ABSTRACT
Yacon (Smallanthus sonchifolius) tubers and leaves have been used widely as foodstuff and as remedy for urinary ailments, muscle pain, hyperlipidemia and diabetes mellitus. Recent studies have investigated on isolating active components for their anti-cancer potential against melanoma, cervical cancer and colon cancer. In this study, the cytotoxicity potential of hexane, methanol and DCM extracts of yacon leaves was assessed against MCF-7 (breast cancer), HT-29 (colon cancer) and HDFn (normal human dermal fibroblast) cell lines by using AlamarBlue® assay. Results showed significant reduction in cellular viability of MCF-7 cell lines caused by hexane, methanol and DCM extracts in a dose dependent manner, with DCM being the most potent. The DCM extract also produced significant cytotoxic activity against HT-29 cells, with IC50 lower than 5-fluorouracil. Effect on HDFn showed that three yacon extracts produced significantly lower cytotoxicity compared to drug controls with the DCM extract showing the least toxicity.
Key words: Yacon, alamar, breast, colon, cancer, MCF-7, HT-29..
Cancer is one of the leading causes of mortality not only in the Philippines but worldwide, with an incidence rate that is observed to be linearly increasing through time (Cancer Research UK, 2016). Numerous studies have investigated on every aspect of malignancy, including types, causes, clinical presentation, pathologic basis, genetics, prognosis, diagnosis and treatment. These scientific inquiries have led to significant improvements on cancer management. However, the modern era is still facing the constant dilemma of treatment toxicity. Yacon (Smallanthus sonchifolius Poepp.&Endl.) is a perennial plant that forms underground tuberous roots.This member of the sunflower family (Asteraceae) is a native herb found in the Andean regions, and is currently being cultivated in the Mountain Province. Fresh yacon tubers are edible, yellowish white, crisp and juicy similar to apple or sinkamas, with sweetness that increases with storage. The root crops are usually eaten raw, but can also be prepared into syrups, jams and other foodstuff (Graefe et al., 2004). Aside from household consumption as food, there are a number of ethnomedical uses for yacon. The tubers were eaten raw in South America as diuretic for urinary ailments. Similarly, in Bolivia, decoctions of the leaves were used as home remedy for cystitis, kidney and even liver problems. Peruvians alternatively prepare leaves into a warm poultice for treatment of muscle and joint pains (Graefe et al., 2004). In Brazil, leaves of yacon were taken in the form of tea for control of diabetes (Genta et al., 2009).
Yacon tubers consist mainly of fructans with a structure that is of the inulin type, that is, β(2→1) fructofuranosylsaccharose (Ojansivu et al., 2011). This content makes yacon tubers marketable as sucrose substitutes and are considered dietetic. Additionally, fructans have favorable influence on the human intestinal flora and can modify certain types of lipid disorders. Since humans have no enzyme capable of hydrolyzing the β(2→1) bond, these fructans also serve as dietary fiber (Ojansivu et al., 2011). Recently, oligofructans have been classified as prebiotics (Pedreschi et al., 2003). These compounds are transported to the colon and fermented by selected species of gut micro-flora, especially Bifidobacterium and Lactobacillus, both indicators of a balanced gut flora. The prebiotic effect of yacon tuber extracts has been demonstrated by their fermentation by these gut bacteria, Lactobacillus plantarum, Lactobacillus acidophilus and Bifidobacterium bifidum (Valentova and Ulrichová, 2003). Studies have shown that prebiotic consumption favorably modifies gut flora composition and its metabolic activities. Perhaps in a similar manner, yacon tuber consumption also modulates lipid metabolism, calcium absorption and immune response. β(2→1) fructans are related to β-glucans, native polysaccharides found in yeast and fungi, serving as non-specific immunostimulators (Valentová et al., 2003). They bind to macrophages, activate them and initiate the immunity cascade. β-glucans are recommended for the treatment of immunity defects, infections, allergies, chronic fatigue syndrome, high cholesterol levels, stomach problems and as an adjuvant in carcinoma therapy. Yacon tubers are also rich in free fructose, glucose and sucrose (Valentova et al., 2006).
The fructooligosaccharides (FOS) extracted from yacon roots were also found to have hypolipidemic effects on diabetic rats. A significant decrease in fasting plasma triacylglycerol and very low-density lipoprotein levels were observed, along with increased insulin-positive pancreatic cell mass distributed in small cell clusters within the exocrine parenchyma (Habib et al., 2011). The positive metabolic effects of yacon root extracts were further tested in diabetes. Aqueous extracts were effective in controlling water and food consumption, hyperglycemia and dyslipidemia, and promote the reduction of liver enzymes, suggesting a hepatoprotective effect in rats with drug-induced diabetes mellitus type 1 (Ornelas et al., 2013). FOS extracted from yacon roots was also found to have preventive effect against Salmonella typhimurium enteric infection. When given orally up to 30 days, FOS from yacon enhanced non-specific immunity, such as increasing the total IgA, which improves the immunological intestinal barrier, thereby preventing pathologic colonization by S. typhimurium (Velez et al., 2013). The high concentration of fructans in yacon roots was also discovered to have potential for colon cancer prevention. A significant reduction in number and multiplicity of aberrant crypt foci and in number of invasive adenocarcinomas was observed in the groups orally treated with 1% yacon and its symbiotic formulation (yacon plus L. casei) (de Moura et al., 2012). Extracts of yacon were also shown to inhibit progression of acute pancreatitis. The inhibitory effect of 1% of yacon extract on dibutyltin dichloride-induced pancreatitis in rats was interpreted based on decreased levels of inflammatory mediators, such as tumor growth factor and cyclooxygenase-2, in yacon-treated subjects (Choi et al., 2012).
Yacon leaves were also extensively studied for physiologic effects on animals. A review on the characteristics of yacon as a functional food (Delgado et al., 2013) states that yacon leaves contain several phenolic compounds that enhance growth of intestinal bacteria with good metabolic properties, inhibiting the attack of pathogens. Hydro-ethanolic crude extracts (400 mg/kg) of yacon leaves given orally to diabetic Wistar rats for 3, 7, 10 and 14 days were shown to significantly decrease fasting and post-prandial serum glucose (Baroni et al., 2008). This finding was further confirmed by another study that utilized methanol, butanol and chloroform extracts, given to Wistar rats at 50, 10 and 20 mg/kg body weight for eight weeks (Genta et al., 2010). This study measured for oral glucose tolerance test and serum insulin, aside from fasting and post-prandial blood glucose. Results showed effective hypoglycemic activity and increased insulin levels. Another study utilized normoglycemic mice and concluded that 100 mg/kg oral dose of yacon leaf tea extract and ent-kaurenoic fraction were both effective in lowering blood glucose levels (Raga et al., 2010). The methanolic extract of yacon leaves yielded ent-kaurenoic acid and related diterpenoid substances. Recently, ent-kaurenoic acid from yacon was found to possess significant antibacterial and antifungal activities (Padla et al., 2012). Extracts of leaves were also found to have in vivo radical scavenging activity. Peroxidation of lipids was significantly inhibited, protecting the liver of rats against oxidative injury (Valentova et al., 2003).
Sesquiterpene lactones, namely, enhydrin, uvedalin and sonchifolin, were also isolated from the leaves of yacon (Siriwan et al., 2011). Sesquiterpene lactones are plant products extensively studied for their wide array of biological activities, such as anti-inflammatory, neurocytotoxic and anticancer potentials (Cho et al., 2004). The ones isolated from yacon leaves, specifically enhydrin and uvedalin, are demonstrated to have potent anticancer activity against cervical cancer cell line, specifically by inducing apoptosis-mediated proliferation inhibition via caspase and deactivation of NF-κB (Siriwan et al., 2011). Another study have also shown chemopreventive properties of the sesquiterpene lactones isolated from yacon leaves, with enhydrin, uvedalin and sonchifolin showing stronger chemopreventive activity than parthenolide (Siriwan et al., 2011). The latter is a reference sesquiterpene lactone that has been proven to possess potent chemopreventive properties and is now included in cancer clinical trials (Ghantous et al., 2010). A study exploring on trypanocidal activity of sesquiterpene lactones isolated from yacon revealed that enhydrin, uvedalin and polymatin B efficiently inhibited both the epimastigote and the replicative intracellular amastigotes of Trypanosoma cruzi (Frank et al., 2013).
Yacon has also been investigated on its action against colon cancer and melanoma. Scientists used 1,2-dimethylhydrazine to induce colon carcinogenesis in male Wistar rats. Those administered with dried extract of yacon root and a mixture of yacon with a probiotic showed significant reduction in number and multiplicity of aberrant crypt foci and decreased number of invasive adenocarcinomas (De Moura et al., 2012). Another study investigated the anti-oxidant and anti-cancer activities of different organic solvent fractions of yacon root. Hexane fractions showed high growth inhibitory activities against cancer cells (Min et al., 2012). Another study explored the potential of yacon for melanin synthesis inhibition. Yacon leaf extracts exhibited significant anti-melanogenic activity to suppress melanin synthesis in mouse B16 melanoma cells (Ishikawa et al., 2010).
This study aims to establish reliable data on the anticancer activity of yacon extracts, specifically against breast and colon cancer cell lines. Future scientific ventures on acute toxicity, subacute toxicity and human clinical investigations on yacon will greatly benefit from the output of this study. The information generated from this research can also be used in further identification of active components, which will eventually aid in the discovery and synthesis of a novel, plant-derived drug with superior cytotoxic activity and acceptable side effect profile.
Collection of plant
Yacon leaves were collected from a farm in Misamis Oriental under the management of Doalnara Multi-Purpose Cooperative. Samples of the leaves were sent to the Bureau of Plant Industry for taxonomic identification. The leaves were cleaned and shade dried for more than 4 weeks in average ambient temperature of 32°C and humidity of 64%. The dried materials were ground into powder using a blender and stored in airtight plastic containers and labeled accordingly.
Preparation of plant extract
The finely ground leaves of S. sonchifolius (278.62 g) was exhaustively extracted for six consecutive days (two days for each type of solvent) with solvents in increasing polarity starting with hexane, followed by dichloromethane, and lastly with methanol. For every extraction, the collected crude extracts were concentrated in vacuo using a Buchi rotavapor at a maintained temperature of 45°C. Each extraction afforded three crude extracts labeled as SsH for the hexane extract, SsD for the dichloromethane extract, and SsM for the methanol extract. Small amounts of each crude extract (0.2029 g for SsM, 0.6832 g for SsD, and 0.3888 for SsH) were prepared into 100 µg/ml using 0.2% dimethyl sulfoxide (DMSO) in complete Dulbecco’s modified eagle medium (DMEM) as solvent. This working concentration was then serially diluted (two-fold) to 50, 25, 12.5, 6.25, 3.125, 1.563 and 0.781 µg/ml during treatment on the different cell lines.
Cell culture
Three cell lines were used for this study, namely, breast cancer (MCF-7), colon cancer (HT-29) and normal human neonatal dermal fibroblast (HDFn) cells. The cells were maintained in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, USA) and 1% antibiotic antimycotic (Invitrogen, USA) in tissue culture flasks (Falcon, USA) and incubated at 37°C, 5% CO2 and 95% relative humidity. Cell counts were obtained by the trypan blue exclusion method to calculate cell densities to a final of 1 × 104 viable cells per ml. Experiments were performed in flat bottom 96-well microplates (Falcon, USA) seeded with cell densities of 1 × 103 cells per well. The cells were incubated for 24 h before the drug or plant extracts were added. Untreated cells served as negative controls while 0.2% DMSO in complete DMEM as negative vehicle control. After treatment, the cells were incubated for 48 h prior to analysis with the AlamarBlue® assay (Sankara et al., 2013).
AlamarBlue® assay
Ten microliters of AlamarBlue® was aseptically added to each well. The plates were shaken carefully to thoroughly mix the contents. These were then further incubated at 37°C, 5% CO2 and 95% relative humidity for 4 h. Viable cells in culture reduce blue resazurin in AlamarBlue® into red resorufin, which has maximum absorbance measured at 570 nm using a microplate reader (ELx800, Biotek, USA) (Fotakis et al., 2006; Sankara et al., 2013).
Methotrexate, colchicine, tamoxifen and 5-fluorouracil were used as standard drug controls. Concentrations of these drugs were prepared similarly as that of the extracts using complete DMEM as solvent. The assay was done in triplicates. The percentage of inhibited growth was computed as: 100 – [(absorbance of treated cells/absorbance of untreated cells) × 100].
Statistical analysis
The data were expressed as mean optical density ± standard deviation (SD). Analysis of variance (ANOVA) was used to assess significant differences between controls and plant extracts. IC50 for extracts and controls were computed from the generated dose-response curves.
MCF-7 Cell Line
The next set of tables and figures shows the cytotoxicity effect of increasing concentrations of yacon leaves extracts and controls on breast cancer cells. The measured optical densities are tabulated in Table 1, and plotted against concentration in Figure 1. The computed percentages of cell viability inhibition are shown in Table 2 and Figure 2. There is an observed linear decrease in optical density and increase in cellular growth inhibition with increasing concentration of the three extracts. ANOVA analysis revealed significant difference (p value < 0.001) from negative control for the three extracts at concentrations 12.5, 25 (except for hexane extract), 50 and 100 µg/ml, and no significant difference from the positive controls at all concentration levels, except for tamoxifen.
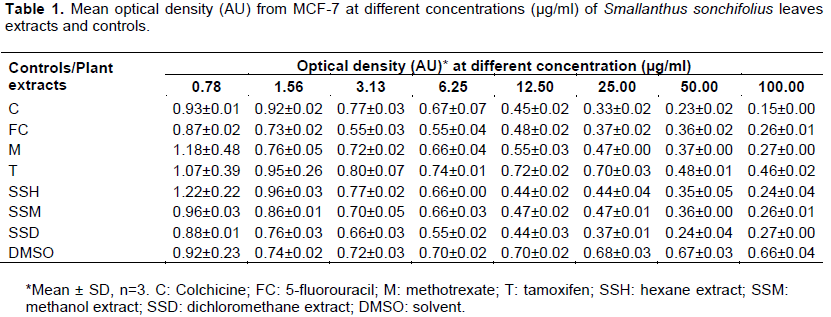
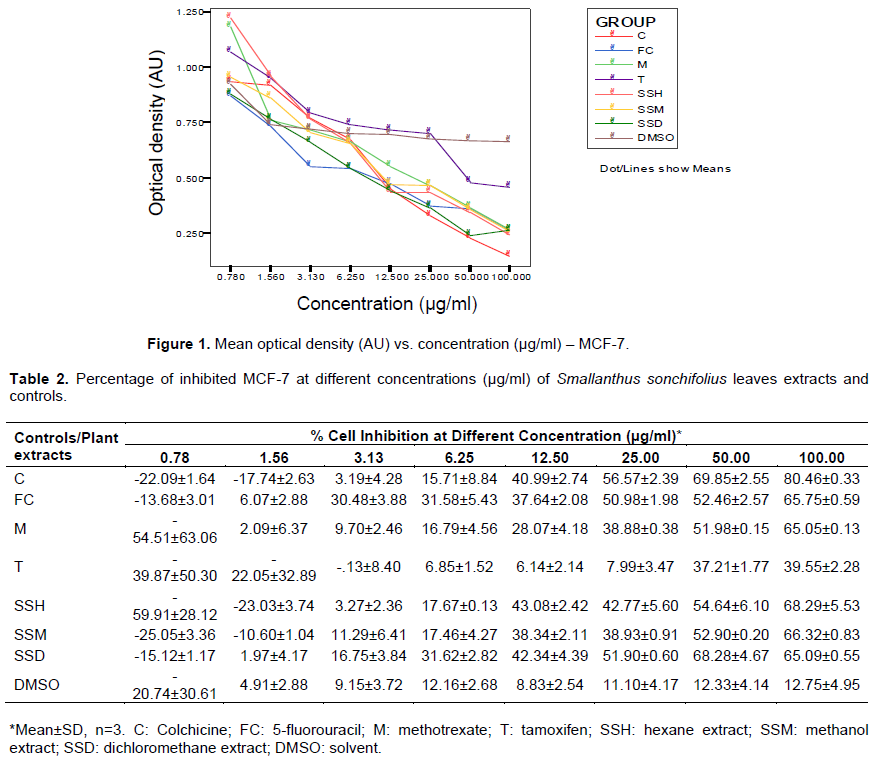
The IC50 were computed using log-linear regression dose-response curve and are shown in Table 3, along with measure of linearity (r2), slope and their respective confidence intervals. The three extracts significantly reduced viability of cells in dose-dependent manner, with the DCM extract being the most potent. The IC50 for the hexane and methanol extracts were 32.08 and 37.44µg/ml,respectively.These values are higher than the computed IC50 for colchicine and 5-fluorouracil, but are significantly lower than the IC50 of tamoxifen and methotrexate. The DCM extract has the lowest IC50 at 23.77 µg/ml, which is significantly lower than the positive controls, except for colchicine.
HT-29 cell line
Table 4 and Figure 3 show cytotoxic effect of the Yacon leaves extracts and drug controls on colon cancer cells. Decreasing optical densities were observed in a linear fashion after treatment with increasing concentrations of the three Yacon extracts. Percentage cell growth inhibition was computed based on these values, and are shown in Table 5 and plotted against increasing concentrations in Figure 4. The DCM extract significantly reduced cell viability in a dose-dependent manner. ANOVA analysis showed significant difference (p < 0.001) of optical density and cell viability from the negative control, and no significant difference from positive controls (5-flurouracil and colchicine) starting from 3.125 µg/ml concentration of DCM extract.

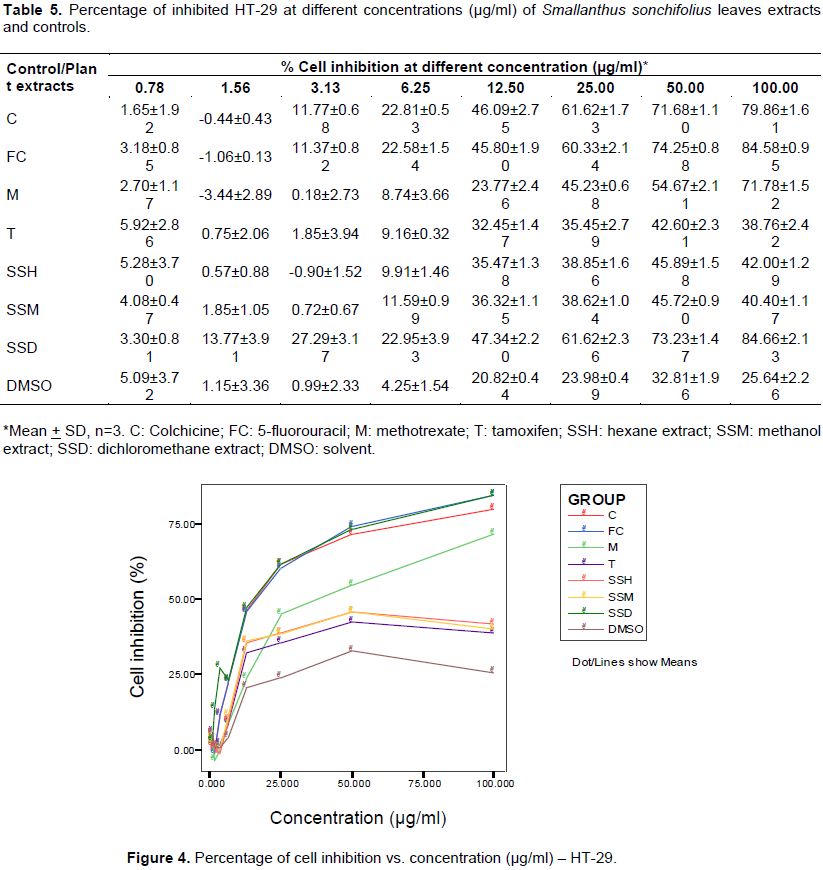
Dose-response curve parameters were generated using log-linear regression to compute for the IC50 (Table 6). Hexane and methanol extracts did not exhibit significant cytotoxicity (IC50 > 100 µg/ml). On the other hand, the IC50 for the DCM extract is 14.32 µg/ml, which is lower than all the positive drug controls, including 5-fluorouracil. This indicates potent cytotoxicity effect produced by the DCM extract.
HDFn cell line
Effect on normal human cellline was assessed by treating HDFn with the same concentrations of Yacon extracts and controls used for MCF-7 and HT-29 cell lines. Tables 7 and 8 (plotted as Figures 5 and 6, respectively) show the cytotoxic effects of the extracts and controls on the normal cells. Dose-response curve parameters were generated using log-linear regression to compute for the IC50 (Table 9). Results showed significant higher cytotoxicity effect of the drug controls on normal cells compared to the plant extracts. Data suggest that the Yacon extracts are non-cytotoxic to HDFn normal cells (IC50 > 100 µg/ml).
Cancer treatment almost always includes chemotherapy and/or radiation, and these cytotoxic processes can lead to life threatening conditions such as severe immune deficiency, cardiomyopathy and development of treatment-related malignancy. The search for safer treatment options continues to be an unrelenting challenge for the scientific community. Recent researches have ventured on plant products, uncovering several anti-cancer potentials from different extracts.
Similar to the findings of previous experiments done by De Moura et al. (2012) and Ishikawa et al. (2010), results of this study showed strong potential of the three yacon extracts to be further investigated as cytotoxic agents against breast cancer. The strong cytotoxic activity of DCM extract against colon cancer also warrants further investigation. The IC50 for hexane, methanol and DCM extracts against MCF-7 were 32.08, 37.44 and 23.77 µg/ml, respectively. These are acceptable IC50 levels against MCF-7 compared to that observed from drug controls (colchicine with 22.61 µg/ml, 5-fluorouracil with 28.77 µg/ml, methotrexate with 39.06 µg/ml and tamoxifen with 182.42 µg/ml). The IC50 for hexane, methanol and DCM extracts against HT-29 were 116.96, 122.00 and 14.32 µg/ml, respectively. The IC50 observed from hexane and methanol extracts are significantly higher than the drug controls, indicating poor cytotoxic activity for this cancer cell line. However, the low IC50 of DCM (14.32 µg/ml) is noteworthy, as it is significantly lower compared to the IC50 values from all the drug controls (colchicine with 17.93 µg/ml, 5-fluorouracil with 16.86 µg/ml, methotrexate with 41.53 µg/ml and tamoxifen with 179.89 µg/ml).
The three yacon extracts were also observed to be significantly non-cytotoxic to normal HDFn cells. The IC50 for colchicine, 5-fluorouracil and methotrexate were 7.38, 5.40 and 6.49 µg/ml, respectively. Tamoxifen produced the highest IC50 for the drug controls at 242.19 µg/ml, but this value is still lower than those observed from the plant extracts. The IC50 for hexane, methanol and DCM extracts were 252.23, 601.82 and 678.76 µg/ml, respectively. Further investigations should be done on protective effect and mechanism of action of yacon extracts against these cancer cells (Siriwan et al., 2011; Choi et al., 2004).
The DCM extract outstandingly produced lower IC50 levels compared to drug controls against MCF-7 (except to colchicine) and HT-29. This extract showed the lowest IC50 against HT-29 (14.32 µg/ml), even lower than the IC50 observed from the current drug of choice against colon cancer, 5-fluorouracil (16.86 µg/ml).
Conclusively, results of this study feature the potential anti-cancer activity of Yacon extracts, most exceptionally the DCM extract. These extracts showed significant cytotoxic effect against breast (hexane, methanol and DCM extracts) and colon cancer cells (DCM extract), while exhibiting non-cytotoxic activities on the normal human cells compared to existing cytotoxic drugs. Results of this study merit further investigation particularly on the cytotoxic mechanisms of the extracts, which can also be utilized for development of new medicine against cancer.
The authors have not declared any conflict of interests.
REFERENCES
|
Baroni S, Suzuki-Kemmelmeieret F, Caparroz-Assef SM, Nakamura RC, Bersani-Amado CA (2008). Effect of crude extracts of leaves of Smallanthus sonchifolius (yacon) on glycemia in diabetic rats. Braz. J. Pharm. Sci. 44(Suppl 3):521-530.
Crossref
|
|
|
|
Cancer Research UK (2016). Cancer Statistics Worldwide. Available at:
View Accessed June 2016.
|
|
|
|
|
Cho JY, Kim AR, Jung JH, Chun T, Rhee MH, Yoo ES (2004). Cytotoxic and pro-apoptotic activities of cynaropicrin, a sesquiterpene lactone, on the viability of leukocyte cancer cell lines. Eur. J. Pharm. 492:85-94.
Crossref
|
|
|
|
|
Choi NH, Kim JB, Kim JT, Park IS (2012). The Effects of Yacon (Smallanthus sonchifolius) Extract on Pancreatic Fibrosis in the Rat. J. Life Sci. 22(7):904-911.
Crossref
|
|
|
|
|
De Moura NA, Caetano BF, Sivieri K, Urbano LH, Cabello C, Rodrigues MA, Barbisan LF (2012). Protective effects of yacon (Smallanthus sonchifolius) intake on experimental colon carcinogenesis. Food Chem Toxicol. 50(8):2902-2910.
Crossref
|
|
|
|
|
Delgado G, Choque T, da Silva WM, Tamashiro C, Maróstica MR, Pastore GM (2013). Yacon (Smallanthus sonchifolius): A Functional Food. Plant Foods Hum. Nutr. 68:222-228.
Crossref
|
|
|
|
|
Fotakis G, Timbrell JA (2006). In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 160(2):171-177. Cite author name in body of work.
Crossref
|
|
|
|
|
Frank FM, Ulloa J, Cazorla SI, Maravilla G, Malchiodi EL, Grau A, Martino V, Catalán C, Muschietti LV (2013). Trypanocidal Activity of Smallanthus sonchifolius: Identification of Active Sesquiterpene Lactones by Bioassay-Guided Fractionation. Hindawi Publishing Corporation, Evid-Based Complement. Altern. Med. 2013:627898.
|
|
|
|
|
Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A, Sánchez S (2009). Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 28(2):182-187.
Crossref
|
|
|
|
|
Genta SB, Cabrera WM, Mercado MI, Grau A, Catalán CA, Sánchez SS (2010). Hypoglycemic activity of leaf organic extracts from Smallanthus sonchifolius: constituents of the most active fractions. Chem. Biol. Interact.185:143-152.
Crossref
|
|
|
|
|
Ghantous AH, Gali-Muhtasib H, Vuorela NA, Saliba and Darwiche N (2010). What made sesquiterpene lactones reach cancer clinical trials. Drug. Discov. Today 15:668-678.
Crossref
|
|
|
|
|
Graefe S, Hermann M, Manrique I, Golombek S, Buerkert A (2004). Effects of post-harvest treatment on the carbohydrate composition of yacon roots in the Peruvian Andes. Field Crop Res. 86:157-165.
Crossref
|
|
|
|
|
Habib NC, Honoré SM, Genta SB, Sánchez SS (2011). Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: biochemical approach. ChemBiol. Interact. 194(1):31-9.
Crossref
|
|
|
|
|
Ishikawa T, Hyakutake T, Gondou Y, Sakai M, Kinoshita R, Tottori, J, Kunitake H, Kusumoto KI (2010). Anti-Melanogenic Activity of Yacon Leaves in Mouse Melanoma Cells. Anim. Cell Technol: Basic Appl. Aspects 16:359-364.
Crossref
|
|
|
|
|
Min KJ, Cheon JU, Cha CG (2012). Anti-oxidative and Anti-cancer Activities of Extracting of Yacon. Korean J. Food Sci. Anim. Resour. 32(2):190-197.
|
|
|
|
|
Ojansivu I, Ferreira CL, Salminen S (2011). Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends Food Sci. Technol. 22:40-46.
Crossref
|
|
|
|
|
Ornelas G, Oliveira C, Bragab P, Fernandes AAH (2013). Improvement of biochemical parameters in type 1 diabetic rats after the roots aqueous extract of yacon [Smallanthus sonchifolius (Poepp. & Endl.)] treatment. Food Chem. Toxicol. 59:256-260.
Crossref
|
|
|
|
|
Padla EP, Solis LT, Ragasa CY (2012). Antibacterial and antifungal properties of ent-kaurenoic acid from Smallanthus sonchifolius. Chin. J. Nat. Med. 10(6):408-414.
Crossref
|
|
|
|
|
Pedreschi R, Campos D, Noratto G, Chirinos G, Cisneros-Zeval (2003). Andean yacon root (Smallanthus sonchifolius Poepp. & Endl.) Fructooligosaccharides as a potential novel source of prebiotics. J. Agric. Food Chem. 51:5278-84.
Crossref
|
|
|
|
|
Raga DD, Alimboyoguen AB, del Fierro RS, Ragasa CY (2010). Hypoglycemic effects of tea extracts and ent-kaurenoic acid from Smallanthus sonchifolius. Nat. Prod. Res. 24(18):1771-1782.
Crossref
|
|
|
|
|
Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella T, Minor L (2013). Cell Viability Assays. In: Assay Guidance Manual; Sittampalam GS, Coussens NP, Brimacombe K, Grossman A, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Iversen PW, Li Z, McGee J, McManus O, Minor L, Napper A, Peltier JM, Riss T, Trask OJ Jr, Weidner J (eds,). Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences. Available at:
View
|
|
|
|
|
Sankara A, Naresh J, Kumar L, Mokkapati A (2013). In vitro anti-cancer activities of few plant extracts against MCF-7 and HT-29 cell lines. Int. J. Pharm. Sci. 3(2):185-188. Cite author name in body of work.
|
|
|
|
|
Siriwan D, Miyawaki C, Miyamoto T, Naruse T, Okazaki K and Tamura H (2011). Chemopreventive Activity of Sesquiterpene Lactones (SLs) from Yacon against TPA-induced Raji Cells Deformation. Pak. J. Biol. Sci. 14:605-609.
Crossref
|
|
|
|
|
Siriwan D, Naruse T, Tamura H (2011). Effect of epoxides and [α]-methylene-[γ]-lactone skeleton of sesquiterpenes from yacon (Smallanthus sonchifolius) leaves on caspase-dependent apoptosis and nf-[κ]b inhibition in human cervical cancer cells. Fitoterapia 10:10-16.
|
|
|
|
|
Valentova K, Cvak L, Muck A, Ulrichova J, Simanek V (2003). Antioxidant activity of extracts from the leaves of Smallanthus sonchifolius. Eur. J. Nutr. 42:61-66.
Crossref
|
|
|
|
|
Valentova K, Lebeda A, Dolezalová I, Jirovský D, Simonovska B, Vovk I, Kosina P, Gasmanová N, Dziechciarková M, Ulrichová J (2006). The biological and chemical variability of yacon. J. Agric. Food Chem. 54(4):1347-52.
Crossref
|
|
|
|
|
Valentova K, Ulrichová J (2003). Smallanthus sonchifolius and Lepidium meyenii - prospective Andean crops for the prevention of chronic diseases. Biomed Pap Med. Fac. Univ. Palacky Olomouc Czech Repub. 147(2):119-30.
Crossref
|
|
|
|
|
Velez E, Castillo N, Mesón O, Grau A, BibasBonet ME, Perdigón G (2013). Study of the effect exerted by fructooligosaccharides from yacon (Smallanthus sonchifolius) root flour in an intestinal infection model with Salmonella typhimurium. Br. J. Nutr. 109(11):1971-9.
Crossref
|
|