ABSTRACT
The leaves of Spondias mombin L. consumed as infusion in Ivory Coast are used to treat gastrointestinal disorders such as diarrhea. In this study, the leaves’ extract, after purification by the ethyl acetate method, was evaluated for its effect on growth and cell viability of two Gram positive bacteria (Staphylococcus aureus ATCC25923 and Listeria monocytogenes ATCC25051) and four Gram negative bacteria (Pseudomonas aeruginosa ATCC27853, Escherichia coli ATCC25922, Salmonella enteritidis ATCC27042 and Klebssiela pneumonia ATCC27001) which are foodborne pathogens. This evaluation was performed by following their growth by a spectrophotometric method in Mueller-Hinton broth contained amount of antibacterial fraction ranging from 0.5 to 3%. The total inhibition of the Gram positive species’ growth was observed in the medium at 2.5% of antibacterial fraction, while that of the Gram negative species was observed in the medium at 3% of antibacterial fraction after 24 h of incubation. The test of the percentage in reduction of Alamar blue indicating cell viability showed that in the medium at 2.5% of antibacterial fraction, around 3% of cells were still alive for the two Gram positive bacteria after two days of incubation. At these same concentration and incubation time, around 7% of the cells were still alive for the four Gram negative bacteria. These results suggest that different classes of compounds in the extract are likely responsible for the bactericidal activities. Thus, it can be used as a natural preservative and an alternative to current chemical preservatives used in food which are harmful for human health.
Key words: Antibacterial fraction, bacteria, cell viability, foodborne pathogens, growth rate.
The multiple cases of diseases due to food consummation highlight the importance of food safety, particularly in Africa, South-East Asia and other regions (South America and East Europe). Indeed, foods can become
contaminated at multiple points along the food’s journey from production to consumption (Heaton and Jones, 2008). In summary, there are over 250 infectious and non-infectious agents that could contaminate food and many recognized food vehicles (Newman et al., 2015). It has been shown that the main causes of foodborne illness are bacteria (66%), chemicals (26%), virus (4%) and parasites (4%) (Khare et al., 2018). Prior to this study, Mead et al. (1999) indicated that bacteria are the causative agents of foodborne illness in 60% of cases requiring hospitalization. These authors have also shown that, almost all reported cases of foodborne illness are caused by bacteria or by their toxins. These toxins are commonly produced in food before it is eaten and cannot be detected by taste, odor or color. In addition to bacteria, all the microorganisms capable of producing toxins are also capable of causing foodborne illnesses (Ramesh and Rao, 1987). This foodborne illness is often used to describe any disease caused by eating contaminated foods or drinks (Sudershan et al., 2014). Despite the data gaps and limitations of the estimates, it is apparent that the global burden of foodborne diseases is considerable, and affects individuals of all ages, particularly children under 5 years of age and persons living in low-income regions of the world (WHO, 2015). Thus, food is capable of supplying consumers with nutrients, and also capable of supporting the growth of contaminating microorganisms. The consummation of food contaminated by microorganisms such as bacteria leads to gastrointestinal disorders such as stomach upset, stomach pain, dysentery and especially diarrhea most of the time (Global Burden Diseases, 2015). Therefore, innovative technologies are urgently needed to reduce the risks of contamination of food by pathogens microorganisms.
For many years now, it has been clear that the most effective means to avoid contamination of food by microorganisms is the prevention of this contamination of food by pathogens microorganisms (Bullerman, 1977). After the application of good practices of production and conservation of food which are not totally efficient, chemical preservatives are most of the time used for the inhibition of microorganisms’ development in food. However, they have become less favored by regulators due to the toxicological risks (Directive 91/414/CEE). Therefore, the use of natural substances capable on one hand of inhibiting microorganisms’ development including foodborne pathogens and on the other hand killing these foodborne pathogens is of a great importance.
Nature around us provided everything of necessity of mankind. Among the plants around us, there is Spondias mombin Linn which belongs to the family of Anacardiacae (broadleaf plants). It grows in the rain forest and in the coastal area. It can reach a height of 15 to 22 m. The trunk has deep incisions in the bark, which often produces a brown resinous substance. The leaves and the flowers are at the end of the branches. Before the tree starts to flower, it strips itself from most of the leaves (Ayoka et al., 2008; Tiburski et al., 2011). Young leaves are cooked as greens. A decoction of the young leaves is a remedy for diarrhea and dysentery. The juice of crushed leaves and the powder of dried leaves are used as poultices on wounds and inflammations (Ayoka et al., 2008; Akoua-Koffi et al., 1993). Infusion of the leaves has been used since long time, without any report of collateral effects, due to its activity (Corthout et al., 1991). These leaves have been shown to possess antimicrobial (Abo et al., 1999) and anti-viral (Corthout et al., 1992) properties.
However, even if the infusion of these leaves has been used since long time, without any report of collateral effects, the determination of the minimum concentration of leaves’ extract which can kill pathogens bacteria is of a great importance because as a low concentration of a substance could affect the intestinal microbiota of consumers, a high concentration of a substance which possesses antimicrobial activities could also affect this intestinal microbiota of consumers.
Thus, in this study, the extract of the leaves of S. mombin L. consumed in Ivory Coast was evaluated for its bactericidal effect against foodborne pathogens for a contribution to the research for natural substance which can be used as alternative to current chemical preservatives used in food and also in feed. This natural substance could also be used to find out a solution to overcome the resistance of bacteria towards antibiotics by determining the minimum bactericidal concentration of this extract.
In this study, the leaves of S. mombin L. were used. These leaves were collected from rural zone of the central part (Daoukro) located at 250 km north of Abidjan, the economic capital of Ivory Coast. The mean of temperature of this rural zone was 27.4°C with a mean of rainfall of 826.4 mm. Six foodborne bacterial strains (Pseudomonas aeruginosa ATCC27853, Staphylococcus aureus ATCC25923, Listeria monocytogenes ATCC25051, Escherichia coli ATCC25922, Salmonella enteritidis ATCC27042 and Klebssiela pneumonia ATCC27001) from the Pasteur Institute of Abidjan, Ivory Coast were also used.
The choice of these strains was due to the fact that, they were frequently isolated in food and feed sold on Ivorian markets. The bacterial culture medium used in this study was the medium Mueller-Hinton (broth and agar medium).
Preparation of the leaves extract
The method used was that used by Kouadio et al. (2013). S. mombin L. leaves were dried in the shelter of the sun. These dried leaves were ground and 30 g of the obtained homogenate were added to 150 ml of 100% ethanol. The mixture was boiled in water bath at 80°C for 1 h under gentle stirring. The resulting mixture was centrifuged at 2000 rpm for 10 min. The supernatant was then filtered through Whatman paper with 2.5 µm as pore size. The resulting solution was lyophilized. The lyophilisate obtained was dissolved into 15 ml of distilled water and shaken until total dissolution. In order to fractionate the suspension homogenized and used the fraction containing the compounds which could inhibit the bacteria strain tested, the method of fractioning by ethyl acetate was used. This fractioning of the extract was made by adding to the homogenate obtained, 15 ml of ethyl acetate 100%. The resulting mixture was shaken during 1 min. and centrifuged at 2000 rpm for 10 min. Aqueous and ethyl acetate phases were obtained. The ethyl acetate phase was recovered into a new tube. To the remaining aqueous phase, 15 ml of ethyl acetate were added again, shaken and centrifuged as described earlier. This fractioning was done three times. The three ethyl acetate phases were put into the same tube and the aqueous phase into another tube and then, these two solutions obtained were dried under fume hood. The residues of the aqueous and ethyl acetate phases were dissolved, respectively into distilled water and ethyl acetate and then filtrated separately onto 0.20 μm cutoff membranes to eliminate eventual contaminants. These aqueous and ethyl acetate fractions were evaluated for their antibacterial activities in order to use the one containing the antibacterial compounds for the evaluation of its effect on bacteria growth and bacteria cells viability. The concentration of the tested extracts was 0.35 g/ml.
Preparation of the tested species
The preparation of the tested species was done according to the method of CLSI (1999). Quantity of 1 ml of each strain previously stored in glycerol 15% at -20°C was thawed in 9 ml of liquid Mueller-Hinton medium. The obtained suspension was firstly incubated at 30°C for 8 h. In a second step, 1 ml each of the microbial suspension obtained after 8 h of incubation was put in 9 ml of Mueller-Hinton. The whole was incubated at 30°C overnight. The absorbance of this second culture was measured with a spectrophotometer at 630 nm. The optical density was adjusted at 0.6 by diluting (1/10) and the microbial suspension was used for the inhibition of bacteria growth and the bioassay analysis (test for the determination of the percentage of reduced of Alamar Blue).
Evaluation of the antibacterial activities of the fractions obtained after purification of the leaves extract by the ethyl acetate method
Each bacteria suspension was sprayed onto the Mueller-Hinton medium with 0.1 ml. A disc of 1 cm of diameter was impregnated with 100 μl of each fraction of the extract and put onto the medium inoculated. Each medium with impregnated disc was incubated at 30°C. The disc around which any bacteria growth was observed was identified as the disc impregnated with the fraction containing the antibacterial compounds.
Monitoring of bacteria growth
The fraction containing the antibacterial compounds was added to the liquid medium of Mueller-Hinton (v/v) to obtain mediums with different concentrations of 0.5, 1, 1.5, 2, 2.5 and 3%. Medium without antibacterial fraction was also used as positive control to validate the effect of the antibacterial fraction on bacteria species. These solutions at different concentrations were prepared in order to determine the minimum concentration of a total inhibition of the bacteria growth. The analysis was carried out by putting a quantity of 300 μl of each concentration in separate wells of the micro-plate without any strain (negative control). Each solution was placed in at least three wells of the micro-plate. In a second step, the microbial strains tested (30 μl each) are cultured in other separate wells of the micro-plate contained the different concentrations mentioned earlier (270 μl each). These cultures were made in at least three wells of the micro-plate. The seeded plate was cultured in the Bioscreen apparatus at 30°C and at a wavelength of 600 nm for 24 h. The measurement of the optical density expressing microbial growth was taken every 30 min. The experiment was carried out three times.
Bioassay analysis
The experiment was conducted over a span of 3 days. After each 24 h of incubation, 700 μl of liquid medium of Mueller-Hinton and 300 μl of Alamar blue reagent were added into each tube. The final concentration of the Alamar blue reagent into each test-tube was 10%. Then, the microbial suspension with the Alamar blue reagent was incubated at 37°C for 4 h. A liquid medium of Mueller-Hinton without the microbial suspension but containing Alamar blue reagent was also incubated.
After this incubation time, 100 μl of each suspension was put into separate wells of a micro-plate and the absorbance was monitored at 570 nm using 600 nm as a reference wavelength in an apparatus Bio-Teck ELISA (Kouadio et al., 2011).
Statistical analysis
The statistical analysis of data was done by Analysis of Variance (ANOVA) using 5% level of significance. Statistics for each analysis are based on cases with no missing data for any variable in the analysis. The statistical package used is IBM SPSS Statistics version 20. Tukey's Multiple Comparison test was used to identify these differences.
Identification of the fraction containing the antibacterial compounds (saponins, anthraquinone, glycosides)
The results showed that the antibacterial compounds derived from S. momin leaves were water-soluble compounds. Indeed, the discs around which any bacterial growth was observed were those impregnated with the aqueous fraction (Figure 1). Thus, as the infusion of these leaves was prepared using water, these antibacterial compounds are in it. This suggests that consuming the infusion of the leaves means consuming antibacterial compounds of from these leaves.
Monitoring of growth of bacteria species
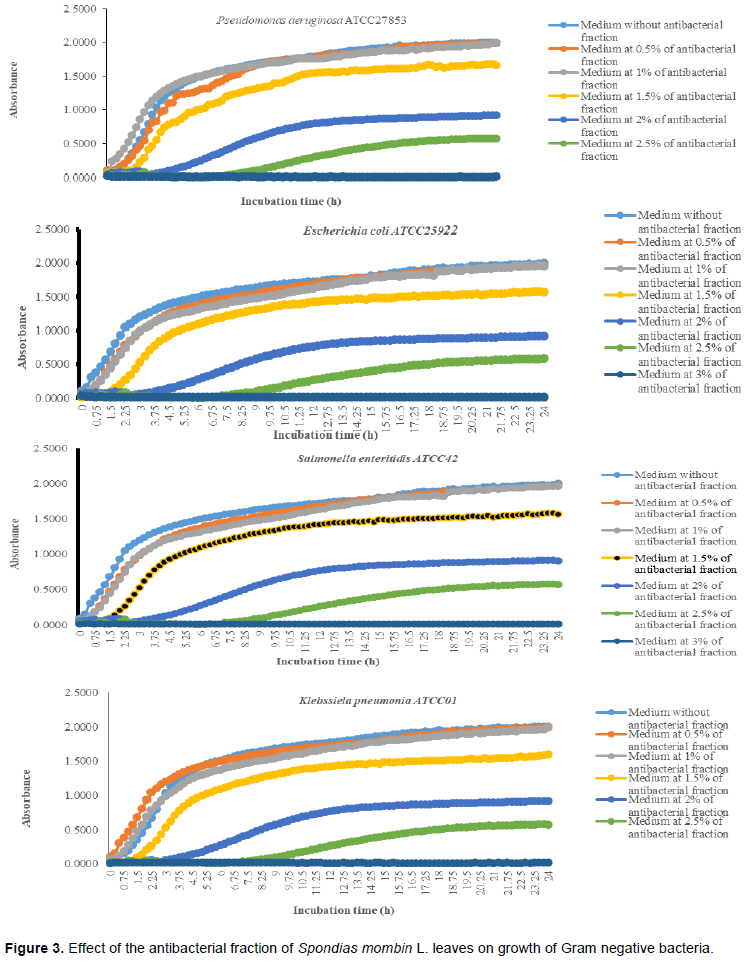
It was noted that with the increases in the antibacterial fraction content in the medium, a decrease of growth in a dose-dependent manner was noted (Table 1). Indeed, from the medium without antibacterial fraction to the medium at 0.5% of antibacterial fraction, no significant reduction of the growth was observed for the Gram positive bacteria (S. aureus ATCC25923 and L. monocytogenes ATCC25051) (P>0.05). However, from the medium at 1% of antibacterial fraction, the significant reduction of the growth of these Gram positive bacteria was observed with a total inhibition of their growth observed from the medium at 2.5% of antibacterial fraction (Figure 2). For the Gram negative bacteria (P. aeruginosa ATCC27853, E. coli ATCC25922, S. enteritidis ATCC27042 and K. pneumonia ATCC27001), no significant reduction of the growth was observed from the medium without antibacterial fraction to the medium at 1% of antibacterial fraction (P>0.05).
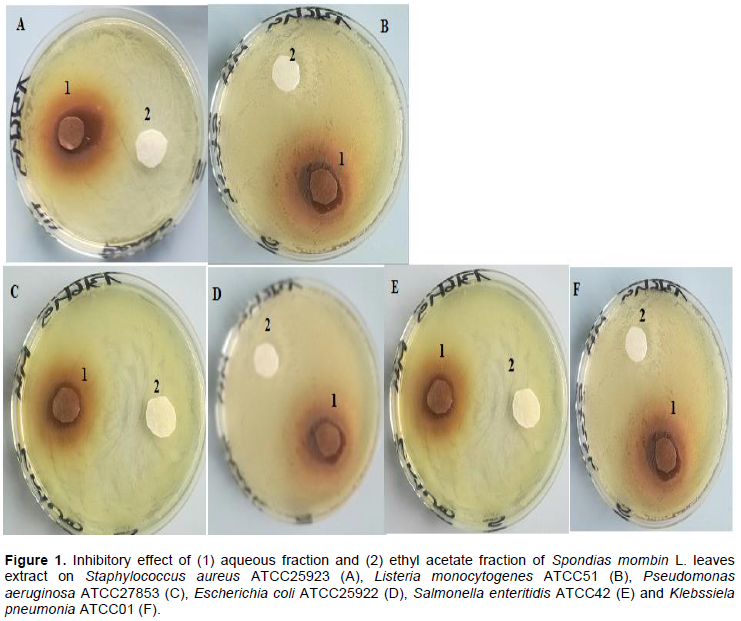
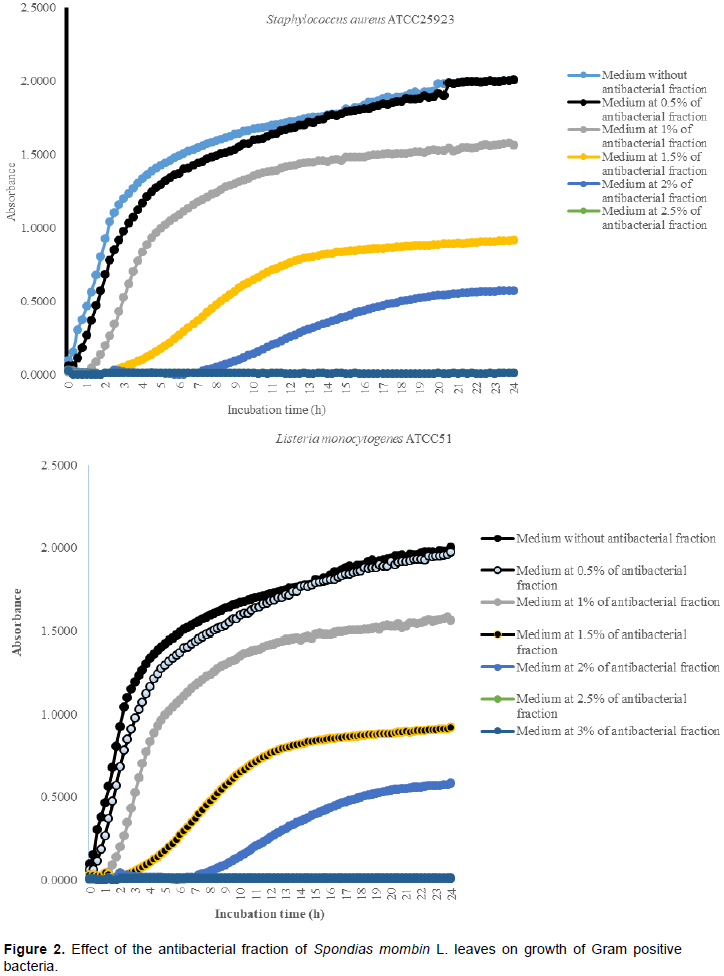
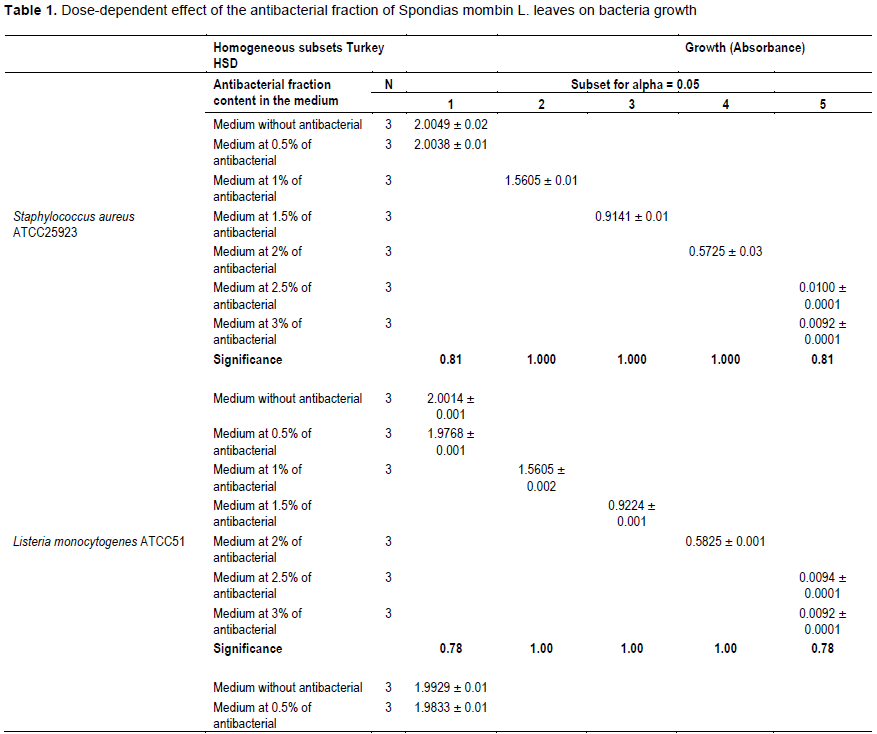
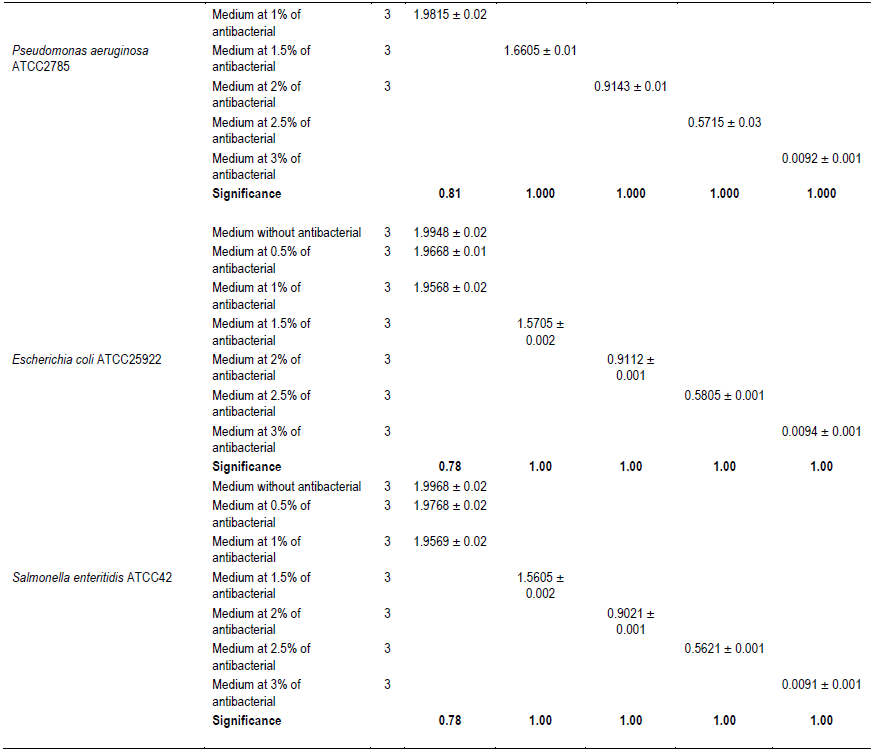
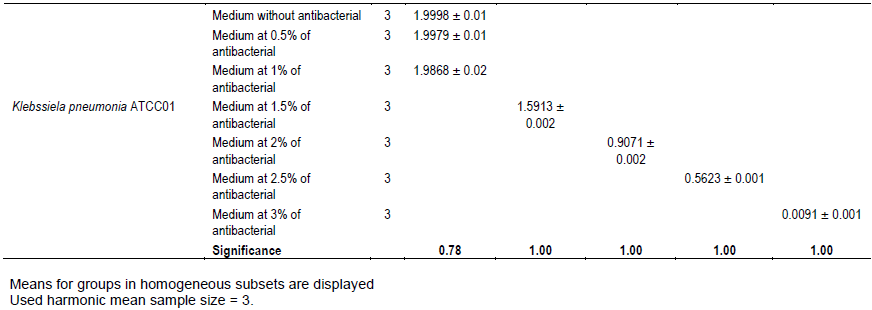
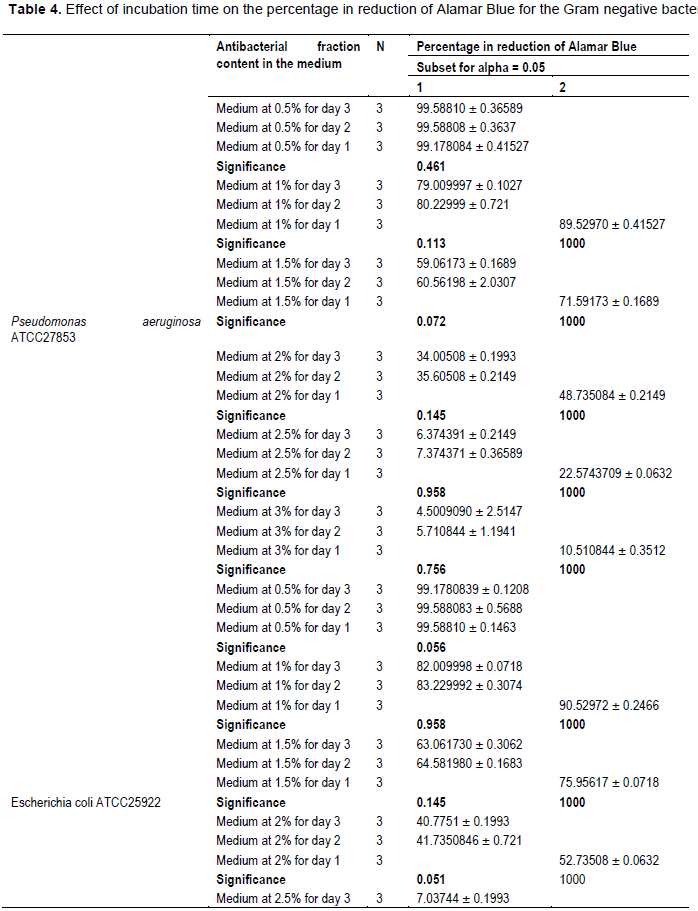
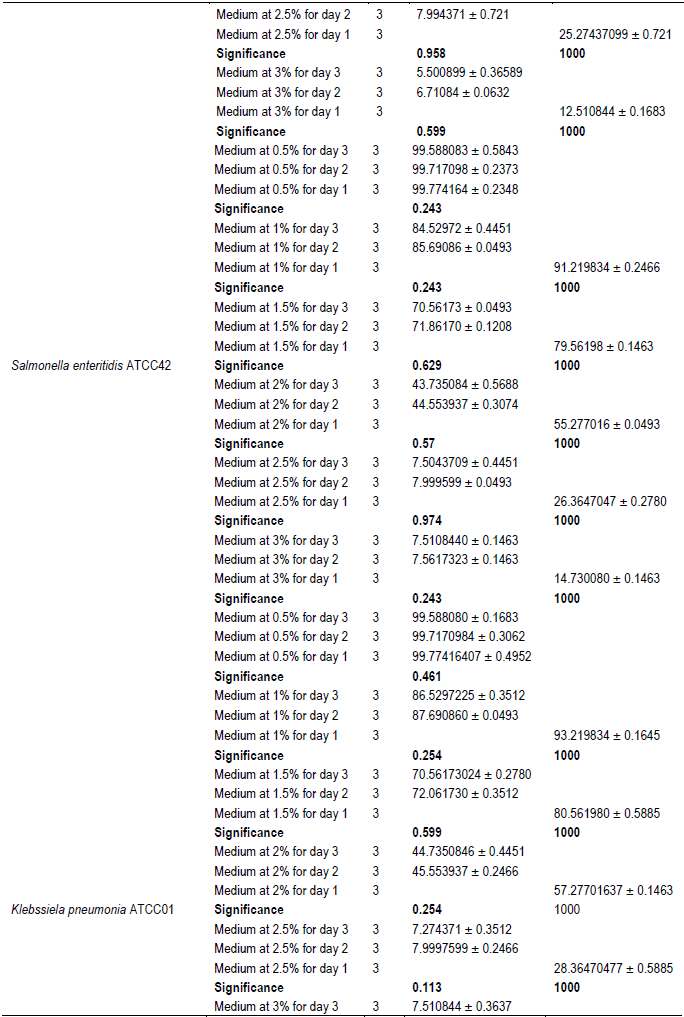
However, a significant reduction of the growth of these Gram negative bacteria was noted from the medium at 1.5% of antibacterial fraction (P<0.05). The total inhibition of their growth was observed in the medium at 3% of antibacterial fraction (Figure 3). These results show on one hand, the effective inhibitory effect of the leaves extract on the proliferation of the six bacteria species tested and on the other hand, they show that different classes of compounds are likely responsible for the antibacterial activities. The sensitivity of the Gram negative strains and the Gram positive strains tested appeared very different. Indeed, the more sensitive species to the leaves extract were S. aureus and L. monocytogenes. Previous studies on the leaves of this plant (Abo et al., 1999; Rodrigues and Hasse, 2000) have also reported that their extract exhibited antibacterial activities. However, these authors reported that S. mombin leaves possess significant antibacterial activities on Bacillus cereus, Streptococcus pyogenes and Mycobacterium fortuitum. This showed a large antibacterial activities spectrum for the leaves of this plant that could be explained by their compounds content (saponins and anthraquinone glycosides). Indeed, the composition of an extract could impact the biological proprieties of this extract. This fact was shown by Corthout et al. (1994) who demonstrated that the phenolic acid, 6-alkenyl-salicylic acid of this plant extract is responsible for the antibacterial activities. For Coates et al. (1994), the anacardis acid derivative from the hexane extract of the plant was shown to possess beta lactamase inhibitory properties.
Thus, further studies should be carried out on the antibacterial fraction obtained in our present study in order to find out all the compounds responsible of the inhibitory effect on the Gram positive bacteria and those responsible of the inhibitory effect on the Gram negative bacteria.
Evaluation of the effect of the antibacterial fraction on cells viability of bacteria
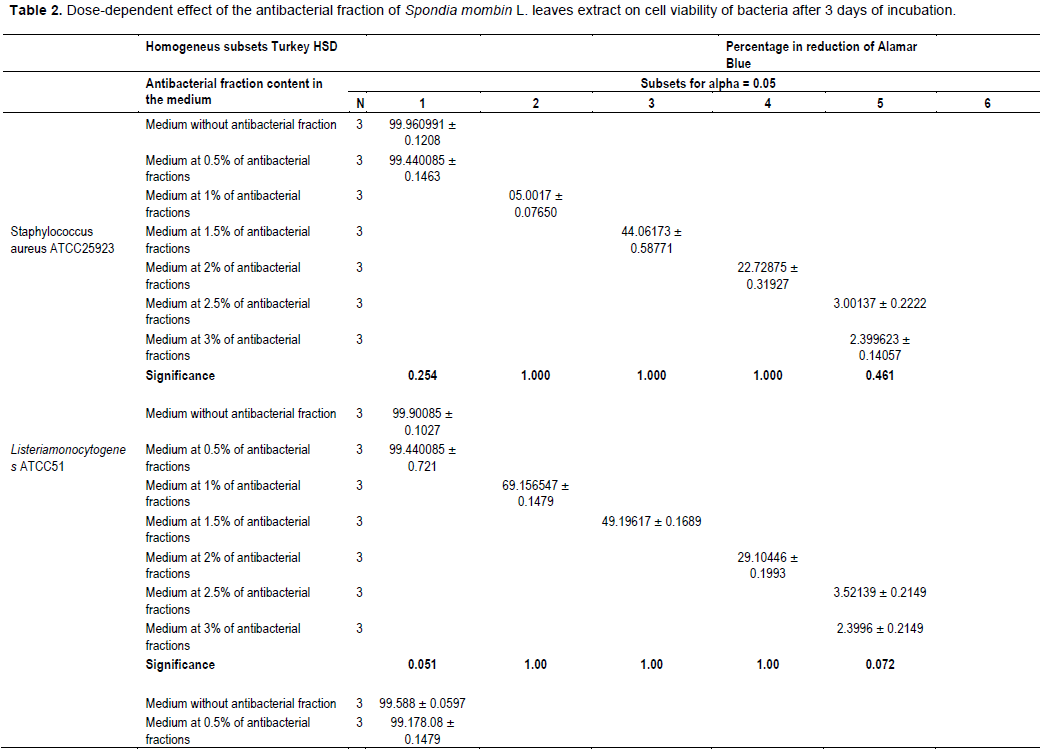
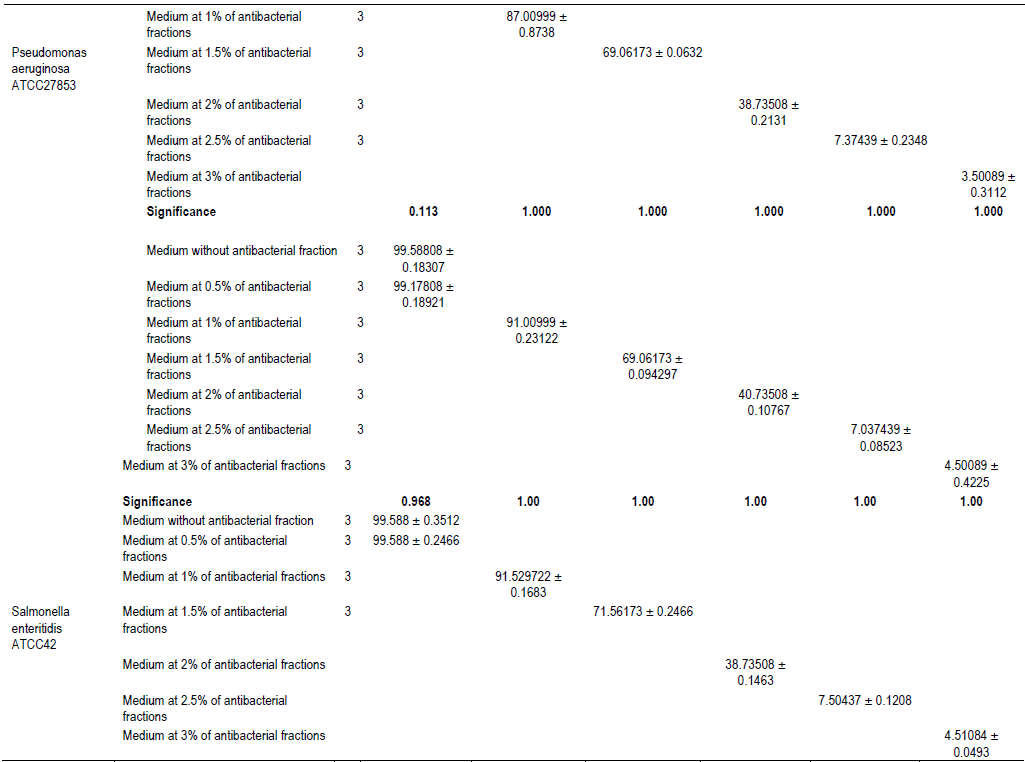
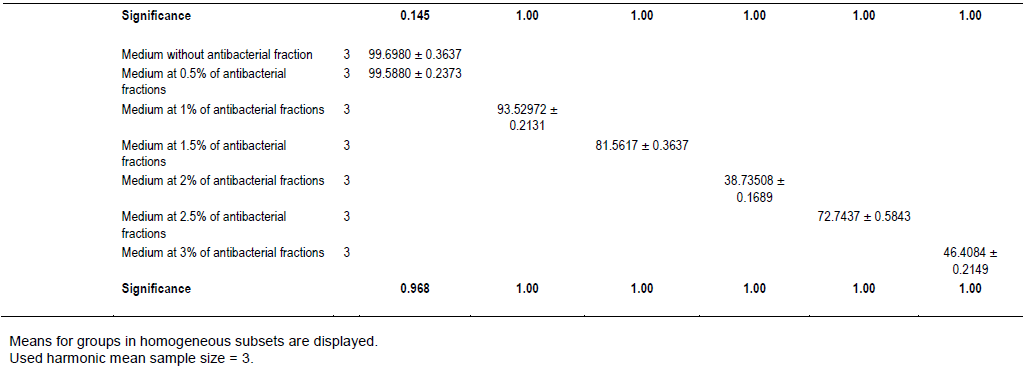
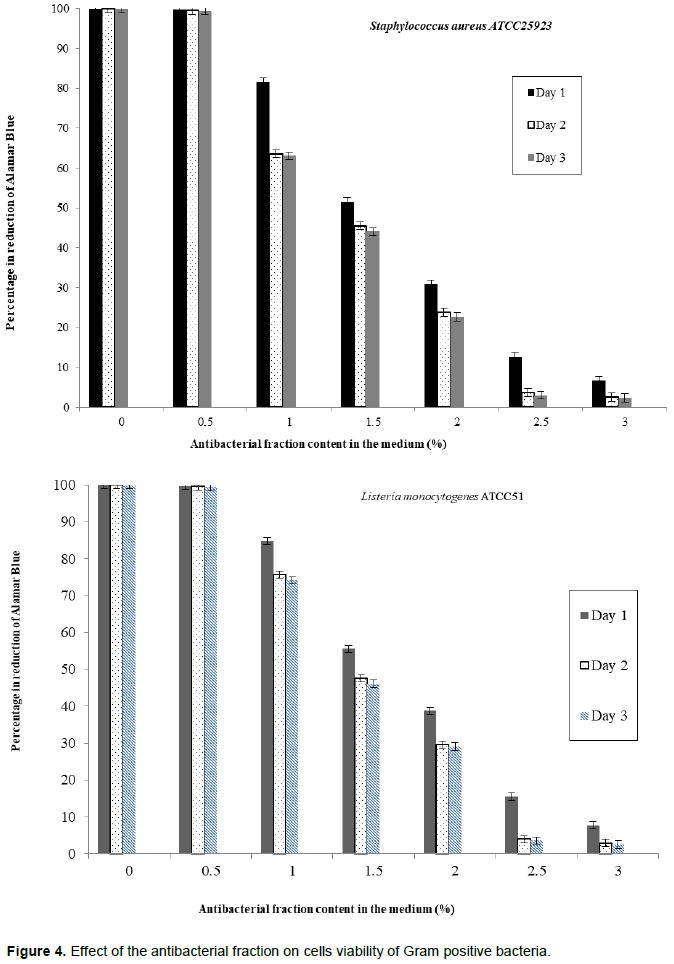
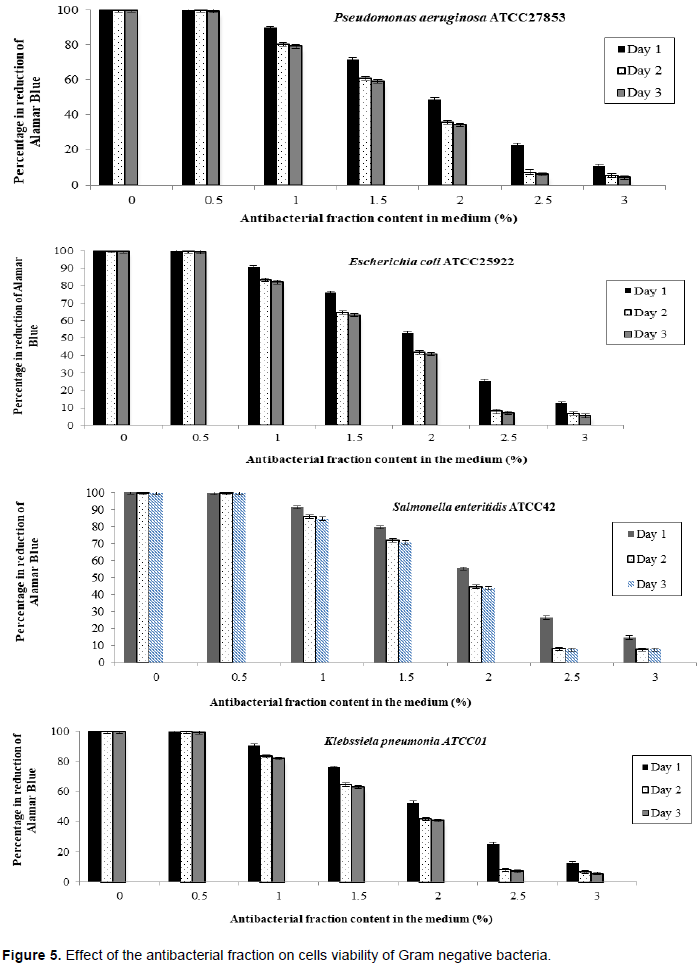
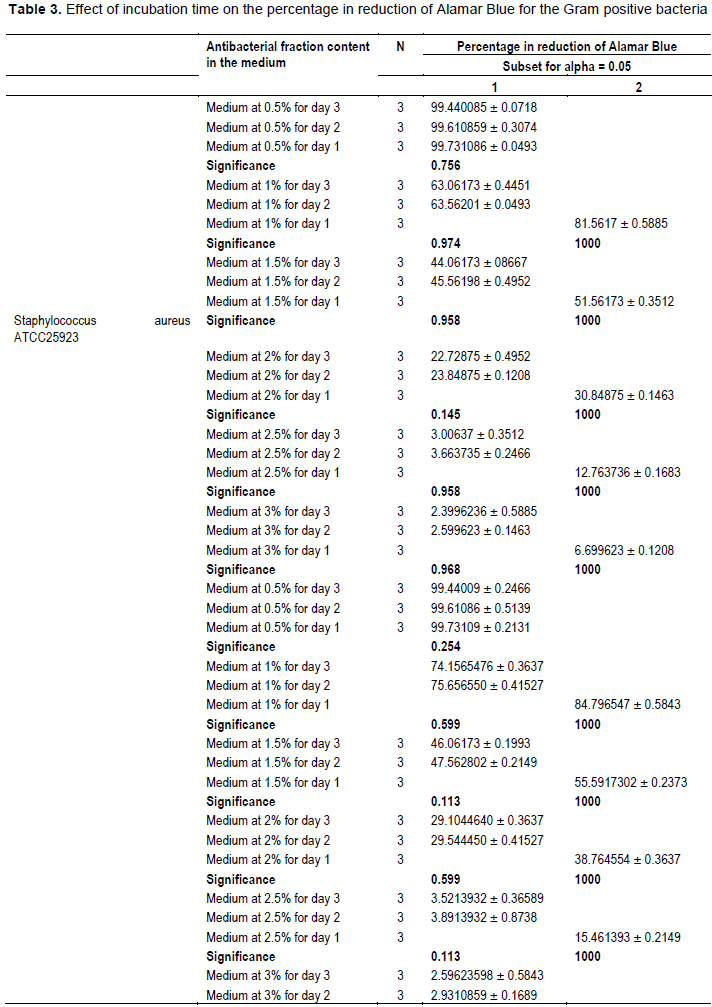
The evaluation of the effect of this antibacterial fraction on cells viability of the bacteria species showed that with the increase in the antibacterial fraction content in the medium, a decrease of the surviving cells in a dose-dependent manner was noted (Table 2). As it is noted (Table 2), the decrease of the surviving cells was influenced significantly by the antibacterial fraction content in the medium (P<0.05). Indeed, the determination of the percentage in reduction of Alamar blue indicating cells viability which was around 100% in the medium without antimicrobial fraction, decreased to reach values around 3% in the medium at 2.5% of antibacterial fraction after two days of incubation for theGram positive bacteria tested (S. aureus ATCC25923 and L. monocytogenes ATCC25051) (Figure 4). However, for the Gram negative bacterial tested (P. aeruginosa ATCC27853, E. coli ATCC25922, S. enteritidis ATCC27042 and K. pneumonia ATCC27001), around 7% of cells were still alive after two days of incubation at the same antibacterial fraction content in the medium (Figure 5). Thus, the reduction of the growth when the extract content in the medium increased as mentioned earlier could be explained by the death of the bacteria’s cells. It is noted that the Gram positive bacteria were more sensitive to the extract than the Gram negative bacteria. As it is known, Gram positive bacteria have no outer lipid membrane whilst Gram negative bacteria have an outer lipid membrane (Madigan and Martinko, 2006). The outer lipid membrane of the Gram negative bacteria could be one of the reasons of the much slower penetration of the extract in their cells compared to Gram positive bacteria. The reduction of surviving cells was also influenced significantly by the incubation time (P<0.05). Indeed, from 24 to 48 h, the percentage of surviving cells decreased significantly (Tables 3 and 4). However, from 48 to 72 h, the reduction of this percentage of surviving cells was not significant (P>0.05). This suggests that the use of this antibacterial fraction against pathogens bacteria could be done during48 h. This reduction of surviving cells was observed the medium at 1% of antibacterial fraction. This already in indicates that the minimum killing concentration could be this value. For many years now, these leaves were eaten in many African countries toxicological risks for consumers without any toxic effect noted. Therefore, to avoid the due to the use of chemical preservatives and additives in food and feed, this extract could be used. It could also be used to overcome the resistance of bacteria against antibiotics. This antibacterial fraction of S. mombin leaves extract could be used at the concentration of 2.5% or above as a natural bactericidal substance in alternative in chemical bactericidal.


This investigation revealed that S. mombin L. leaves possess significant antibacterial activities due to their inhibitory effect on bacteria proliferation and their capacity of killing them. It highlights the discovery of natural substances for the research for alternative in chemical preservatives in food and feed that could inhibit bacterial growth without killing them. Further research must be carried out on this antibacterial extract in order to find out the different classes of antibacterial compounds.
The authors have not declared any conflict of interests.
The authors gratefully acknowledge the Laboratory of Biochemistry and Food Sciences, UFR Biosciences, University Félix Houphouët-Boigny, Abidjan (Ivory Coast) for the funding.
REFERENCES
|
Abo KA, Ogunleye VO, Ashidi JS (1999). Antimicrobial potential of Spondias mombin, Croton zambesicus and Zygotritonia crocea. Phytotherapy Research 13:494- 497.
Crossref
|
|
|
|
Akoua-Koffi G, Faye-Kette H, Kouakou K (1993). Intérêt d'uti¬lisation d'un test au latex (Rotalex) pour le dépistage de rotavirus dans les selles diarrhéiques à Abidjan. Medecine d'Afrique Noire 40(10):599-602.
|
|
|
|
|
Ayoka AO Akomolafe RO, Akinsomisoye OS, Ukponmwan OE (2008). Medicinal and Economic Value of Spondias mombin. African Journal of Biomedical Research 11:129-136.
Crossref
|
|
|
|
|
Bullerman LB (1977). Incidence and control of mycotoxins producing molds in domestic and imported cheeses. Annals of Nutrition and Food 31:435-446.
|
|
|
|
|
Clinical and laboratory standards institute: CLSI (1999). ''Methods for determining bactericidal activity of antimicrobial agent: approved guideline'', Document M26-A, Wayne, PA: CLSI.
View
|
|
|
|
|
Coates NJ, Gilpin ML, Gwynn MN, Lewis DE, Milner PH, Spear SR, Tyler JW (1994). SB-202742 a novel beta-lactamase inhibitor isolated from Spondias mombin. Journal of Natural Products 57:654-657.
Crossref
|
|
|
|
|
Corthout J, Pieters LA, Claeys M, Vanden Berghe DA, Viletinck AJ (1991). Antiviral; Ellagitannins from Spondias mombin. Phytochemistry 30:1190.
Crossref
|
|
|
|
|
Corthout J, Pieters LA, Claeys M, Vanden Berghe DA, Viletinck AJ (1992). Antiviral caffeoyl: esters from Spondias mombin. Photochemistry 31:79-81.
Crossref
|
|
|
|
|
Corthout J, Pieters LA, Claeys M, Vanden Berghe DA, Viletinck AJ (1994). Antibacterial and molluscicidal phenolic acid from Spondias mombin. Planta Medica 6:460-463.
Crossref
|
|
|
|
|
Directive 91/414/CEE du Conseil du 15 juillet 1991 concernant la mise sur le marché des produits phytopharmaceutiques. Official Journal, L 230 du 19.8.1991, P 1.
View
|
|
|
|
|
Global Burden Diseases Statistics (2015). Diarrhea Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and etiologies of diarrhea diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infectious Diseases 17:909-948.
|
|
|
|
|
Heaton JC, Jones K (2008). Microbial Contamination of Fruit and Vegetables and the behavior of Enteropathogens in the Phyllosphere, A Review. Journal of Applied Microbiology 104:613-626.
Crossref
|
|
|
|
|
Khare S, Tonk A, Rawat A (2018). Foodborne diseases outbreak in India: A review. International Journal of Food Science and Nutrition 3(3):09-10.
|
|
|
|
|
Kouadio AI, Oulahal N, Nguyen Thi PI, ADT I, Degraeve P (2011). Study of the antimicrobial activities of Solanum indicum ssp. Distichum (Schumach. and Thonning 1827) fruits ("gnangnan" berries) from a tropical humid zone (Côte d'Ivoire). 2011. International Journal of Biological and Chemical Sciences (International Journal of Biological and Chemical Sciences) 5(3):1190-1200.
Crossref
|
|
|
|
|
Kouadio IA, Koffi LB, Dosso MB (2013). Prevention of crops contamination by fungi and mycotoxins using natural substances derived from Lycopersicon esculentum Mill. Leaves. Journal of Food Security 1(2):16-26.
|
|
|
|
|
Madigan MT, Martinko JM (2006). Brock Biology of Microorganisms (11th ed.). Pearson Prentice Hall. ISBN 978-0131443297.
|
|
|
|
|
Mead PS, Slutsker L, Dietz V, Mccaig LF, Bresee JS, Shapiro C., Griffin PM, Tauxe RV (1999). Food-related illness and death in the United States. Emerging Infectious Diseases 5:607-625.
Crossref
|
|
|
|
|
Newman KL, Leon JS, Rebolledo PA, Scallan E (2015). The Impact of Socioeconomic status of foodborne illness in high income countries: A systematic Review. HHS Public Access 143(12):2473-2485.
Crossref
|
|
|
|
|
Ramesh VB, Rao RN (1987). Foodborne diseases in India. The Indian Journal of Pediatrics 54(4):553-562.
Crossref
|
|
|
|
|
Rodrigues KF, Hasse M (2000). Antimicrobial activities of secondary metabolites produced by endophytic fungi from Spondias mombin. Journal of Basic Microbiology 40:261-267.
Crossref
|
|
|
|
|
Sudershan RV, Naveen Kumar R, Bhaskar V, Polasa K (2014). Foodborne Infections and Intoxications in Hyderabad India. Epidemiology Research International, 5.
Crossref
|
|
|
|
|
TiburskI JH, Rosenthal A, Deliza R, Ronoel L, Godoy DO, Pacheco S (2011). Nutritional properties of yellow mombin (Spondias mombin L.). Food Research International. 44:2326-2331.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2015). WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007- 2015.
View
|
|