ABSTRACT
Prevalence and incidence of foodborne illness in developing countries has risen in recent times as a result of increased demand for Ready-To- Eat (RTE) foods. The current study assessed the microbiological quality of khebab sold at selected areas within the Kumasi metropolis. A total of 36 khebab samples were purchased and analyzed for Total Viable Count (TVC), Total Coliforms Count (TCC) and Thermotolerant Coliforms Count (TTC). The results showed that the mean TVC, TCC and TTC in beef khebab at the different locations ranged from 6.91- 7.23 Log10 CFU/g, 7.25-9.23 Log10 MPN/g and 4.97-7.75 Log10 MPN/g respectively. For chevon khebab, it ranged from 6.83-7.25 Log10 CFU/g, 7.98 - 9.23 Log10 MPN/g and 6.61-8.81 Log10 MPN/g respectively. That of gizzard khebab ranged from 6.89-7.30 Log10 CFU/g, 7.98-9.23 Log10 MPN/g and 6.89-7.53 Log10 MPN/g respectively. The mean TVC, TCC and TTC for the beef khebab were not significant (p = 0.680, 0.055 and 0.070) respectively. For the chevon, the TVC and TCC were not significant (p = 0.547 and 0.121) respectively but that of the TTC was significant (p = 0.034). The mean TVC, TCC and TTC of the gizzard were not significant (p = 0.794, 0.056 and 0.822) respectively at the different locations. These mean microbial loads (TVC, TCC and TTC) in the khebab samples exceeded the standard acceptable limits (? 5 Log CFU/g and ? 2 Log10 MPN/g). Since the microbial loads exceeded the standard acceptable limits, it could put consumers at high risk of contracting foodborne infection. This result should prompt the relevant institutions responsible for ensuring food safety in the metropolis to strictly enforce the standard regulations on food safety practices as well as carry out adequate monitoring to avoid possible foodborne infections.
Key words: Khebabs, total viable count, total coliforms count, thermotolerant coliforms count.
Street food vending plays an important role in the financial growth of people living in the cities of Ghana and other developing nations (Osei Mensah et al., 2016). In addition, the economy of a nation gets bolstered through the taxes paid by these vendors (Okoye, 2020; Anyidoho, 2013). The industry provides large collection of foods that are relatively cheap, nutritious and easy to come by to thousands of consumers every day (Tigari and Shalini, 2020; Bellia et al., 2016; Monney et al., 2014; Tambekar et al., 2008).
Despite its important role, foodborne infections of microbial source are the main health risks associated with its consumption (Gizaw, 2019; Cho et al., 2011; El-Shenawy et al., 2011; Campos et al., 2015; Mensah et al., 2002). The total number of outpatients reported cases of foodborne infections in Ghana is about 420,000 per year, with an annual death rate estimated at 65,000 and total cost to the economy at $69 million (Graphic Online, 2015; Mahami and Odonkor, 2012; MOFA and World Bank, 2007). The problem of foodborne infection in developing countries is mainly due to failure of the street food vendors to comply with the standard guidelines during preparation of the food and secondary contamination after preparation (Feglo and Sakyi, 2012; Tavakoli, 2008). Additionally, lack of food safety training for vendors have equally contributed to the causes of these foodborne infections since majority of them operate in poor hygienic conditions (Umar et al., 2018). Estrada-Garcia et al. (2004) indicated that street foods are the commonest source of foodborne disease outbreaks. It is worth noting that food safety issues are not restricted to only developing countries. A significant number of food related outbreaks have been reported in most developed countries. In the selected countries in USA and Europe, rates of death are as high as 3000 and 4654 annually (Scallan et al., 2011a, b; WHO, 2017).
Khebab belongs to these Ready-To-Eat (RTE) foods. It is popularly known in Ghana as ‘chinchinga’ which is highly patronized. Among the RTE foods, there are indications that khebab may be the most patronized (Panozzo et al., 2015). As such, it is usually sold at the street corners, markets, beaches, lorry terminals, beer bars and restaurants. The khebab is usually made from either beef, pork, chevon, mutton, chicken or other sources of meat. The meat is marinated with a preparation called ‘suya’ spice and char-grilled. Suya is a spicy meat skewer which is a popular food item in West Africa. During the processing of the khebab, it is extensively manipulated by the vendor key among being the constant touching and turning of the meat whilst being grilled. Such activities have a potential for the introduction of high microbial contamination. In addition, some portions of the meat stuck on the stick and may not be well-cooked. As a result, there may be an increased risk of pathogen survival not only by cross-contamination, but also by undercooking. There have been several reported incidences of foodborne disease outbreaks associated with khebab consumption (ACMSF, 2004; Evans et al., 1999; Little and Gillespie, 2008). For instance, in England and Wales, several foodborne diseases outbreaks associated with khebabs have been reported by Meldrum et al. (2009). Additionally, Synnott et al. (1993) also reported an outbreak of Salmonella mikawasima which was associated with doner khebabs consumption. Recent studies have indicated unsatisfactory loads of bacteria ? 105 cfu/g in khebab samples (Durmaz et al., 2015; Lopašovský et al., 2016). At KNUST for instance, majority of students, staff and indigenous people residing on KNUST campus and its environs patronize these ready-to-eat foods (Ababio and Adi, 2012). The Ghana Standard Board has indicated an acceptable limit of ?5 Log CFU/g for bacteria in khebab. As such, any khebab beyond this threshold when consumed may have dire public health implications.
Even though khebab is one of the most patronized of such foods, the microbial profile as well as load has not yet been established. Therefore, the present study estimated the load and further profile bacteria contaminants in kehbab sold on the campus of a tertiary institution and its surrounding communities in Ghana.
Study design
A cross-sectional study was conducted to assess the microbiological quality of ready-to-eat meat (khebab) sold in KNUST campus and its environs.
Study location
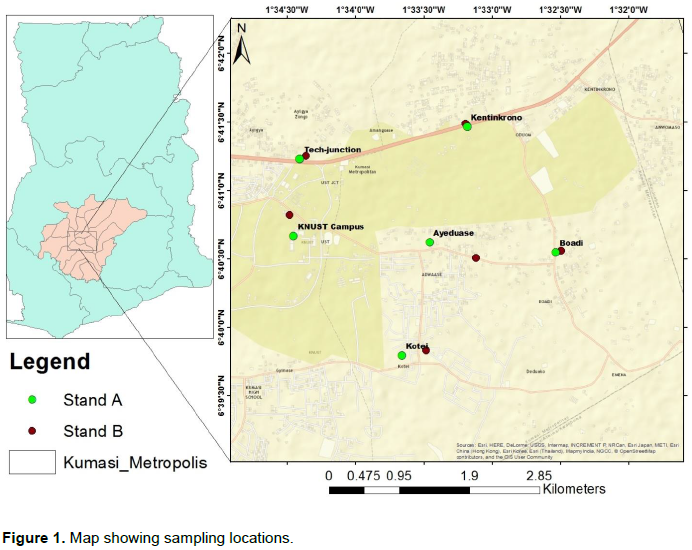
The study was conducted at the Kwame Nkrumah University of Science and Technology (KNUST) campus and selected areas including Ayigya, Kotei, Ayeduase, Boadi and Kentinkrono in the Ashanti region of Ghana (Figure 1).
Sample collection
RTE khebab made from beef, chevon and gizzard were purchased from each vendor in KNUST campus, Ayigya, Kotei, Ayeduase, Boadi and Kentinkrono. At each location, the khebabs were purchased from two vendors. About one hundred grams of the three khebab samples (beef, chevon and gizzard) were placed in labeled sterile ziploc bags. The samples were kept in an ice chest with ice packs and transported immediately to the laboratory for analysis.
Microbiological analysis
Total viable count (TVC)
The total viable count (TVC) was carried out using the pour plate technique with plate count agar (PCA) (Oxoid CM 0325; Oxoid Ltd Basingstoke, Hamsphire, England). A stock dilution was prepared by placing 10 g of khebab sample into 90 ml sterilized buffered peptone water (Oxoid CM 0009; Oxoid Ltd Basingstoke, Hamsphire, England) and pulsified for 15 s. The stock was serially diluted by transferring 1 ml of it into 9 ml sterile peptone water and successfully transferred in a similar manner to obtain dilution of 10-2 to 10-5. Aliquots of 1 ml from each of the dilutions were put into labeled Petri dishes and about 10 ml of molten (45°C) plate count agar was added. The plates were swirled slowly for uniform mixing and allowed to solidify and sealed with parafilm and incubated at 36°C (±1) for 24 h. After the incubation, colonies between 30 and 300 were counted using the colony counter (Stuart colony counter, UK) and the average colonies were calculated, from which the total viable count was estimated using the formula:
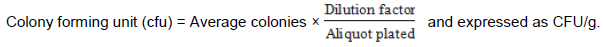
Total and thermotolerant coliforms
The Most Probable Number (MPN) method was employed to determine the total and thermotolerant coliforms in the khebab samples. One milliliter aliquots from each of the dilutions prepared were inoculated into a 5 ml sterile MacConkey Broth (Oxoid CM 0085; Oxoid Ltd Basingstoke, Hamsphire, England) and incubated at 36°C (±1) for total coliforms and 44°C for Thermotolerant coliforms for 24 h. The tubes that showed colour change from purple to yellow after 24 h were identified as positive for both total and Thermotolerant coliforms. Counts per gram were calculated from the MPN tables. The total number of organisms in the samples was estimated using the formula:
MPN/g = Number of organisms obtained from MPN table × Dilution factor of middle set of tubes (https://www.jlindquist.com/generalmicro/102dil3.html).
Statistical analysis
The data obtained were analyzed by Genstat statistical software (version 12) (Payne et al., 2009). The means of bacterial load (TVC and MPN) of samples collected from the various sites were compared using One-way Analysis of Variance (ANOVA). Significant differences were assessed at 5% level of significance (p = 0.05). Where there was significant difference, means were separated using the Fishers protected least significant difference (LSD) procedure to identify the source of difference. The data were log arithmetically (Log10) transformed to minimize the variations associated with the enumeration techniques.
Microbial load of beef khebab samples from the six locations
In general, the khebab samples analyzed showed varying loads of microbes. Table 1 shows that KNUST campus recorded the highest mean TVC (7.23 Log10 CFU/g), while Kotei recorded the least (6.91 Log10 CFU/g). The results showed no significant differences (p = 0.680) among the mean TVC loads (Table 1). For the total coliform count, Ayigya recorded the highest mean load (9.23 Log10 MPN/g) whereas Boadi recorded the least (5.25 Log10 MPN/g). Again, KNUST campus recorded the highest thermotolerant coliform count (7.75 Log10 MPN/g) while Boadi recorded the least (4.97 Log10 MPN/g). There were no statistical differences (p = 0.055 and 0.070) among the mean loads for the total and thermotolerant coliform counts, respectively.
Microbial load of chevon khebab samples from the six locations
Khebabs from KNUST campus recorded the highest mean TVC of 7.25 Log10 CFU/g, whereas those from Kotei recorded the least mean TVC load of 6.83 Log10 CFU/g (Table 2). The differences in the mean loads were not significant (p = 0.55). Likewise, khebabs from KNUST campus recorded the highest mean total coliform load of 9.23 Log10 MPN/g while those from Kotei and Ayeduase recorded the least (7.98 Log10 MPN/g). Similarly, khebabs on KNUST campus recorded the highest mean total coliforms load of 8.18 Log10 MPN/g while those of Kotei showed the least (6.61 Log10 MPN/g). There were no significant differences (p = 0.12) among the mean loads of the total coliform count recorded. However, the mean load of thermotolerant coliforms recorded for those from KNUST campus was significantly higher than that of Kotei and Ayeduase (p = 0.043).
Microbial load of gizzard khebab samples from the six locations
For total viable count, khebabs from Ayigya recorded the highest mean load of 7.30 Log10 CFU/g while those from KNUST campus recorded the least (6.88 Log10 CFU/g). Samples from Ayigya also recorded the highest mean load of total coliforms of 9.23 Log10 MPN/g, while samples from Ayeduase recorded the least mean load of total coliforms (7.98 Log10 MPN/g) (Table 3). Kentinkrono recorded the highest mean thermotolerant coliform load of 7.53 Log10 MPN/g, while the lowest mean load of 6.89 Log10 MPN/g was observed in Kotei for thermotolerant coliform count.
Though the mean TVC load obtained for the beef, chevon and gizzard khebab samples were different; they showed no significant differences (p = 0.680, 0.545 and 0.794), respectively at the different sampling locations. However, the TVC for the different khebabs in all the locations exceeded the Ghana Standard Board acceptable limit of ?5 Log CFU/g. This could mean similar hygienic and processing methods were undertaken by the vendors at the different locations. Since the mean TVC of the khebabs were above the standard acceptable limit, they were unsatisfactory for human consumption (NSW Food Authority, 2009). It also suggests the existence of microbial risk associated with the different khebabs in the different locations. Our results on TVC can be compared to a similar study by Manguiat and Fang (2013) and Ologhobo et al. (2010) who reported unsatisfactory levels of aerobic plate count (APC) in hot grilled chicken and Nigerian roasted chicken ‘suya’, respectively. Shaltout et al. (2015) also reported unsatisfactory levels of aerobic plate count (APC) in street vended meat products sandwiches in Kalyobia Governorate. Further, the study agree with findings of Wimalasekara and Gunasena (2016) who reported unacceptable levels of TVC in RTE Khebab and Ham with Log10CFU ranging (4.34-8.53) and (6.42-8.53), respectively.
The mean total coliform count (TCC) and thermotolerant coliform count (TTC) of beef, chevon and gizzard khebabs at the different locations were all above the standard acceptable limit of ?2 Log MPN/g. Consumers are therefore at risk of contracting pathogenic bacteria infections such as typhoid fever (Salmonella species), dysentery (Shigella species), cholera (Vibrio cholera), etc. In Ghana, from 2009 to 2013, there were four main clinically diagnosed foodborne diseases which include cholera (59.8%), typhoid fever (16.5%), dysentery (shigellosis) (2.6%) and viral hepatitis (1.6%) (Osei-Tutu and Anto, 2016). Although the mean TCC and TTC for khebab from different meats were different at each location, they were not significantly different (p ? 0.05) except for the mean TTC of the chevon khebab which showed significant differences among KNUST campus, Kotei and Ayeduase (p = 0.034). Similarity in bacterial loads of samples collected at the various locations could be attributed to the fact that the khebab vendors were operating under similar environmental conditions. Similar study by Manguiat and Fang (2013) reported unsatisfactory levels of coliforms (4.4 Log CFU/g) in hot grilled chicken in Laguna, Taiwan. Agbodaze et al. (2005) also reported unsatisfactory levels of faecal coliforms in khebabs sampled from Accra central (4.4 Log CFU/g), Osu (3.98 Log CFU/g) and Nima (3.80 Log MPN/g) all in Ghana. Wimalasekara and Gunasena (2016) also reported unacceptable levels of total coliforms in RTE Khebab and Ham with Log10 MPN ranging (0.95-3.04) and (2.66-3.04), respectively as well as unacceptable levels of faecal coliforms ranging (0.60-3.04) and (2.66-3.04), respectively.
The high mean TVC, TCC and TTC load observed in the beef, chevon and gizzard khebab could be as a result of poor storage conditions and poor handling processes by the vendor as suggested by NSW Food Authority (2009). According to Ababio and Adi (2012) and Fang et al. (2003), most RTE food vendors are not educated and lack the knowledge in hygienic practices and safety of the food product and this may lead to their contamination.
Factors such as educational level, knowledge in hygienic practices such as frequent washing of hands, protection of food from flies and dust, etc. have been reported as determinants of foodborne infections (Amegah et al., 2020; Monney et al., 2013; Yi?it and Duran, 1997). Elsewhere, there are indications that majority of khebab vendors do not keep the khebab in the display cabinet and therefore are exposed to the mercy of the environment and this could be a route through which the khebab can be contaminated by polluted air and dust as well as flies which are in the environment. The metal bars used in roasting the khebabs if not properly cleaned could also introduce microorganisms into the meat and cause contamination. Schroeder et al. (2005) and Mboto et al. (2012) indicated that the presence of high faecal coliforms in foods depicts poor hygienic practices of handling the meat during slaughtering and processing. Other factors such as cross contamination from the skin, mouth or nose of the handlers could also introduce pathogens into the meat. Contamination may also arise from the spices used by vendors as well as the heating process, if not done properly to kill or minimize the loads of microorganisms.
The study in general has demonstrated that street vended khebab sold on KNUST campus, Ayigya, Kotei, Ayeduase, Kentinkrono and Boadi were significantly contaminated with varying loads of microbes. Microbial levels were above the Ghana Standard Board and Brazilian food sanitation standard acceptable bacterial limits established for total viable count (TVC), total and thermotolerant coliform count. Thus, consumers are at a high risk of contracting infections and therefore food vendors should be given frequent training such as seminars, workshops and public education on the consequences of poor personal and sanitation hygiene. Additionally, the Municipal Health Inspectors must monitor the activities of these Ready-To-Eat food vendors to ensure strict adherence to the safety regulations.
The authors have not declared any conflict of interests.
REFERENCES
|
Ababio PF, Adi DD (2012). Evaluating food safety practices among food handlers in the Kumasi Metropolis. Internet Journal of Food Safety 14(2):35-43.
|
|
|
|
Advisory Committee on the Microbiological Safety of Food (ACMSF) (2004). Microbiological Control of Doner Kebabs. Food Standards Agency, London.
|
|
|
|
|
Agbodaze D, Nmai PN, Robertson F, Yeboah-Manu D, Owusu-Darko K, Addo K (2005). Microbiological quality of khebab consumed in the Accra metropolis. Ghana Medical Journal 39(2):46-49.
Crossref
|
|
|
|
|
Amegah KE, Addo HO, Ashinyo ME, Fiagbe L, Akpanya S, Akoriyea SK, Dubik SD (2020). Determinants of hand hygiene practice at critical times among food handlers in educational institutions of the Sagnarigu municipality of Ghana: a cross-sectional study. Environmental Health Insights.
Crossref
|
|
|
|
|
Anyidoho NA (2013). Informal Economy Monitoring Study: Street Vendors in Accra, Ghana. Manchester, United Kingdom: Women in Informal Employment: Globalizing and Organizing.
|
|
|
|
|
Bellia C, Pilato M, Seraphin H (2016). Street food and food safety: a driver for tourism? Calitatea 17(S1):20-27.
|
|
|
|
|
Campos J, Gil J, Mourao J, Peixe L, Antunes P (2015). Ready-to-eat street-vended food as a potential vehicle of bacterial pathogens and antimicrobial resistance: an exploratory study in Porto region. Portugal International Journal of Food Microbiology 206:1-6.
Crossref
|
|
|
|
|
Cho JI, Cheung CY, Lee SM, Ko SI, Kim KH, Hwang IS, Kim SH, Cho SY, Lim CJ, Lee KH, Kim KS, Ha SD (2011). Assessment of microbial contamination levels of street-vended foods in Korea. Journal of Food Safety 31:41-47.
Crossref
|
|
|
|
|
Durmaz H, Aygun O, Sancak H (2015). The microbiological quality of grilled meats (Kebab) and salads consumed in Sanliurfa restaurants. International Journal of Science and Technology Research 1(1):297-302.
|
|
|
|
|
El-Shenawy M, El-Shenawy M, Mañes J, Soriano JM (2011). Listeria spp. in street vended ready-to-eat foods. Interdisciplinary Perspective on Infectious Disease.
Crossref
|
|
|
|
|
Estrada-Garcia T, Lopez-Sancedo C, Zamarripa- Ayala B, Thompson MR, Gutierrez L (2004). Prevalence of Escherichia coli and Salmonella spp. in street-vended food of open markets (tianguis) and general hygienic and trading practices in Mexico City. Epidemiology and Infection 132(6):1181-1184.
Crossref
|
|
|
|
|
Evans MR, Salmon RL, Nehaul L, Mably S, Wafford L, Nolan-Farrell MZ, Gardner D, Ribeiro CD (1999). An outbreak of Salmonella typhimurium DT 170 associated with kebab meat and yoghurt relish. Epidemiology and Infection 122(3):377-383.
Crossref
|
|
|
|
|
Fang T, Que-Kim W, Chia-Wei L, Min-Ju H, Tzu-Hui W (2003). Microbiological quality of 18oC ready-to-eat foods products sold in Tawian. International Journal of Food Microbiology 80(3):241-250.
Crossref
|
|
|
|
|
Feglo P, Sakyi K (2012). Bacterial contamination of street vending food in Kumasi, Ghana. Journal of Biomedical Science 1(1):1-8.
|
|
|
|
|
Gizaw Z (2019). Public health risks related to food safety issues in the food market: A systematic literature review. Environmental Health and Preventive Medicine 24(1):68.
Crossref
|
|
|
|
|
Graphic Online (2015). View
|
|
|
|
|
Little CL, Gillespie IA (2008). Prepared salads and public health. Journal of Applied Microbiology 105(6):1729-1743.
Crossref
|
|
|
|
|
Lopašovský L, Terentjeva M, Kunová S, Zele?áková L, Ka?ániová M (2016). Microbiological quality of ready-to-eat foods produced in Slovakia. Journal of Microbiology Biotechnology and Food Science 5(1):31-35.
Crossref
|
|
|
|
|
Mahami T, Odonkor ST (2012). Food Safety Risks Associated with Tertiary Students in Self Catering Hostels in Accra Ghana. International Journal of Biology, Pharmacy and Allied Sciences 1(4):537-550.
|
|
|
|
|
Manguiat LS, Fang TJ (2013). Microbiological quality of chicken- and pork-based street-vended foods from Taichung, Taiwan, and Laguna, Philippines. Food Microbiology 36(1):57-62.
Crossref
|
|
|
|
|
Mboto CI, Agbo BE, Ikpoh IS, Agbor RB, Udoh DI, Ambo EE, Ekim MA (2012). Bacteriological study of raw meat of Calabar Abattoir with public health and veterinary importance. Journal of Microbiology and Biotechnology Research 2(4):529-532.
|
|
|
|
|
Meldrum RJ, Little CL, Sagoo S, Mithani V, McLauchlin J, De Pinna E (2009). Assessment of the microbiological safety of salad vegetables and sauces from kebab take-away restaurants in the United Kingdom. Food Microbiology 26(6):573-577.
Crossref
|
|
|
|
|
Mensah P, Yeboah-Manu D, Owusu-Darko K, Ablordey A (2002). Street foods in Accra, Ghana: How safe are they?. Bulletin: World Health Organization 80(7):546-554.
|
|
|
|
|
Ministry of Food and Agriculture (MOFA)/World Bank (2007). Review of food safety in Ghana. Accessed (16th February, 2018).
|
|
|
|
|
Monney I, Agyei D, Ewoenam BS, Priscilla C, Nyaw S (2014). Food hygiene and safety practices among street food vendors: an assessment of compliance, institutional and legislative framework in Ghana. Food and Public Health 4(6):306-315.
|
|
|
|
|
Monney I, Agyei D, Owusu W (2013). Hygienic practices among food vendors in educational institutions in Ghana: the case of Konongo. Foods 2(3):282-294.
Crossref
|
|
|
|
|
New South Wales (NSW) Food Authority. (2009). Microbiological quality guide for ready-to-eat foods e A guide to interpreting microbiological results. Newington, NSW.
|
|
|
|
|
Okoye V (2020). Street Vendor Exclusion in "Modern" Market Planning: A Case Study from Kumasi, Ghana. WIEGO Resource Document No. 15. Manchester, UK: WIEGO.
|
|
|
|
|
Ologhobo AD, Omojola AB, Ofongo ST, Moiforay S, Jibir M (2010). Safety of street vended meat products e chicken and beef 'suya'. African Journal of Biotechnology 9(26):4091-4095.
|
|
|
|
|
Osei Mensah J, Ohene-Yankyera K, Aidoo R (2016). Constraints to growth of micro and small-scale enterprises in Ghana: A case of street food enterprises. Journal of Development and Agricultural Economics 8(10):241-250.
Crossref
|
|
|
|
|
Osei-Tutu B, Anto F (2016). Trends of reported foodborne diseases at the Ridge Hospital, Accra, Ghana: a retrospective review of routine data from 2009-2013. BMC Infectious Diseases 16(1):139.
Crossref
|
|
|
|
|
Panozzo M, Magro L, Erle I, Ferrarini S, Murari R, Novelli E, Masaro S (2015). Nutritional quality of preparations based on Döner Kebab sold in two towns of Veneto Region, Italy: preliminary results. Italian Journal of Food Safety 4(2).
Crossref
|
|
|
|
|
Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2009). GenStat for Windows. 12th Edition, VSN International, Hemel Hempstead.
|
|
|
|
|
Scallan E, Griffn P, Angulo F, Tauxe R, Hoekstra R (2011b). Foodborne illness acquiredin the United States-unspecifed agents. Emerging Infectious Disease 17(1):16-22.
Crossref
|
|
|
|
|
Scallan E, Hoekstra RM, Angulo F, Tauxe R, Widdowson M, Roy SL, Jones JL, Griffin PM (2011a). Foodborne illness acquired in the United States-major pathogens. Emerging Infectious Disease 17:7-15.
Crossref
|
|
|
|
|
Schroeder CM, Naugle AL, Schlosser WD, Hogue AT, Angulo FJ, Rose JS (2005). Estimate of illnesses from Salmonella enteritidis in eggs, United States, 2000. Emerging infectious Diseases 8(10):2385-2388.
|
|
|
|
|
Shaltout FA, El-Shater AH, Wafaa MA (2015). Bacteriological assessment of Street Vended Meat Products sandwiches in kalyobia Governorate. Journal of Benha veterinary Medicine 28(2):58-66.
Crossref
|
|
|
|
|
Synnott M, Morse DL, Maguire H, Majid F, Plummer M, Leicester M, Threlfall EJ, Cowden J (1993). An outbreak of Salmonella mikawasima associated with doner kebabs. Epidemiology and Infection 111:473-481.
Crossref
|
|
|
|
|
Tambekar DK, Jaiswal VJ, Dhanorkar DV, Gulhane PB, Dudhane MN (2008). Identification of microbiological hazards and safety of ready-to-eat food vended in streets of Amravati city, India. Journal of Applied Biosciences 7:197-201.
|
|
|
|
|
Tavakoli HR (2008). Food microbiology and control of food production and distribution centers. 2nd edition. Tehran: Marz-e-Danesh Publication.
|
|
|
|
|
Tigari H, Shalini S (2020). Socio-Economic Condition of Urban StreetFood Vendors. Shanlax International Journal of Economics 8(3):67-74.
Crossref
|
|
|
|
|
Umar AA, Sambo MN, Sabitu K, Iliyasu Z, Sufiyan MB, Hamza KL (2018). Personal and food hygiene practices among street-food vendors in Sabon-Gari local government area of Kaduna State, Nigeria. Archives of Medicine and Surgery 3:77-83.
Crossref
|
|
|
|
|
Wimalasekara SGMRL, Gunasena GDDK (2016). Microbiological quality of ready-to-eat meat based food available in temporary food outlets in Gall Face Green, Colombo, Sri Lanka. International Journal of Agriculture Forestry and Plantation 4:38-44.
|
|
|
|
|
World Health Organization (WHO) (2017). The burden of foodborne diseases in the WHO European region.
|
|
|
|
|
Yi?it V, Duran T (1997). Institutional Nutrition Technology I (in Turkish). Ekin Publishing, Istanbul, Turkey.
|
|