves produced by the probe create bubbles around the sample matrix and eventually burst and release temperature and pressure to the surface. This destroys the matrix surface and the analytes are dissolved in the extraction solvent as they encounter it. After the extraction step, the mixture was filtered to obtain the extract which was then concentrated, and optimized for analysis by HPLC, although this was not performed at the laboratory.
Identification of anacardic acids (HPLC-MS and HPLC-DAD)
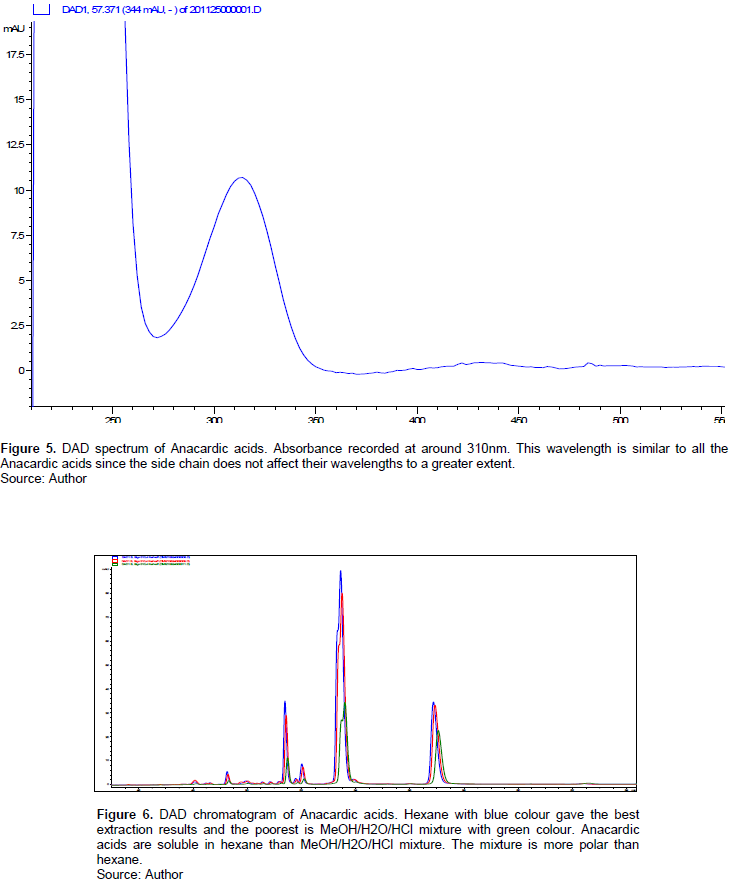
The analytes identification was done using both HPLC-MS and HPLC-DAD. Although this was not performed at the laboratory, a standard compound of the anacardic acids was purchased, and after injecting it into the HPLC system, the retention times, UV absorption spectra, and mass spectra were recorded. After the HPLC analysis of the unknown anacardic acids, the retention times, UV spectra, and mass spectra were compared to the standard to identify the unknown anacardic acids. In this experiment, the results of the unknown anacardic acids were compared to the results in the literature for qualitative and quantitative analysis. Considering the HPLC-DAD, the diode array detector gave a UV/vis spectra and chromatogram. The UV/vis spectra has an absorption at around 310 nm which is typical for anacardic acids as recorded in the literature and observed from the spectra in Figure 3. The HPLC chromatogram gave peaks in retention time range of 50 to 60 min which is similar to the range recorded in the literature and helped in the identification purposes. These chromatogram peaks correspond to monoene, diene, and triene anacardic acids; the main peaks of anacardic acids. Considering the HPLC-MS, the mass spectra m/z values of the unknown anacardic acids were compared to m/z values of the anacardic acids in the literature which also helped in the identification of the unknown anacardic acids.
Quantification of anacardic acids (HPLC-DAD)
For quantification purpose, although not performed at the laboratory, stock solution of the anacardic standards can be prepared and diluted to different concentrations or each solution with different concentration can be prepared separately. The alkyl chain of the anacardic acids do not affect the wavelength to a greater extent; therefore only one anacardic acid standard can be used for all the anacardic acids present in the pistachio hull. The different concentrations can be prepared for a maximum of about 6 experiments. The standard is injected into the HPLC-DAD system and the absorbance recorded for each concentration injected. A calibration curve is prepared by plotting the absorbance of each of the spectra curves for the maximum experiments versus the different concentrations. Hence, from the absorbance of the unknown anacardic acids, and by plotting on the standard calibration curve, the concentration of the unknown anacardic acids can be found.
RESULTS AND DISCUSSION
The solvents used in the extraction were hexane; ethyl acetate; and MeOH/H2O/HCl. MeOH/H2O/HCl gave the poorest results as observed from the HPLC chromatogram in Figure 6. The solvents were chosen based on their polarity considering the polarities of the Anacardic acids. The peaks correspond to monoene, diene and triene Anacardic acids with double bond in the alkyl chains of 1, 2 and 3 respectively. Hexane which is mostly non- polar gave the highest peaks for the anacardic acids followed by ethyl acetate. The limited solubility of the anacardic acids in the MeOH/H2O/HCl mixture led to the poorest results (Sevcan et al., 2017). The polarity of anacardic acid depends on the length of the alkyl group side chain and whether the alkyl group is saturated or unsaturated. Increasing the unsaturation increases the polarity of the anacardic acid-increasing the side chain length decreases polarity. In this work, four saturated anacardic acids were detected with varying side chain length (Table 2). Anacardic acid 11:0 (The ratio attached to the Anacardic acids name correspond tohe number of carbon chains in the alkyl group attached to the Salicylic acid (first number) and the number of double bonds present in the alkyl chain (second number) has the shortest side chain and 17:0 has the highest side chain. The anacardic acids in increasing polarity are: 17:0, 15:0, 13:0, 11:0, 17:1, 16:1, 15:1, 13:1, 13:2 and 15:3. Anacardic acid 15:3 has the highest degree of unsaturation; the remaining have one or two degree of unsaturation with varying side chain and fairly less polar. This explains the more efficient extraction with hexane. In the work of Edoga et al. (2006), hexane was used as extraction solvent for extracting polyphenols from cashew nut shell. They obtained 30% Anacardic acid after separation from other polyphenols. Chitra et al. (2020) also used hexane and methanol to extract cashew nut shell liquid from cashew nut. The cashew nut shell liquid contains Anacardic acid and other polyphenols.
Maria et al. (2012) also obtained greater concentration of monounsaturated Anacardic acid using supercritical CO2 (40°C, 300 bar, 4 h). This supports the argument that, the efficiency of extraction depends on the degree of unsaturation and polarity of the extracting solvent. The penetration of the solvent in the Anacardic acid solute is less efficient and extraction is poor. Considering the two extraction techniques utilized in this work, the ultrasound probe gave a better extraction results compared to the bath. This could be due to the excessive temperature and pressure exerted when the bubble bursts on the sample matrix surface. Ultrasound probe uses sound waves to create bubbles of the extracting solvent which bursts and releases pressure, increasing the penetration in the solute. The HPLC-DAD (Figure 5) gave an absorbance of around 310 nm and HPLC chromatogram (Figure 6) of retention time range of 50-60 min of anacardic acids. This wavelength is similar to all Anacardic acids due to less effect of the side chain in its absorbance-hence only one Anacardic acid standard was used for quantification. The HPLC-MS (Figure 7) gave the mass spectra of the Anacardic acids as recorded in Table 2. The most abundant m/z ratio is 373.2 which is also reported in the literature as 373; it corresponds to Anacardic acid 17:1 with an absorbance of 312 nm.

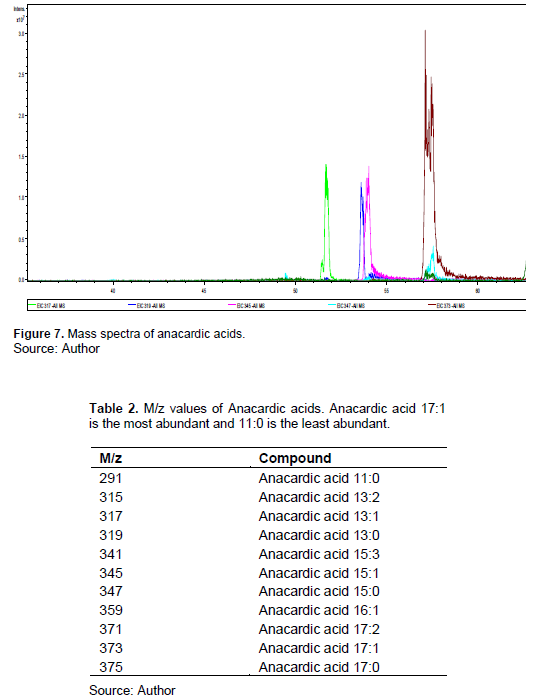
Anacardic acid 11:0 gave the lowest m/z ratio of 291 and UV/vis absorption of 308 nm. Anacardic acid 15:0 and 17:1 have very close results with retention time of 92 and 92.2 min and absorbance of 311 and 312 nm, respectively but different m/z values. To improve the analytical method, the column and stationary phase can be changed in order to improve the chromatographic performance. Also, the time for the extraction of the Anacardic acids using the MeOH/H2O/HCl mixture must be lowered in order to prevent the extraction of the polar matrixes, thus gallic acid, β-glucogallin, penta-O-galloyl-β-D glucose, quercetin 3-O-galactoside, and quercetin 3-O-glucoside. With HPLC, the diode array detector can measure the entire wavelength range which is an advantage. In a case where the retention time of two analytes is very close, by comparing the UV/vis spectrum and also mass spectra, the analytes can be identified. Also, UHPLC is another alternative which is faster than HPLC and with small column particle sizes could improve the resolution of the peaks. Moreover, a replicate extraction for each solvent could be performed for better results. Maceration method using methanol only as extraction solvent could also be an alternative. In this case, the pistachio hull is broken into smaller pieces and soaked with the methanol in an airtight container (Aliefman et al., 2021).
CONCLUSION
In this work, the extraction of Anacardic acids was achieved using ultrasound-assisted extraction, both probe and water bath. Ultrasound probe was the most efficient extraction technique compared to bath as sound wave generates bubbles in extraction solvent which burst and increases penetration in the solute to extract the Anacardic acid with greater pressure and temperature. Hexane as extraction solvent gave the highest gas chromatogram peaks of Anacardic acids due to greater solubility of the Anacardic acids. Mixture of methanol, water and hydrochloric acid gave the poorest gas chromatogram peaks as the Anacardic acid is less polar and solubility decreases. Ethyl acetate gave an intermediate result due to less polarity compared to the mixture of solvents. Anacardic acid in pistachio hull has biological benefit to health.
CONFLICT OF INTERESTS
The author has not declared any conflicts of interests.
ACKNOWLEDGEMENT
All chromatograms, mass spectrum and table of results were obtained from Dr. Manuela Cortese-Laboratory technician at University of Camerino.
REFERENCES
|
Aliefman H, Jamaluddin IN, Loka SH, dan Ayu Rizki M (2021). Anacardic acid isolation from cashew seed skin. Jurnal Pijar Mipa 16(3):418-421. |
|
|
Chitra DV, Mothil S, Sineha L, Rahul Rajan AT, Santhosh P (2020). Extraction of polyphenols from cashew nut (Anacardium occidentale) shells using rotocel extractor. AIP Conference Proceedings. Crossref |
|
|
Edoga MO, FADIPE AL, Edoga RN (2006). Extraction of polyphenols from cashew nutshell. Leonardo Electronic Journal of Practices and Technologies 9:1-6. |
|
|
Hemshekhar M, Sebastin Santhosh M, Kemparaju K, Girish KS (2012). Emerging Roles of Anacardic Acid and Its Derivatives: A Pharmacological Overview. Basic and Clinical Pharmacology and Toxicology 110(2):122-132. |
|
|
Kumar PP, Paramashivappa PJ, Vithayathil PJ, Subra Rao PV (2002). Process for isolation of cardanol from technical cashew (Anacardium occidentale) nutshell liquid. Journal of Agricultural Food Chemistry 50(16):4705-4718. |
|
|
Maria Y, Ngoc YT, Yi-Hsu J (2012). Effect of extraction methods on characteristic and composition of Indonesian cashew nut shell liquid. Industrial Crops and Products 35(1):230-236. |
|
|
National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 167551, Anacardic acid. Retrieved February 13, 2022 from |
|
|
Syed AA, Isa Baba K, Mohsina B (2020). Innovative Spectrophotometric Methods for the Determination of Anacardic Acid. Research and Scientific Innovation Society 3(IA):5-8. |
|
|
Sevcan E, Özlem GÜ, Reinhold C, Ralf MS (2017). Determination of pistachio (Pistacia vera L.) hull (exo- and mesocarp) phenolics by HPLC-DAD-ESI/MSn and UHPLC-DAD-ELSD after ultrasound-assisted extraction. Journal of Food Composition and Analysis 62:103-114. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0