ABSTRACT
Fatty acid profile of Manketti oil extracted by screw press (SPE), supercritical fluid, Soxhlet (SE) and mechanical shaking (MS) extractions determined by chromatography (GC). Eight fatty acids were identified using authentic standards; two were calculated by equivalent chain lengths (ECL) values; α-eleostearic acid was identified by GC and spectroscopic techniques while three could not be identified. The results indicate that, extractions using hexane, SE and MS, had higher concentrations of total saturated fats of 15.71 and 16.27%, respectively, compared to SPE and SFE with 15.51 and 15.13%, respectively. On the other hand, SPE and SFE respectively had concentrations of unsaturated fats of 82.55 and 82.90%, compared to SE and MS, which had 82.08 and 81.86%, respectively. SPE and SFE had significantly higher (P < 0.05) concentrations of α-eleostearic acid of 27.01 and 26.86% compared to SE and MS with 26.28 and 26.11%. Hence, Manketti oil that is relatively rich in unsaturated fats can be obtained by SPE and SFE. Additionally, SPE and SFE oil is free of organic solvent residues, hence is suitable for edible purposes. The existence of α-eleostearic acid in this Manketti sample has been proven by GC, Gas chromatography-mass spectrometry (GC-MS), infrared (IR), ultraviolet (UV) and nuclear magnetic resonance (NMR) techniques. The results strongly agree with literature. The result is important since α-eleostearic acid is known to have the ability to lower antitumor and hepatic cholesterol; so Manketti oil could be used for neutraceutical purposes.
Key words: Manketti oil, fatty acids, screw-press, supercritical fluid, solvent extraction, α eleostearic acid.
Manketti (Schinziophyton rautanenii) is a deciduous and dioecious tree; it is a member of the family, Euphobiaceae (Graz, 2002). The fruit and nut of Manketti are referred to as the staple food of the people in South-western Zambia, North-western Namibia and North-western Botswana (Lee, 1973). It has been reported that it is edible when fresh and that the local population extracts oil from the nuts used for cooking (Engelter and Wehmeyer, 1970; Davis et al., 1983). The fatty acid composition of Manketti oil is reportedly similar to that of maize oil (Zea mays) (Mitei et al., 2008). The oil is used in modern cosmetics and skin care products because of its presumed healing and nourishing properties, in the form of a body rub and skin moisturizer (Van Wyk and Gericke, 2008; Athar and Nasir, 2005). The high degree of unsaturation of the oil renders it suitable for making paints and vanishes (Booth and Wickens, 1988).
The uses of vegetable oils for edible or industrial applications depend among other things on their fatty acid composition. The process of obtaining oil from oilseeds or nuts is an essential processing operation and the process used directly affects the quantity and quality of oil obtained from the oilseeds. The effect of different extraction methods on fatty acid composition has been reported previously. Supercritical CO2 (SC-CO2), screw press extraction and solvent extraction were compared for the extraction of flaxseed oil (Pradhan et al., 2010). SC‑CO2 gave higher concentrations of unsaturated fatty acids compared to conventional methods of screw press and solvent extractions. The screw press extraction was reported to expel oil that had higher levels of polyunsaturated fatty acids than that obtained by Soxhlet extraction. Furthermore, proton nuclear magnetic resonance (NMR) studies of oil extracted by SCâ€CO2 had a higher percentage of olefinic, allylic, methylene and allylic methylene protons. This was attributed to a higher percentage of unsaturated and conjugated fatty acids in SC-CO2 extracted oil. On the other hand, Soxhlet extracted oil showed higher percentages of -CH3 and -CH2 protons and this was attributed to a high recovery of saturated fats. The effect of Soxhlet and maceration extraction methods using different solvent polarities on fatty acid composition of Pistachio oil has also been evaluated (Abdolshahi et al., 2014). Soxhlet extraction using ethylacetate solvent gave an extract with the highest unsaturated fatty acid content of 89% and also high concentrations of oleic (52%) and linolenic (0.39%) acids. The maceration method gave a higher concentration of linoleic acid (36%). Supercritical fluid extraction (SFE) with supercritical carbon dioxide and Soxhlet extraction, with chloroform and methanol as solvents, of brown seaweed lipids were compared (Cheung et al., 1998). Higher recoveries of lipid and fatty acid contents were reported with the SFE method compared to the Soxhlet method.
Conjugated linolenic acids (CLN) are a group of positional and geometric isomers of octadecatrienoic acids that contain three conjugated double bonds. The CLN isomers have been reported in several seed oils including Tung oil which has been reported to have α‑eleostearic acid (c9,t11,t13-18:3) and β-eleostearic acid (t9,t11,t13-18:3); Punica granatum L. oil with mainly punicic acid (c9,t11,c13‑18:3) and balsam pear seed oil with mainly α‑eleostearic acid (Suzuki et al., 2001). These CLNs have been reported to have anti-tumor activity (Igarashi and Miyazawa, 2000; Yasui et al., 2006; Kohno et al., 2004). Punicic and α-eleostearic acids have been reported to decrease hepatic cholesterol level (Yang et al., 2005) and seed oils with CLN including α-eleostearic acid can be used as food supplements or in food products for cancer prevention (Shinohara et al., 2012).
Despite the use of Manketti oil for food (Engelter and Wehmeyer, 1970), cosmetics (Van Wyk and Gericke, 2008) as well as other applications, there is no literature with regards to the influence of extraction method on fatty acid composition of the oil extracted from the nuts. In addition, although Manketti oil has been reported to have α-eleostearic acid (Chisholm and Hopkins, 1966; Juliani et al., 2007), experimental evidence for its existence has not been adequately reported in literature. The general objective of this study is to extract Manketti oil and analyze its fatty acid profile. The specific objectives were to extract the oil by four different methods, namely, mechanical screw-press (SPE), supercritical fluid (SFE), Soxhlet (SE) with hexane as solvent and mechanical shaking (MS) with hexane extractions from the nuts, in order to evaluate the influence of extraction methods on fatty acid profiles of the oil. In addition, experimental evidence for the existence of α-eleostearic acid in Manketti oil will be proved through gas chromatography (GC), gas chromatography-mass spectrometry (GC-MS), infrared (IR), ultraviolet (UV), and nuclear magnetic resonance (NMR) techniques by comparing experimental results of this study with results in literature for seed oils that are known to have α-eleostearic acid.
All solvents and chemicals used, unless otherwise stated, were of analytical reagent grade and they were used without further purification. The solvents and chemicals were obtained from SMM instruments (Pty) Ltd and Sigma Aldrich (South Africa). The methylester standards of palmitate (16:0), palmitoleate (16:1∆9), stearate (18:1∆9), oleate (18:0), linoleate (18:2∆9,12), αâ€linolenate (18:3∆9,12,15), γâ€linolenate (18:3∆6,9,12), arachidate and 11‑eicosanoate (20:1∆11) were from Nu-Chek Prep, Inc. (Elysian, Minnesota, USA). Heptane used was of chromatographic quality.
Manketti nuts, originating from the Okavango region in Namibia, were bought through the Department of Forestry in Namibia and received by courier in South Africa. Here they were stored in a freezer (at -18°C) until required for analysis. The oilseeds were cleaned to remove all foreign materials, sand, metal or plastic. The oilseeds were pressed with very low pressure on the screw press expeller (Destek Engineering, Pretoria, South Africa) to reduce them to a small size. The broken nuts were used for SFE, SE and MS extractions.
Screw press extraction
A screw press expeller (Destek Engineering, Pretoria, South Africa) was used for mechanical expression of oil from Manketti oilseeds. Extraction procedure was based on previous method (Gwatidzo et al., 2014). Briefly, before full pressing, the machine was run at low pressure, with nuts, to raise the temperature to about 85°C. Once heated, full pressing was done at a feed rate of 5 kg/h with screw rotating at 50 rpm.
Supercritical fluid extraction
For SFE, a home-built pilot-plant was used and the procedure was according to previous method (Gwatidzo et al., 2014). About 10 g of ground nuts was carefully weighed and transferred to an extraction cell. The flow rate of carbon dioxide was kept at 2.5 mL/min and the sample was extracted for 2 h at 350 atm and 60°C. No solvent or modifier was used with CO2.
Soxhlet extraction
About 200 g of ground Manketti nuts was transferred to a cellulose extraction thimble and stoppered with a cotton wool plug. The thimble was placed in a Soxhlet extraction apparatus that was attached to a 500-mL round-bottomed flask, which had about 300 mL of solvent. The method was optimized for different times and solvent. The optimum extraction time was found to be 6 h
and hexane was found to be more appropriate for producing highest oil yields. The extraction was done for 6 h, in triplicate, and thereafter the hexane was removed from the extract under reduced pressure at 70°C using a rotary evaporator (Büchi, Flawil, Switzerland). The oil obtained was stored at -18°C until required for analysis.
Extraction by mechanical shaking
About 100 g of carefully weighed ground Manketti nuts was mixed with 300 mL hexane (1:3, w:v) in a 1-l Schott bottle. The bottle was mounted on reciprocating platform shaker and shaken continuously for 6 h, which was the shortest time for effective oil extraction. After the samples were filtered under vacuum, hexane was removed under reduced pressure at 35°C. This oil obtained by mechanical shaking (MS) was stored at -18°C until required for analysis.
Preparation of fatty acid methyl esters
Fatty acid methylesters (FAMEs) were prepared according to the IUPAC method (Dieffenbacher and Pocklington, 1987). About 350 mg of Manketti oil was introduced into a 50 mL round bottom flask. Methanolic NaOH (6 mL, 0.5 M) was added to the flask together with boiling chips. The mixture was boiled under reflux for about 15 min. 7 mL of methanolic boron trifluoride solution was then added and boiled for 1 min. 5 mL of HEPTANE was added and boiled for another 1 min. The flask was removed from the heat source. The condenser was removed and a small amount of saturated NaCl was added with gentle shaking by rotating several times. More saturated NaCl was added to the neck of the flask and the contents were allowed to separate. The upper heptane layer was collected, dried with anhydrous sodium sulphate to remove traces of water and filtered. This sample contained 7 to 17% FAMEs and was injected directly into the GC.
Preparation of maleic anhydride adduct
The maleic anhydride adduct was prepared according to a previous method by Tsevegsuren et al. (1998). FAMEs were prepared according to the IUPAC method just described. Since the mixture of FAMEs prepared in this manner is known to be 7 to 17% pure, 1 mL of the sample was assumed to have 0.17 g of FAME. To this mixture of FAME was added 0.24 g of maleic anhydride refluxed in toluene under nitrogen for 2 h. The toluene was removed and the FAME reconstituted in heptane and injected. The chromatograms obtained before and after reaction of the mixture of FAME of Manketti oil with maleic anhydride were compared.
Preparation of picolinyl esters
The picolinyl esters were prepared according to a previous method (Destaillats and Angers, 2002). Oil samples (10 mg) were dissolved in dry dichloromethane (1 mL) then allowed to react with a mixture of potassium tert-butoxide in tetrahydrofuran (100 µL, 1.0 M) and 3‑hydroxymethylpyridine (200 µL), at room temperature, for about 2 min. Dilute sodium bicarbonate solution was then added (2.5%, 1 mL) and the organic layer was collected, dried with anhydrous sodium sulphate and stored under nitrogen for analysis by GC-MS.
Determination of fatty acid methyl esters by gas chromatography-flame ionization detector (GC-FID)
FAMEs, in heptanes, were analysed in a Varian CP-3800 gas chromatograph equipped with a FID. An Rtx ®-2330 capillary column (30 m × 0.25 mm i.d., 0.2 µm film thickness; Restek Corporation, Bellefonte, PA) was used for the separation of the FAME. 1 µL samples were injected using a Varian CP-8400 autosampler and a split-splitless injector with a split ratio of 1:50. Injector and detector temperatures were set at 300 and 280°C, respectively. The oven temperature was initially set at 80°C and held for 5 min. It was then ramped at 10°C/min to 150°C and held for another 5 min. It was finally ramped to 270°C at 10°C /min and held for 20 min. Helium of 99.99% purity (Afrox, Africa) was used as carrier gas at a flow rate of 2.5 mL/min. The analyses were done in triplicate.
Determination of picolinyl esters of fatty acids by GC-MS
An Agilent Technologies 7890A gas chromatographic system equipped with a 7693 autosampler, split less injector and a 5975C mass selective detector was used for GC-MS analysis. Conditions employed were based on a previous method (Destaillats and Angers, 2002). An ionization voltage of 70 eV at 250°C was used. The interface temperature was maintained at 250°C and He was used as carrier gas under constant flow of 1 mL/min. The same column used for the separation of FAMEs was used for picolinyl esters. The oven temperature was kept at 200°C for 10 min, then increased to 240°C at 5°C/min for 20 min and then increased to 260°C at 5°C /minute.
Fourier transform infrared spectroscopy
Fourier transform infrared spectrum (FTIR) was determined from Manketti oil films on NaBr plates using a Perkin Elmer FTIR spectrometer (Perkin Elmer Spectrum 5, USA) with a focusing attachment to check the presence of trans unsaturated fatty acids.
Ultraviolet absorption spectroscopy
A 100 mL sample of 1% (v/v) Manketti oil was prepared. A UV spectrum of the oil was taken from a solution of the oil in ethanol using a UNICAM UV/Vis Spectrometer UV2 (UNICAM, England) with a focusing attachment to confirm the presence of any conjugated fatty acids.
Nuclear magnetic resonance analysis
Both 13C NMR and proton NMR (1H NMR) spectra of the oils were acquired at 400 MHz using a Varian Mercury 400 spectrometer. About 200 mg of the oil sample was dissolved in 700 µL CDCl3 and this solution was placed in a 5 mm diameter NMR tube. The acquisition parameters for 13C NMR spectrum were: Spectral width of 2500 Hz, number of scans 256, acquisition time 1.311 s, 1.0 s delay, 45° pulse angle with full proton decoupling and for 1H NMR, spectral width 6410 Hz, acquisition time 2.556 s, 45° pulse angle, 1.0 s delay and 8 scans.
Data analysis
Homogenized broken Manketti nut sample was extracted by each of the extraction methods. For each of the oils obtained by the extraction method, three independent oil samples were taken for the preparation of FAMEs that were injected into the GC-FID instrument.
Data obtained were analysed by STATISTICA 7.0-2004 software (StatSoft, Inc.). A 95% confidence level (P ≤ 0.05) was applied for all statistical analyses. Analysis of variance (ANOVA) was used to determine if differences in fatty acid composition obtained from the different extraction methods were significant. Differences between results from individual fatty acid contents of the extracts were tested for significance using Duncan’s new multiple range test.
Fatty acid composition of Manketti oils
Analysis of fatty acid composition of Manketti oil extracted by the different methods yielded 14 peaks (Figure 1 and Table 1). The insert in the chromatogram (Figure 1) shows the expanded view of the chromato-gram. The amount of each fatty acid expressed as a percentage of its peak area to the total peak area of all fatty acids in the oil is illustrated in Table 1. A total of eight fatty acids were identified using authentic standards, two peaks, 3 and 6, were identified by comparing their calculated equivalent chain len gth values with literature and presence of α‑eleostearic acid was confirmed by GC, GC-MS and spectroscopic techniques.

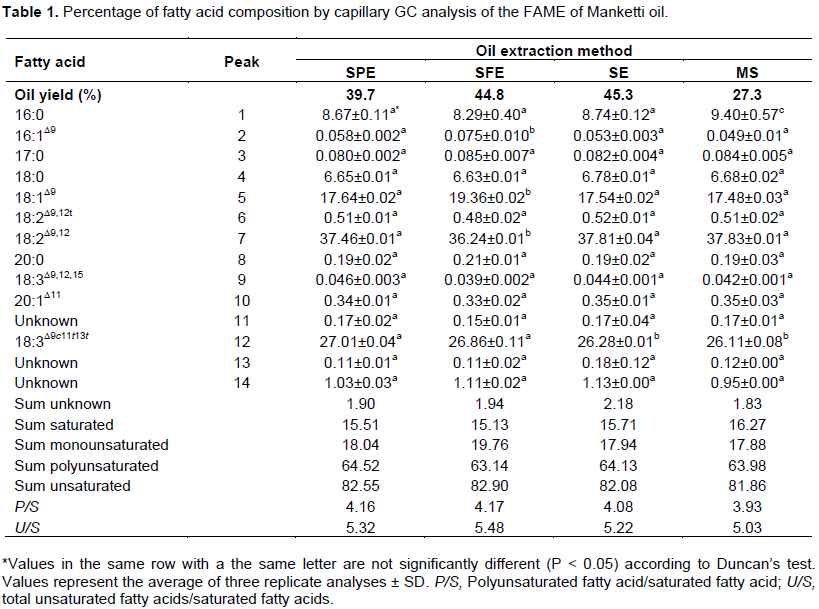
Duncan’s new multiple range test shows that the four extraction methods are not significantly different from each other, in terms of amount of fatty acid extracted, for most of the fatty acids (Table 1). SE and MS respectively gave 15.71 and 16.27% total saturated fatty acids compared to SPE and SFE that respectively gave 15.51 and 15.13%. On the other hand, SPE and SFE gave extracts that had 82.55 and 82.90% total unsaturated fatty acids, respectively, compared to SE and MS with 82.08 and 81.86%, respectively. These results are consistent with a previous report of Pradhan et al. (2010). In the cited study, fatty acid composition of flaxseed oil extracted by supercritical carbon dioxide had higher amounts of unsaturated fatty acids compared to the other two conventional methods. In addition, screw press method expelled oil with higher amount of poly-unsaturated fatty acids compared to that extracted by Soxhlet (Pradhan et al., 2010). This is also consistent with this study, that the total polyunsaturated fatty acids extracted by SPE (64.52%) are greater than SE (64.13%); however, in this study SFE (63.14%) gave lower concentrations of polyunsaturated fatty acids (Table 1).
Furthermore, 1H-NMR studies conducted on the flaxseed oil that was extracted by supercritical carbon dioxide gave higher percentages of olefinic, allylic methine and allylic methylene protons, which was attributed to higher percentages of unsaturated and conjugated fatty acids. On the other hand, higher percentages of -CH3 and -CH2 protons were reported in Soxhlet extracted oil and attributed to high recovery of saturated fatty acids. The results in this study indicate that the total percentage of olefinic, allylic methine and allylic methylene protons was high in SFE oil, followed by SPE. This may suggest that the percentage of unsaturation together with conjugated unsaturated fatty acids was slightly higher in SFE and SPE extracted oil compared to SE and MS extractions (Table 2). The slightly higher percentage of -CH3 and â€CH2 protons, seen in oils extracted by SE and MS, may suggest that slightly more saturated fatty acids are extracted by these two methods compared to SFE and SPE (Table 2).

For most of the individual fatty acids, there was no significant difference (P < 0.05) in composition of oil extracted by the different methods according to Duncan’s test. The major exceptions, however, were noted with C16:0, C18:1, C18:2 and C18:3. SE, MS and SPE oil yielded the same amount of linoleic acid since the amounts were not statistically significant. The linoleic acid in oil obtained from SFE was significantly different from all the other methods. This shows
that SFE extracted oil has less of this fatty acid compared to the other extraction methods used.
SPE and SFE extracted oil with significantly more α-eleostearic acid compared to SE and MS methods which had significantly less of this fatty acid. MS oil had the greatest amount of palmitic acid (16:0). Although there was no significant difference in the amount of palmitic acid in oil extracted by other methods, the results generally indicate that hexane extracted oils have more of this fatty acid than SPE and SFE oils. SFE method extracted oil had a higher percentage of oleic acid (18:1) in comparison to the other oils, which contained values of similar magnitude. The results also show that, although, all the methods extract oil with similar amounts of stearic acid (18:0) SE and MS extracted oil with slightly high concentrations of this fatty acid, SFE oil having the least. Other fatty acids that have been found in this study that have not been reported before, although minor, include palmitoleic, linolenic and arachidic acids. There were also three peaks that were not identified.
These results indicate that SPE and SFE are the methods of choice if Manketti oil relatively richer in unsaturated fatty acids is desired. Additionally, these methods are desirable also since the oil obtained is free of solvent residues, hence suitable for edible purposes.Oil extracted by SE and MS also has relatively high concentrations of unsaturated fatty acids though lower than the former methods; however, the oil extracts contain some organic solvent residues which are undesirable for edible or pharmaceutical applications.
Experimental evidence for the existence of α-eleostearic acid
GC-FID of FAMEs of Manketti oil after reaction with maleic anhydride
The existence of α-eleostearic acid in Manketti oil has been reported by some researchers (Chisholm and Hopkins, 1966; Chander, 2010). Its existence in Manketti oil in this study was confirmed by spectroscopic and chromatographic studies.
The presence of conjugated unsaturation was confirmed by a GC peak which decreased by about 50% after reaction of the mixed fatty acid methylesters of Manketti oil with maleic anhydride (Figure 2). This indicated that the α-eleostearic acid formed a maleic anhydride adduct by the Diels-Alder reaction (Tsevegsuren et al., 1998). Some of the methyl ester of α‑eleostearic acid reacts with maleic anhydride to form maleic anhydride adduct, which when filtered off and the fatty acid solution reinjected, a reduction of the GC peak of methyl α‑eleostearate is observed (Figure 2).
GC-MS of picolinyl esters of Manketti oil
GC-MS of picolinyl esters of Manketti oil gave peaks of only the major fatty acids including α-eleostearic acid. For α-eleostearic acid, the spectrum had the expected molecular ion at m/z 369 (Figure 3). The first ion peak at m/z 354 was obtained by loss of terminal methyl group (M-15). This was followed by a series of ion peaks 14 amu apart starting at m/z 340 and ending at m/z 312, indicating cleavage at three successive methylene groups, followed by a gap of 26 amu to m/z 286. This gap indicates the position of the terminal double bond between carbon 13 and carbon 14. This is then followed by two consecutive gaps of 26 amu to m/z 260 and 234, respectively. This is indicative of the second and third conjugated double bonds between carbon 11 and carbon 12 as well as carbon 9 and carbon 10, respectively. After this there is also a series of ion peaks 14 amu apart. This data show the presence of three conjugated bonds between carbons 9 and 14 (Figure 3). The data, however, does not tell the configuration of the double bonds.

Infrared spectrum of Manketti oil
The infrared (IR) spectrum of Manketti oil had a structure that is in agreement with the structure deduced from 1H-NMR analysis. The FTIR spectrum of Manketti oil had two characteristic absorption bands at 964 and 992 cm-1 (Figure 4). Previous work on isomers of eleostearic acid attributed a strong spectral band at 993 cm-1 to β-eleostearic acid (trans, trans, trans) and a doublet with a strong band at 991 cm-1 and a weaker band at 963 cm-1 corresponding to α-eleostearic acid (cis, trans, trans) (Prashantha et al., 2009). Therefore the corresponding doublet in the spectrum of Manketti oil (Figure 4) confirmed the presence of α‑eleostearic acid.
UV spectrum of Manketti oil
Manketti oil showed characteristic absorbance peaks in the UVC (100-290 nm) range between 240 and 290 nm. The UV spectrum of Manketti oil gave three absorption peaks with maxima at about 259, 268 and 279 nm (Figure 5). This is indicative of a cis and trans configuration as has been previously described in oils that contain α-eleostearic acid (Dyer et al., 2002). The UV spectrum of Manketti oil is also consistent with that of pure α-eleostearic acid and a seed oil of Recinodendron heudelloti that is known to have α-eleostearic acid (Nzali et al., 2012).
NMR spectrum of Manketti oil
NMR spectroscopy, an important technique for structural analysis, was used to identify α‑eleostearic acid detected by GC. The 13C NMR spectrum of Manketti oil had chemical shifts characteristic of conjugated double bonds (Table 3 and Figure 6). The chemical shifts were 128.71 (C9, c, t, t), 132.76 (C10, c, t, t), 125.88 (C11, c, t, t), 134.94 (C12, c, t, t) 131.56 (C13, c, t, t), 130.56 (C14, c, t, t). The 13C-NMR chemical shifts correspond to the cis, trans, trans configuration as reported previously for Ricinocarpus bowmanii and Ricinocarpus tuberculatus (Rao et al., 1991). The chemical shifts of the corresponding carbon atoms also compare very well with those of Parinari montana (Chrysobalanaceae) (Spitzer et al., 1992), Tragopogon and Calendula (Chisholm and Hopkins, 1960), seed oils that are known to have α‑eleostearic acid. This confirms its presence in Manketti oil.


Proton NMR of Manketti oil showed a complex multiplet in the 5.3 to 6.5 ppm region which is due to protons of conjugated dienes and trienes in triacylglycerols according to a previous report (Sbihi et al., 2015) (Figure 7). The 1H-NMR signals could be assigned as follows: δ = 6.36 (J = 12.39, J = 10.69, 1H, H-13), δ = 6.15 (J = 13.10, J = 10.92, J = 13.86, J = 10.13, 2H, H-11, H12), δ = 5.97 (J = 10.92, J = 10.13, 1H, H‑10), δ = 5.7 (J = 13.82, J = 7.31, 1H, H-14). J-values were calculated from the expanded 1H-NMR spectrum in the region of 5.6 to 6.5 ppm. A J-value of 6 to 11 Hz corresponds to a cis configuration and that of 11 to 15 Hz corresponds to a trans configuration (Yurawecz et al., 1999). As a result 9 and 10H were identified as cis configurations while 11 and 12H, 13 and 14H were assigned trans configurations. In comparison to other conjugated linoleic acids that have known configurations such as β-eleostearic acid (9t, 11t, 13t), punicic (9c, 11t, 13c), catalpic (9t, 11t, 13c), calendic (8t, 10t, 12c), jacaric (8c, 10t, 12c) acids, only α-eleostearic acid has a c, t, t configuration.

Seed oils with α-eleostearic acid have been used as drying oils, suitable for making varnishes, coatings, alkyd resins for paints (Mertzger and Bornscheue, 2006) as well as in cosmetic formulations (Gunstone, 2005). α‑Eleostearic acid has been reported to have antioxidative and anticancer activity and has been suggested for use as a therapeutic agent for the treatment of cancer (Zhang et al., 2012) and also for lowering hepatic cholesterol (Yang et al., 2005). Manketti oil, with a significant amount of α-eleostearic acid, could possibly be used for these industrial and nutraceutical applications.
Results have shown that extraction techniques that utilize hexane solvent (SE and MS) gave rise to extracts that had slightly higher concentrations of saturated fatty acids compared to SPE and SFE which on the other hand produced extracts with higher concentrations of unsaturated fatty acids. These results are important if the oil is desired for edible or pharmaceutical uses. For edible purposes oil richer in unsaturated fatty acids is desirable than that with saturated fatty
acids . Saturated fatty acids increase the risk of cardiovascular diseases. Furthermore, oil obtained by SPE and SFE is cleaner and free of organic solvent residues and hence suitable for pharmaceutical applications. SPE and SFE extracted oil was slightly richer in unsaturated and conjugated fatty acids while SE and MS had slightly more saturated fatty acids. Significantly higher concentrations of α-eleostearic acid were observed with SFE and SPE extraction methods compared to SE and MS methods. This shows SFE and SPE are the methods of choice to obtain oil extracts rich in this fatty acid which has important health benefits.
Experimental evidence for the existence of α-eleostearic acid has been proved by chromatographic and spectroscopic techniques. Therefore, this sample of Manketti oil has α‑eleostearic acid, and may be used for nutraceutical applications. Fatty acids that have been found in this study have not been reported before, although minor, were palmitoleic, linolenic and arachidic acids.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdolshahi A, Mortazavi SA, Naibandi S, Shabani AA, Elhmai AH, Taheri M, Heydari Majd M (2014). Effect of solvent and extraction techniques on the fatty acid composition of Pistachio oil. Iran Food Sci. Technol. J. 10(2).
|
|
|
|
Athar M, Nasir SM (2005). Taxonomic perspective of plant species yielding vegetable oils used in cosmetics and skin care products. Afr. J. Biotechnol. 4:36.
|
|
|
|
Booth FEM, Wickens GE (1988). Non-timber uses of selected arid zone trees and shrubs in Africa. FAO Conservation Guide 19. Bookmark:
View
|
|
|
|
Chander AK (2010). Characterisation and oxidative stability of speciality plant seed oils. PhD Thesis, Aston University.
|
|
|
|
Cheung PCK, Leung AYH, Ang PO (1998). Comparison of Supercritical Carbon Dioxide and Soxhlet Extraction of Lipids from a Brown Seaweed, Sargasum hermiphylum (Turn.) C. Ag. J Agric. Food Chem. 46(10):4228-4232.
Crossref
|
|
|
|
Chisholm MJ, Hopkins CY (1960). Conjugated fatty acids of Tragopogon and Calendula seed oils. Can. J. Chem. 38(12):2500-2507.
Crossref
|
|
|
|
Chisholm MJ, Hopkins CY (1966). Kamlolenic Acid and Other Conjugated Fatty Acids in Certain Seed Oils. J. Am. Oil Chem. Soc. 43:390-392.
Crossref
|
|
|
|
Davis JB, Kay DE, Clark V (1983). Plants tolerant of arid, or semi-arid, conditions with non-food constituents of potential use. Report of the Tropical Products Institute G 150, London Bookmark.
|
|
|
|
Destaillats F, Angers P (2002). One step methodology for the synthesis of fatty acid picolinyl esters from intact lipids. J. Am. Oil Chem. Soc. 79:253-256.
Crossref
|
|
|
|
Dieffenbacher A, Pocklington WD (1987). Standard methods for the analysis of oils, fats and derivatives. IUPAC, Blackwell Scientific Publications, Oxford Bookmark:
View
|
|
|
|
Dyer JM, Chapital DC, Kuan JC, Mullen, RC, Turner T, McKeon TA, Pepperman AB (2002). Molecular analysis of a bifunctional fatty acid conjugase/desaturase from Tung: Implications for the evolution of plant fatty acid diversity. Plant Physiol. 130:2027-2038.
Crossref
|
|
|
|
Engelter C, Wehmeyer AS (1970). Fatty acid composition of oils of some edible seeds of wild plants. J. Agric. Food Chem. 18:25.
Crossref
|
|
|
|
Graz FP (2002). Description and Ecology of Schinziophyton rautanenni (Schinz) Radcl.-Sm in Namibia. Dinteria 27:19-35.
|
|
|
|
Gunstone FD (2005). Bailey's Industrial Oil and Fat Products. Shahidi F (ed.). Sixth Edition, John Wiley & Sons, USA.
Crossref
|
|
|
|
Gwatidzo L, Botha BM, McCrindle RI (2014). Extraction and identification of phytosterols in Manketti (Schinziophyton rautanenii) nut oil. J. Am. Oil Chem. Soc. 94:783 794.
Crossref
|
|
|
|
Igarashi M, Miyazawa T (2000). New recognised effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 148:173-179.
Crossref
|
|
|
|
Juliani HR, Koroch AR, Simon JE (2007). Mungongo cold pressed oil (Schinziophyton rautanenii): A new natural product with potential cosmetic applications. Acta Hortic. 756:407.
Crossref
|
|
|
|
Kohno H, Suzuki R, Yasui K, Hokosawa M, Miyashita K, Tanaka T (2004). Pomogranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 95(6):481-486
Crossref
|
|
|
|
Lee RB (1973) Mongongo: The ethnography of a major wild food resource. Ecol. Food Nutr. 2:307-341.
Crossref
|
|
|
|
Metzger JO, Bornscheuer U (2006). Lipids as renewable resources: current state of chemical and biotechnological conversion and diversification. Appl. Microbiol. Biot. 71(1):13-22.
Crossref
|
|
|
|
Mitei YC, Ngila JC, Yeboah SO, Wessjohann L, Schimidt J (2008). NMR, GC-MS and ESI-FTICT-MS Profiling of Fatty Acids and Triacylglycerols in Some Botswana Seed Oils. J. Am. Oil Chem. Soc. 85:1021.
Crossref
|
|
|
|
Nzali HG, Tchiegang C, Mignolet E, Turu C, Larondelle Y, Meurens L (2012). Study of the bioconversion of congugated linolenic acid of Ricinodendron heudelotti (Bail.) Seed in Male Rats in Conjugated Linoleic Acid Using UV-Vis and Gas Chromatography. Asian J. Biochem. 7(4):194-205.
Crossref
|
|
|
|
Pradhan RC, Meda V, Rout PK, Naik S, Dalai AK (2010). Supercritical CO2 extraction of fatty oil from flaxseed and comparison with screw press expression and solvent extraction processes. J. Food Eng. 98:393-397.
Crossref
|
|
|
|
Prashantha MAB, Premachandra JK, Amaresinghe ADUS (2009). Composition, physical properties and drying characteristics of seed oil of Momordica charantia cultivated in Sri Lanka. J. Am. Oil Chem. Soc. 86:27-32
Crossref
|
|
|
|
Rao KS, Kaluwin C, Jones GP, Rivett DE, Tucker DJ (1991). New source of α-eleostearic acid: Ricinocarpus bowmanii and Ricinocarpus tuberculatus seed oils. J. Sci. Food Agr. 57(3):427-429.
Crossref
|
|
|
|
Sbihi HM, Nehdi IA, Al-Resayes SI (2015). Characterisation of white Mahlab (Prunus Mahaleb L.) Seed Oil: A Rich Source of α Eleostearic Acid. J. Food Sci. 79(5):C795-C801.
Crossref
|
|
|
|
Shinohara N, Tsuduki J, Ito J, Honma T, Kijima R, Sugawara, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, Mishiyama K, Nakagawa K, Miyazawa T, Ikeda I (2012). Jacaric acid and alpha-linolenic acid with a conjugated triene system has a strong antitumor effect in vitro and in vivo. Biochim. Biophys. Acta 1821:980-988.
Crossref
|
|
|
|
Spitzer V, Marx F, Maia JGS, Pfeilsticker K (1992). Occurence of alpha-eleostearic acid in the seed oil of Parinari montana (Chrysobalanaceae). Fat Sci. Technol. 94:58-60.
Crossref
|
|
|
|
Suzuki R, Noguchu R, Ota T, Abe M, Miyashita K, Kawada T (2001). Cytotoxic effect of conjugated trienoic fatty acid on mouse tumor and human monocytic leukaemia cells. Lipids 36:477-482.
Crossref
|
|
|
|
Tsevegsuren N, Christie WW, Lösel D (1998). Tanacetum (Chrysanthemum) corymbosum Seed Oil - A Rich source of Novel Conjugated Acetylenic Acid. Lipids, 33:723-727.
Crossref
|
|
|
|
Van Wyk B, Gericke N (2008). People's plants. A guide to useful plants of southern Africa. Briza Publications, Pretoria.
|
|
|
|
Yang L, Leung KY, Ying C, Yuang Y, Ratnayake WMN, Chen ZY (2005). α-Linolenic acid but not conjugated linolenic acid is hypocholesterolaemic in hamsters. Brit. J. Nutri. 93:433-438.
Crossref
|
|
|
|
Yasui Y, Hosokawa M, Kohno H, Tanaka T, Miyashita K (2006). Troglitazone and 9cis, 11trans, 13trans-conjugated linolenic acid: composition and antiproliferative and apoptosis-inducing effects on different colon cancer cell lines. Chemotherapy 52(5):220 225.
Crossref
|
|
|
|
Yurawecz MP, Roach JAG, Sehat N, Mossoba MM, Cramer JKG, Fritsche J, Steinhart H, Ku Y (1999). A new conjugated linoleic acid isomer 7 cis, 9 trans-octadecadienoic acid in cow milk, cheese, beef, human milk and adipose tissue. Lipids 33:803-809
Crossref
|
|
|
|
Zhang T, Gao Y, Mao, Y, Zhang Q, Lin C, Lin P, Zhang J, Wang X (2012). Growth inhibition and apoptotic effect of alpha-eleostearic acid on human breast cancer cells. J. Nat. Med. 66:77-84.
View
|









