ABSTRACT
Three different pre-treated shea nut substrates: raw kernels, roasted kernels and shea nut paste from roasted kernels were subjected to treatment with three different industrial enzymes: lipase, pectinase and cellulase, separately and in combination for shea butter extraction. Enzyme mixtures was optimized at pH of 6; 3% enzyme-substrate concentration, 2 h hydrolysis time at a temperature of 60ºC. The results showed that shea nut paste yielded 48% fat with pectinases (P), 52% with lipases (L) and 46% with cellulases (C) and this made it the best substrate for shea butter recovery. The amount of fat percentage wise with the same substrate increased to 52, 54 and 56 at 1:1 P+C, L+C and P+L enzyme combinations respectively. The highest extraction efficiency of 70% was recovered from 1:1:1 combination of all three industrial enzymes. Therefore, enzyme assisted aqueous hydrolysis of shea nut biomass for shea butter production is a promising technology with a great potential (extraction efficiency and safety) to substitute traditional extraction methods.
Key words: Shea kernels, shea butter, traditional extraction, solvent extraction, commercial enzymes.
The importance of the shea tree is considered second to the palm tree (Paulsen, 1981) because of the benefits of its butter to many industries both locally and internationally (Soladoye et al., 1989; Russo and Etherington, 2001; Chaffin, 2004; Ndukwe et al., 2007; Ogbonnaya and Adgidizi, 2008; Akihisa et al., 2010). The significance of the shea butter commodity to the pharmaceutical, cosmetic and food industry cannot be over emphasized. Research into the extraction of the shea butter resource began nearly a century ago, in the former French and British colonies. To date the traditional method of extraction is the preferred choice of most shea butter industries in Ghana (Addaquaye, 2004). This method of shea butter production encompasses many manual unit operations instituting some challenges which render the whole process tedious and laborious (Olanyan and Oje, 2007). In West Africa, the manual procedure employed by rural women in shea butter extraction involves: beating the kernel with pestle and mortar to break the seed into grits, cooking the kernel to accomplish ease of oil recovery, grinding the grits into paste, kneading the paste in water to capture the fat into an emulsion, steaming the combination to separate the fat and skimming off the fat (Addaquaye, 2004).
The final cooling procedure leads to “unrefined Shea Butter”. The traditional village extraction process also known as aqueous extraction method is tedious, time intensive, energy sapping, environmentally unfavorable and generally gives low oil with poor quality (Olaniyan and Oje, 2013). The ineffectiveness of the handling methods decreases the amount of shea butter available in the industry. Alternative to the traditional aqueous extraction methods are other modern methods involving expellers or hydraulic presses, ghanis or mechanical rig (Kaviani et al., 2015; Alenyorege et al., 2015; Al-hassan and Abdul-Malik, 2011; Warra 2011; Olaniyan and Oje, 2007; Southwell and Harris, 1992), chemical extraction (Abdul-Mumeen et al., 2013; Apea and Larbi, 2013; Chen and Diosady, 2003) or preliminary or lab scale enzyme assisted aqueous extraction (Tano-debrah and Ohta, 1995; Tano-debrah and Ohta, 1994; Rosenthal et al., 1996; Otu et al., 2015). The mechanical extraction method has not yielded the desired efficiency (30%) as compared to the enzyme assisted aqueous extraction (58.60 to 72%) (Otu et al., 2015).
To address the problem of low extraction efficiency faced by the shea nut industry, the application of the right enzyme or enzyme consortia for oil recovery from oilseeds has become the most effective tool (Barrios et al., 1990). The use of enzymes has emerged as an effective novel means to improve the oil yield in cold pressing and aqueous extraction techniques (Tano-Deorah and Ohta, 1994) and its significance has come to the attention of many researchers (Cheah et al., 1990; Rosenthal et al., 1996; Hernandez, et al., 2000; Chen and Diosady, 2003; Abdulkarim et al., 2006; Huyanh et al., 2013).The traditional method also described as the hydrothermal extraction method is an improved way of solving the problems associated with the traditional village extraction of oils from oilseeds. It is basically an improvement from the traditional village extraction procedure in which water and heat are used at different stages and at different levels of combination. The equipment required such as mortar and pestle, kneading and boiling pans, seed roasters among others are less costly and easy to obtain.
The improvement notwithstanding, the traditional extraction method is still considered low yielding and about 23% of fat remains in the shea nut cake after a successful extraction (Abdul-Mumeen, 2013). Thus, there is need for continuous development of shea oil extraction methods that are effective, efficient, and easy to embrace at the traditional extraction level. Therefore, the present study investigated the use of commercial enzymes to eliminate technological limitations of the traditional extraction method in the shea butter extraction process. The underlying hypothesis for this research was that energy intensive steps such as roasting can be eliminated and that shea kernels would yield similar quantities of oil regardless of the pre-treatment; unroasted kernels, roasted kernels or kernels roasted and further milled to paste. The information from this study will guide policy discussions peculiar to the shea industry in order to add value to the shea value chain.
The study area
Shea nut kernels were obtained from Tolon in the Tolon District in the Northern Region of Ghana. Tolon is on 156 m elevation at latitude 9.4333 N and longitude 1.0667 W. The Tolon district shares borders with North Gonja to the West, Kumbungu District to the North, Central Gonja to the south and to the East with Sagnerigu District. The Tolon District has a population of seventy-two thousand nine hundred and ninety (72,990) people with 36,630 being female population representing 50.2% (PHC, 2010).
Source of materials
The main raw material for this research was shea nut fruits processed into shea kernels. The shea kernel is the product of the general pre-treatment of the shea nut fruit. Pre-treated shea kernels were obtained from Tolon District in the Northern Region of Ghana. The shea kernels were prepared at three different levels: raw shea kernels (RASK), roasted shea kernels (ROSK) and shea kernel paste (SKEP) (finely milled roasted shea kernels). They were packaged into separated plastic containers and transported to the laboratory and kept at room temperature for further analyses. All chemical and reagents used were of analytical grade.
Commercial enzymes
Commercial pectinase (E6287) from Aspergillus aculeatus, commercial lipase (E0777) from Thermomyces lanuginosus obtained from Sigma-Aldrich (3050 Spruce Street, Saint Louis, MO 63103, USA) and commercial cellulase from Aspergillus niger (obtained from Novozymes, Denmark) were used in the analysis.
Preparation of samples
In general the shea nut kernel was obtained from preliminary pretreatment of the shea nut fruit (Figure 1a, b and c). Three substrates (Figure 2) were then obtained from the shea kernels. The raw and roasted Shea kernels were finely ground and sieved (0.60-0.71 mm) prior to extraction as shown in Figure 2. The paste obtained had lost some amount of water prior to usage and as a result it was re-blended (using a commercial blender) to regain its pasty slurry form (Figure 2).
Substrate moisture content analysis
The moisture content (%) of the kernels were determined according to Mohagir (2010) recommended by AFNOR (1981). In this method, 5 g of ground raw kernels was placed in a dry oven dish, and then dried in an oven at 105±2°C until a constant mass was achieved. The experiment was repeated three times and the average value was taken. The moisture content (Mc ) was calculated on a dry matter basis and expressed in gram per 100 g of initial sample using the formula below:
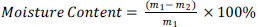
Where, m1 is the mass of the sun dried kernels and m2 is the mass of the oven dried kernels.
Preliminary extraction of shea butter
A preliminary study was carried out using the traditional method according to Tano-Debrah and Ohta (1995) with modification. A 4 x 3 factorial design consisting of 4 kneading time levels (0, 10, 20 and 30 min against 3 boiling time levels: 5, 10 and 15 min were used to determine the optimized kneading and boiling times. The extraction at each kneading-boiling interaction was done in duplicates. The SKEP was used to carry out the preliminary study (Table 1), and optimized conditions were further used for extraction of shea butter from the RASK and ROSK samples. Fifty grams (50 g) of the SKEP was weighed using an electronic weighing balance (Model: AS200) for extraction of shea butter using the traditional extraction method in a laboratory setting. The weighed sample was put into a 250 ml beaker, warm water (40 ml) and cold water (60 ml) were intermittently added and then kneaded to form an emulsion. The total amount of water added was 100 ml to give the desired solid-to-liquid ratio of 1:2 resulting in workable emulsion phase.
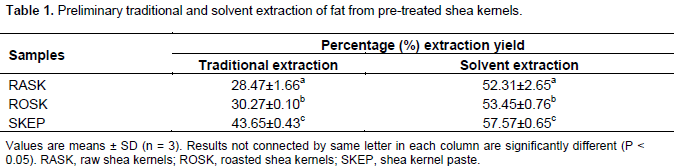
The content of the beaker was treated with 100 ml of boiling water (100°C) on a hot plate, and it was stirred periodically (total stirring time of about 4 min). The beaker with the contents was taken off the hot plate and allowed to cool overnight at room temperature (25°C). A whitish brownish mass was formed which was scooped out onto another beaker. It was washed four times with running tap water and heated again on a hot plate (60°C) to obtain the crude butter. The oil was further heated for 1 h at 100°C using a hot-air oven to expel any residual water. Shea butter extraction yield was then determined. The crude oil was further clarified using vacuum filtration. The clarified oil obtained was transferred into test-tubes with caps and stored in a refrigerator at 4°C for further analyses.
Solvent extraction
Solvent extraction of shea butter was carried out according to the procedure adopted by the AOAC (1984). The RASK, ROSK and the SKEP were subjected to fat extraction by placing two grams (2 g) each of the samples into cellulose-paper cone and the extraction carried out using n-hexane as solvent in a 5 L Soxlet extractor for 8 h. The oil extracted was stored in the refrigerator at 4°C for further analyses of its physico-chemical properties.
Enzyme-assisted traditional extraction (E.A.H.E)
The enzyme-assisted traditional extraction was carried out according to Otu et al. (2015) but not without modifications. An aliquot equivalent to 50 g of shea nut biomass in 600 ml beaker was stirred with water in the ratio of 1:4 wt/vol. The content of the flasks were gently boiled for 5 min, cooled to about 30°C and then extracted at 0.5, 1.0, 2.0, 3.0, and 4.0% enzyme-substrate concentration. The optimization of enzyme-assisted traditional extraction of butter from shea nut biomass was carried out for enzyme concentration, incubation temperature and incubation period. Aluminum foil (Everpack products, Ghana) was used to cover the flasks and then placed in a water bath shaker (Thermo Fisher Scientific manufacturer) and incubated at different temperatures (40°C, 50, 60 and 70°C) and time (1, 2, 3 and 4 H), shaking at 80 rpm. Different set of samples were prepared as control (without enzymes) and incubated along-side the test samples for the same period of time.
The reaction was terminated at 100ºC and the digests were transferred into 600 ml beakers and extracted using the traditional extraction procedure according to Tano-Debrah and Ohta (1995). Hundred milliliters (100 ml) of hot water was added to the mixture and vigorously stirred before cold water was added to reduce the temperature of the mixture to about 30 to 40°C. The mixture was left to stand overnight to settle. The emulsion which formed the top layer was collected into another beaker. Fresh warm water was added and stirred to wash the emulsion and allowed to settle again. The clean emulsion was collected into a beaker and gently boiled until clear oil was obtained. The oil was decanted into another beaker and placed in an oven at 100°C for about 1 h to dry and clarify the butter. It was then decanted into a weighed aluminum dish, cooled and weighed to estimate the percentage yield.
Optimization of parameters for E.A.H.E
Temperature and pH conditions for the control were set at the optimum conditions for the enzymes used in each of the experimental analysis. The optimized conditions for the extraction of shea butter from the SKEP were selected over the RASK and ROSK for extraction of shea butter since it gave the best response.
Extraction yield
The extraction yield was calculated according to mathematical model formulated by Adeeko and Ajibola (1989) and Olaniyan and Oje (2011). Extraction yield (Ey), is usually represented as a percentage. It refers to the amount of oil that can be derived from an oil seed. Oil yield was determined as the ratio of the weight of oil recovered (Wor) to the weight of the crushed seed sample before extraction (Wcss).
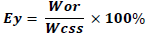
Where, Ey = extraction yield %; Wor = weight of oil recovered;
Wcss = weight of the crushed seed sample before extraction.
Statistical analysis
Values were reported as mean ± SD analyzed individually in triplicate. Two-way analysis of variance was used to determine significant differences between groups, considering a level of significance of less than 5% (P < 0.05) by using the statistical software IBM SPSS STATISTICS 20. Graphs were drawn using Microsoft excel 2013.
The important factors which affected the oil yield during enzymatic extraction constituted the following: pH, type of enzyme, enzyme concentration, moisture content of the substrate, temperature, and incubation time. The effects of these parameters varied according to the differences in the pretreatment levels of the various substrates. The quantity of the oil produced with enzymes was evaluated, compared with the quantity of oil generated by the traditional and solvent extraction methods.
Moisture content % of substrates prior to traditional extraction
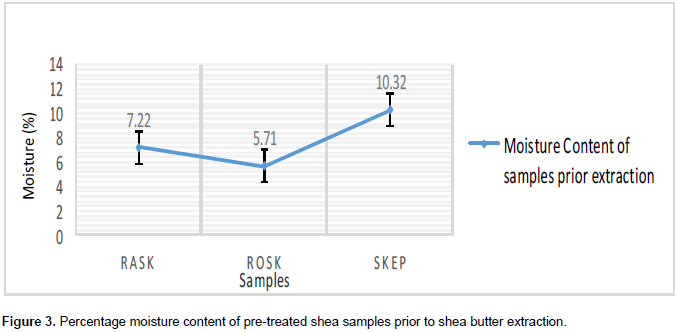
Although all three substrates showed relatively low moisture contents (Figure 3), the ROSK sample showed the lowest moisture content of 5.71%, RASK with 7.22% moisture content and the highest moisture levels was observed with SKEP at 10.32%. The traditional floor drying after 14 days of sun-drying produced moisture content of values ranging from 7 TO 7.5% (Aculey et al., 2012) and this is consistent with the moisture content of the raw shea kernels (7.22%) originally processed by the traditional sun-drying method. Further roasting/heating would allow more dehydration of the kernel and so it is not surprising that moisture content of the roasted kernels plunged down to 5.71%. The phenomenon of the increasing moisture content of roasted kernels turned-out into paste (10.32%) could be attributed to the formation of triacylglycerol (triglycerides) from glycerol and three separate fatty acid chains by condensation during the grinding process. The process of grinding the shea kernel seems to go through three stages chemically: separation of atoms/molecules (bond breaking), rearrangement of atoms/molecules and recombination from which the triglycerides (fat/butter) were formed.
Effect of kneading and boiling time on shea oil recovery (using SKEP sample)
From the preliminary study, boiling time for 10 minand kneading time for 30 min gave the highest oil recovery yield of 42.96% from the SKEP (Figure 4). Subsequent extractions were carried out on the RASK and ROSK samples using these standardized times. A study by Apea and Larbi (2013), on shea butter extraction noted that kneading of Shea nut paste needed 37.4 minaverage time for adequate butter yield (average oil of 493.4 ml/kg) and thus higher oil recovery. The shea nut kernel contains about 52% oil (Adomako, 1985) although, Sachibu et al., (2013) gives an estimation of 60% fat. During a preliminary extraction by the traditional village extraction procedure, 30.29% fat was recovered from the ROSK which had a corresponding moisture content of 5.71%. The RASK which had a moisture content of 7.22% gave 28.47% fat. The shea kernel paste or SKEP, from roasted kernels yielded the highest fat quantity of 43.65% at 10.32% moisture content. The highest fat recovery from the SKEP could be attributed by the wider surface area offered by the paste. In general, as the moisture content decreased, the fat recovery increased and the relation presupposed that the recovery of fat from the shea nut kernel is inversely proportional to the moisture content of the kernel.
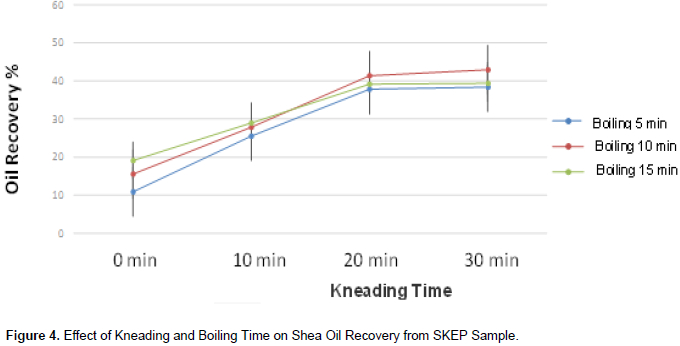
Thus, oil recovery increases with decreasing moisture content (Alenyorege et al., 2015) and the oil yield of a given sample at any given pressure employing the mechanical rig is dependent on the moisture content of that sample (Olaniyan, 2010). Following the use of the solvent extraction method, 57.57 ± 0.65% fat was recovered from shea kernel paste on a dry-weight basis; the highest fat wsarecovered among the pre-treated shea kernel samples. Oil recovery from RASK, ROSK and SKEP respectively, by the traditional procedure, was generally lower (28.47 ± 1.66, 30.27 ± 0.1 and 43.65 ± 0.43%) as compared to oil recovered by solvent extraction methods from the same respective samples (52.31 ± 2.65, 53.45 ± 0.76 and 57.57 ± 0.65) %. Solvent extraction of shea butter oil from the various shea nut meals in general was higher in relation to the traditional extraction. Hexane, the solvent in the case of research, is of low polarity. Esters are of low polarity; hexane was able to dissolve all the esters present in the shea nut biomass which gave rise to high oil recovery (Apea and Larbi, 2013).
The precision and efficiency with the solvent extraction method is remarkable but it is overshadowed with the believe that the product resulting is unwholesome for consumption due to presence of traces of solvent that may be retained in oils extracted (Mbaiguinam et al., 2007). Sachibu et al. (2013) using the traditional village extraction process or the traditional procedure measured the range of extraction rate to be between 20 to 31% and this creates an intercept with the range of the rate of extraction (28.47±1.66 to 43.65±0.43%) but was higher than reported (25%) by Neiss (1983). Apea and Larbi (2013) extracted lower fat (0.2513 ml/g) from unroasted kernels and higher fat (0.3667 ml/g) from roasted kernels employing the mechanical press method. The trend is consistent with the current research although not similar methods were employed. However, Obeng et al. (2010) using a low-pressure (45 kg/cm2) manual screw press employing Intermediate moisture content method (IMC), roasted kernels (65.9%) did not give higher butter yield than raw kernels (68.5%).
But the unique trend of extracting more butter from roasted (33.4%) than unroasted or raw (2.8%) shea kernel was noted (Ajayi, 2013) when an electric food processor was improvised for the kneading. Thus, irrespective of the method of processing, generally, shea butter recovery is higher with kernels with lower moisture content (roasted) than with those with higher moisture content or raw kernels (unroasted). Roasted kernels give higher butter from formation of smooth crystalline structures mainly formed after roasting (Olaniyan and Oje, 2007). The crystalline structures of fats loosens up for extraction after roasting when the roasted kernel is finely pulverized (Olaniyan and Oje, 2013) and this could be the possible reason for recovering lower fat quantity from the ROSK (53.48%) by solvent extraction which had lower surface area as compared to a higher yield (57.57%) from the SKEP by the same method. The SKEP was finely pulverized and exhibited the highest surface area and therefore could be the reason why it gave the best yield compared to both the RASK and ROSK.
Optimization of parameters for enzyme-assisted traditional extraction of shea butter
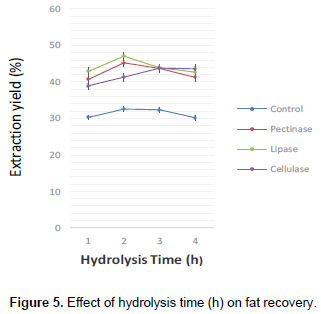
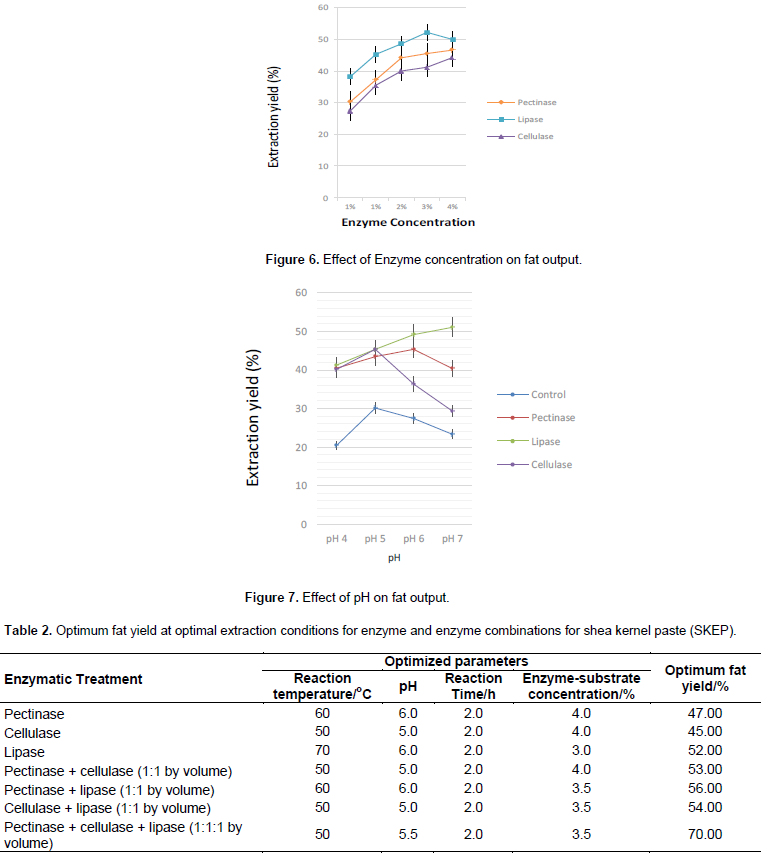
Figures 3, 4, 5 and 6 are illustrations of optimized parameters for optimum fat recovery for enzyme-assisted traditional extraction of shea butter using commercial enzymes. 2 h was the most effective period for hydrolysis of the shea nut substrate (Figure 3) and the best enzyme- substrate concentrations was 3% seed weight basis (Pectinase), 4% seed weight basis (lipase and cellulase) as shown in Figure 4. Fat recovery using cellulase was the best at pH 5 (Figure 5). Lipase and pectinase gave the best fat recovery at pH 7 and pH 6 respectively as shown in Figure 7. The optimized temperature for the best recovery of shea nut fat was at 60°C for pectinase, 70°C for lipase and 50°C for cellulase. However, the best optimized parameters for single and combined enzyme performance for best fat recovery is shown in Table 2.
Enzyme-assisted traditional extraction of shea butter at optimized conditions
Preliminary traditional shea butter extraction showed that the shea kernel paste proved outstanding in shea butter recovery. Nevertheless, the other substrates were still considered for enzymatic extraction of fat. Single enzyme dosage of lipase, pectinase and cellulase for extraction of fat from the SKEP substrate ranged from 52, 47, and 45% respectively. Other substrates (RASK and ROSK) gave lower fat levels. For single enzyme dosage and enzyme mixtures, the optimal conditions used were as shown in Table 2. The percentage fat recovery increased with enzyme combinations and ranged from 56, 54 and 53% for lipase-pectinase, lipase-cellulase and pectinase-cellulase mixtures respectively for SKEP. The fat recoveries from other substrates for enzyme combinations were lower (Figure 8). The best fat recovery was with 1:1:1 combination of all three enzymes on shea kernel paste. The use of enzyme combination gave the best yields a possible indication that enzyme consortia are the best for extraction of oil from fruit nuts. In all, enzyme-enhanced aqueous extraction of shea butter ranged from 22.61 to 69.96%. For single enzyme treatments, lipase gave the highest yield of 53%.
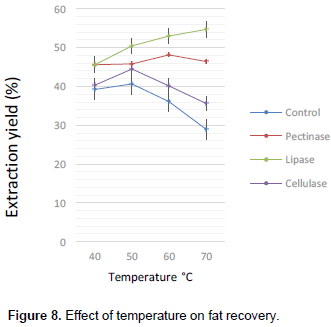
The lipase-pectinase combination offered the highest recovery rate (56%) for two enzyme combinations; and the activity from the three enzymes in combination was outstanding in fat recovery (70%) as shown in Figure 9. The shea nut kernel has 59.04% total lipids, 2.93% pectin, 5.95% cellulose (Tano-Debrah and Ohta, 1994) and this could account for why lipase has been very effective in the current research. The enzyme mixtures (lipase, pectinase and cellulase) had the tendency to break down the substrate whose major components were lipids, pectin and cellulose. Enzyme combinations do better than single enzyme doses for oil recovery (Huyanh et al., 2013) and the highest extraction rate is achieved when the mixture of enzymes are in equal proportions (Olsen, 1988; Dominguez et al., 1995; Lanzani et al., 1975; Cheah et al., 1990; Tano-Debrah and Ohta, 1995; Hernandez et al., 2000; Abdulkarim et al., 2006; Phan, 2008). Enzyme-assisted traditional extraction has thus improved the shea butter extraction by about 200% that is from 25% as was reported by Niess (1983). The statistical analysis showed that all the three samples significantly affected extraction yield (p < 0.05). Although enzymes are substrate specific their activity is often affected by some properties of the substrate such as moisture content.
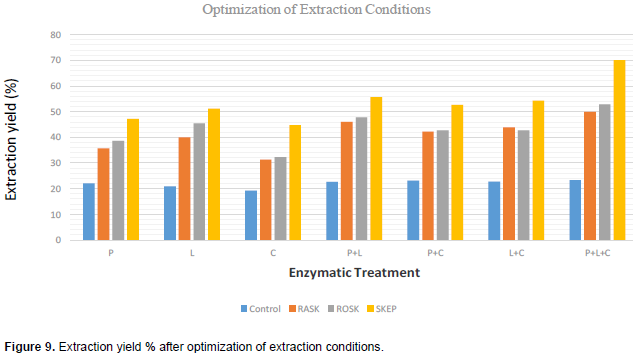
In general, enzymes are suitable for shea butter extraction and the use of enzymes for shea butter extraction will drastically reduce the time spent on the extraction process; kneading especially. The pulverized raw shea kernels gives good fat recovery rate with enzymes but the roasted kernel paste offers outstanding extraction rate with enzymes. Fat recovery was greatly affected by high substrate moisture levels possibly why the raw shea kernels consistently gave lower fat levels as compared to the roasted kernels. Thus, drying of the shea kernel and further roasting remain valuable pre-treatments in the build up to butter extraction from the shea nut kernel. Enzyme-assisted traditional extraction of shea butter using lipase, pectinase and cellulase in 1:1:1 combination was best at pH of 6, 3% enzyme-substrate concentration, 2 h hydrolysis time and at a temperature of 60°C.
Enzyme-assisted traditional extraction of shea butter from shea nut biomass is a promising technology capable of eliminating the drudgery with the mechanical extraction, the safety concerns with the chemical methods and the arduous and cumbersome nature of the traditional village extraction methods. There are still some bottlenecks; the enzymes must be sourced locally so as to reduce the cost of enzymes.
The authors have not declared any conflict of interests.
The Savannah Accelerated Development Authority (SADA) is appreciated for funding this research.
REFERENCES
|
Abdulkarim SM, Lali OM, Muhammed SK, Long K, Ghazali HM (2006). Use of enzymes to enhance oil recovery during aqueous extraction of Moringa oleifera seed oil. J. Food Lipids 13:113-130.
Crossref
|
|
|
|
Abdul-Mumeen I, Zakpaa HD, Mills-Robertso FC (2013). Biochemical and microbiological analysis of shea nut cake: A waste product from shea butter processing. J. Agric. Biotechnol. Sustain. Dev. 5(4):61-68.
Crossref
|
|
|
|
|
Addaquaye J (2004). Shea utter Value Chain: Refining in West Africa, WATH Technical Report No. 3.
|
|
|
|
|
Adeeko KA, Ajibola OO (1989). Processing factors affecting yield and quality of mechanically expressed groundnut oil. J. Agric. Eng. Resour. 5:31-43.
|
|
|
|
|
Ajayi AB (2013). Upgrade of Indigenous Methods of Production of Shea Butter. B.Sc. dissertation submitted to the department of Chemical Engineering. Covenant University, Ogun State-Nigeria.
|
|
|
|
|
Akihisa T, Kojima N, Katoh N, Ichimura Y, Suzuki H, Fukatsu (2010). Triterpene alcohol and fatty acid composition of Shea nut from seven African countries. J. Oleo Sci. 59(7):351-360.
Crossref
|
|
|
|
|
Aculey PC, Lowor ST, Kumi WO, Assuah MK (2012). The effect of traditional primary processing of the shea fruit on the yield and quality. Am. J. Food Technol. 7(2):73-81.
Crossref
|
|
|
|
|
Adomako D (1985). Prospects for the development of the shea nut industry in Ghana. Cocoa Research Institute., Ghana, Technical Bulletin 11:8 -10.
|
|
|
|
|
Association Francaise pour la Normalisation (AFNOR) (1981). Recueil des norms francaises, Corps gras, grains oleagineuses, produits derives", 2ieme edition, Paris, 438.
|
|
|
|
|
Alenyorege EA, Hussein YA, Adongo TA (2015). Extraction Yield, Efficiency and Loss of the Traditional Hot Water Floatation (HWF) Method of Oil Extraction from the Seeds of Allanblackia Floribunda. Int. J. Sci. Technol. Res. 4(02):92-95.
|
|
|
|
|
Al-hassan S, Abdul-Malik A (2011). The role of Grameen Ghana in improving income of women shea butter processors. J. Dev. Agric. Econ. 3(11):537-544.
|
|
|
|
|
Apea OB, Larbi E (2013). Indigenous Technology and Scientific Research as Ingredients for Economic Development: A Case of Shea Butter Industry. J. Contemporary Integrative Ideas 1(1):16-26.
|
|
|
|
|
Association of Official Analytical Chemist (AOAC) (1984). Methods of Analysis of the Association of Official Analytical Chemists, 14th Ed., AOAC, Washington, DC.
|
|
|
|
|
Barrios VA, Olmos DA, Noyola RA, Lopez-Munguia CA (1990). Optimization of an enzymatic process for coconut oil extraction. Oleagineux 45:35-42.
|
|
|
|
|
Chaffin B (2004). Shea Butter Republic. Routledge. New York, NY. Intro, Ch. 1.
|
|
|
|
|
Cheah SC, Augustin MA, Ooi LCL (1990). Enzymatic extraction of palm oil. Palm Oil Res. Bull. 20:30-36.
|
|
|
|
|
Chen B-K, Diosady LL (2003). Enzymatic aqueous processing of coconuts. Int. J. Appl. Sci. Eng. 1:55-61.
|
|
|
|
|
Dominguez H, Nunez MJ, Lema JM (1995). Aqueous processing of sunflower kernels with enzymatic technology. Food Chem. 53:427-434.
Crossref
|
|
|
|
|
Hernandez N, Rodiquez-Alegia ME, Gonzalez F, Lopez-Munguia CA (2000). Enzymatic treatment of rice bran to improve processing. J. Am. Oil Chem. Soc. 77:177-180.
Crossref
|
|
|
|
|
Huyanh CM, Vinh T, Frederic D (2013). Optimization of Enzyme-Assisted Extraction of Oil Rich in Carotenoids from Gac Fruit (Momordica cochinchinensis Spreng.). Food Technol. Biotechnol. 58(4):488-499.
|
|
|
|
|
Kaviani M, Darjani Z, Tomovska J, Mazandarani Z, Shariati MA (2015). Comparing Different Extraction Methods of Sesame Oil. Int. J. Pharm. Res. Allied Sci. 4(2):22-25.
|
|
|
|
|
Lanzani A, Petrini MC, Cozzoli O, Gallavresi P, Carola C, Jacini G (1975). On the use of enzymes for vegetable-oil extraction. A preliminary report. La Riv. Italiana Della Sostanze Grasse 52:226-229.
|
|
|
|
|
Mbaiguinam M, Mbayhoudel K, Djekota C (2007). Physical and Chemical Characteristics of Fruits, Pulps, Kernels and Butter of Shea Butyrospermum parkii (Sapotaceae) from Mandoul, Southern Chad. Asian J. Biochem. 2:101-110.
Crossref
|
|
|
|
|
Mohagir MA (2010). Optimisation of press extraction and decolourisa-tion of shea butter (Vitellaria paradoxa Gaertner F.). Ph.D thesis University of Ngaoundere, Cameroon.
|
|
|
|
|
Neiss T (1983). New shea butter technology for West African Women. GATE Magazine, GEZ Publication. pp 44 .
|
|
|
|
|
Ndukwe IG, Amupitan JO, Isah Y, Adegoke KS (2007). Phytochemical screening of the crude extract of the roots, stem bark and leaves of Vitallaria paradoxa (GAERTN. F). Afr. J. Biotechnol. 6(16):1905-1909.
Crossref
|
|
|
|
|
Obeng GY, Adjaloo MK, Donkor P (2010). Effect of Temperature, Moisture Content, Particle Size and Roasting on Shea Butter Extraction Efficiency. Int. J. Food Eng. 6(2):1-12.
Crossref
|
|
|
|
|
Ogbonnaya C, Adgidizi PP (2008). Evaluation of some Physico-chemical properties of Shea butter (Butyrospermum paradoxum) related to its value for food and industrial utilization. Int. J. Post-Harvest Technol. Innov. 1(3):320-326.
Crossref
|
|
|
|
|
Olaniyan AM (2010). Effect of Extraction Conditions on the Yield and Quantity of Oil from Castor Bean. J. Cereals Oilseeds 1(2):24-33.
|
|
|
|
|
Olaniyan AM, Oje K (2007). Quality Characteristics of Shea Butter Recovered from Shea Kernel through Dry Extraction Process. J. Food Sci. Technol. 44(4):404-407.
|
|
|
|
|
Olaniyan AM, Oje K (2011). Development of model equations for selecting optimum parameters for dry process of shea butter extraction. J. Cereals Oilseeds 2(4):47-56.
|
|
|
|
|
Olaniyan AM, Oje K (2013). Process Conditions Governing Mechanical Expression of Shea Butter from Crushed Shea Kernel under a Uni-axial Compression. International Journal of Engineering Research in Africa. 10:37-48.
Crossref
|
|
|
|
|
Olsen HS (1988). Aqueous enzymatic extraction of oil from seeds. In: Asian food conference proceedings Bankgok, Thailand. Reprinted by: Novo Industry A/S, pp A-0604la.
|
|
|
|
|
Otu SA, Dzogbefia VP, Kpikpi EN, Essuman EK (2015). Comparative Effect of Crude and Commercial Enzyme in Shea Fat Extraction. IOSR J. Biotechnol. Biochem. (IOSR-JBB). 1(3):18-27.
|
|
|
|
|
Paulsen G (1981). Important forest products in Africa other than wood – A preliminary study (Project Report RAF/78/025). FAO, Rome.
|
|
|
|
|
Phan TD (2008). Study of oil extraction process from avocado (Persea americana Mill.) for food and cosmetic application, University Research Report, Nong Lam University, Ho Chi Minh City, Vietnam (in Vietnamese).
|
|
|
|
|
Population and Housing Census (PHC) (2010). Ghana Statistical Service. Accessed May 14th, 2016. [www.statsghana.gov.gh]
|
|
|
|
|
Rosenthal A, Pyle DL, Niranjan K (1996). Aqueous and enzymatic processes for edible oil extraction. Enzy. Microbial Technol. 19:402-420.
Crossref
|
|
|
|
|
Russo L, Etherington T (2001). Non wood news. An info. Bull. non-wood for. products 8:38-39.
|
|
|
|
|
Sachibu M, Enno H, Suglo SBPG (2013). Behind The Butter: An Energy Analysis Of Shea Butter Processing. SNV Ghana. [www.snv.org].
|
|
|
|
|
Soladoye MO, Orhiere SS, Ibimode BM (1989). Ethanobotanical Study of two Indigenous Multipurpose Plants in the Guinea Savanna of Kwara State – Vitellaria paradoxa and Parkia biglobosa. Biennial Conference of Ecological Society of Nigeria. Forestry Research Institute, Ibadan. P 13.
|
|
|
|
|
Southwell KH, Harris R V (1992). Extraction of oil from oilseeds using the hot water flotation method. Trop. sci. 32(3):251-262.
|
|
|
|
|
Tano-Debrah K, Ohta Y (1994). Enzyme-assisted aqueous extraction of fat from kernels of the shea tree, Butyrospermum parkii. J. Am. Oil Chem. Soc. 71(9):979-983.
Crossref
|
|
|
|
|
Tano-Debrah K, Ohta Y (1995). Enzyme-assisted aqueous extraction of shea fat: A rural approach. J. Am. Oil Chem. Soc. 72:251-256.
Crossref
|
|
|
|
|
Warra AA (2011). Sesame (Sesamum indicum L.) Seed oil methods of extraction and its prospects in cosmetic industry: a review. Bayero J. Pure Appl. Sci. 4(2):164-168.
|
|