This objectives of this study were evaluated nutritive values of Bang Chang’s Cayenne pepper (Capsicum annuum var. acuminatum) and investigates its biological characteristics on health promotion, such as, antioxidant activities. The research was conducted by collecting sample, separating dried edible parts, which were determined nutritive values, including proximal analysis of water content, crude protein, crude fat, dietary fiber, total ash content, carbohydrate, total calories, β-carotene, vitamins E and C. The nutritive data show higher nutritive values of Bang Chang’s Cayenne pepper than other Cayenne pepper. Moreover, β-carotene and vitamin E contents of Bang Chang’s Cayenne pepper were higher than other Cayenne pepper. In conclusion, Bang Chang’s Cayenne pepper was quite more good nutritive values than other Cayenne peppers with preferable antioxidant activities and non-toxic effect on Vero cell.
Capsicum annuum in Solanaceae genus is a non-pungent pepper, which is important ingredients in many traditional dishes as decorative vegetables due to their colors (such as green red and yellow) and unique taste. Peppers are found to be sources of bioactive compounds such as vitamin C, vitamin E, provitamin A, carotenoids, phenolics and flavonoids (Materska and Perucka, 2005) with antioxidant activities, which promote reduction of harmful oxidative stress. These compounds could also prevent many diseases that related with free radical oxidation such as cardiovascular disease, cancer and neurological disorders (Shetty and Wahlqvist, 2004). According to various appearances of sweet peppers, relationship between different color sweet peppers and antioxidant activities is of interesting topic. The sweet peppers with difference colors may compose of diverse pigment generators. Carotenoids (including capsanthin, capsorubin and capsanthin) and flavonoids are main pigment compounds in red pepper (Luke, 2000; Sun et al., 2007), while the color of green pepper is from chlorophyll and the carotenoids typical of the chloroplast (Marin et al., 2004). Likewise, yellow sweet pepper consists of α- and β-carotene, zeaxanthin, lutein and β-cryptoxanthin as color generators (Luke, 2000).
Bang Chang’s Cayenne pepper (C. annuum var. acuminatum) or Bang Chang cultivar is originated from Bang Chang sub-district, Amphawa district, SamutSongkhram province, Thailand and had special characteristic for cooking, such as attractive red color or vermillion, aromatic odor after dried, appropriate to cooking and dressing to Thai cuisine.
The aim of this research was to investigate nutritive values, the total phenolic content (TPC) and antioxidant activities from dried pepper extracted in different solvent systems including hexane and ethanol. This research would provide information on the relationship between sweet peppers extracted with different solvent systems and their antioxidant activities, which could be useful for further investigation on isolation of anti-oxidative agents from Bang Chang’s Cayenne peppers.
Sample collection and preparation
The basis data of Bang Chang’s Cayenne peppers, such as, cultivated area, last annual yield of production and local expert’s interview, were supported by Samut Songkram agricultural extension office. The pepper harvest was done during March to April, 2014 which started to sundry during 2 to 3 days. For other, Cayenne peppers were purchased from local market of Bangkok area, which were the “mixture” of common Cayenne pepper cultivars in Thailand including “Phrik-Mun”, “Phrik Chi-Fah”, “Phrik-Jinda” cultivars. All samples were dried in constant weigh and selected edible parts were ground prior to evaluation of nutritive values and biological assays. All assays were carried out in triplicate and the results were described as mean values and standard deviation.
Evaluation of the nutritive values
Proximate analysis
The proximate analysis was carried out according to the methods to be described, or based on the official methods of analysis of AOAC International, 16th ed (AOAC, 1995). The fresh samples were used for the water content determination. The remaining samples were dried at 105°C for 3 h, ground, and then stored in air-tight containers in a cool, dry place for other analyses.
Water content determination
Three to five grams (3 to 5 g) of each sample was dried at 105°C for 3 h. The dried sample was then weighed. The water content was calculated as the percentage on the wet weight basis.
Determination of crude protein
Crude protein was determined by Kjeldahl method (AOAC, 1995), using Buchi Digestion Unit (B-435) and Distillation Unit (B-323) (Buchi, Switzerland). Dried sample (0.2 g) was digested with 20 ml of conc.H2SO4, using 3 g of the selenium and copper sulfate mixture as the catalyst. The digestion was continued for half an hour after the digestion mixture turned clear green. Then 60 ml of 32% sodium hydroxide solution was added, and the mixture was distilled for 3 min. The distillate was collected in a flask containing 60 ml of 2% boric acid solution, with methylene blue and methyl red as the indicators. The distillate was then titrated with 0.1 N H2SO4 solution; the end point was purple. Crude protein was calculated as the percentage on the wet weight basis (N × 6.25).
Determination of crude fat
One gram (1 g) of the dried sample was extracted with 25 ml of petroleum ether in a Goldfisch apparatus (Labconco, U.S.A.) for 3 to 4 h. The petroleum ether extract was evaporated to dryness at 105°C. The residue was weighed and then calculated as the percentage of crude fat on the wet weight basis.
Determination of dietary fiber
Insoluble dietary fiber content was determined according to the AOAC official method 991.42 (AOAC, 1995). Amyloglucosidase (conc.) in the amount of 0.1 ml was used instead of 0.3 ml of the normal strength enzyme. Soluble dietary fiber content was determined according to the AOAC official method by modified as in insoluble dietary fiber determination. The sum of both values was recorded as the total dietary fiber content of each sample.
Determination of total ash content
One gram (1 g) of each sample was ignited in a muffle furnace at 525°C until ash was obtained. The residue was weighed and expressed as total ash on the wet weight basis.
Determination of carbohydrate
The carbohydrate content was obtained by difference, subtracting the water content, crude protein, crude fat, total dietary fiber, and total ash contents from 100% w/w.
Determination of β-carotene, vitamins E and C
a) Measure β-carotene was applied from the method of Munzuroglu et al. (2003). Sample (50 g) was mashed in a homogenizer and 2 g homogenate paste per sample was taken for extraction of β-carotene. To the above homogenates, 4 ml of ethanol were added, vortexed and the mixture centrifuged (MistralÓ 2000) at 2000 rpm for 3 min at 4°C. The supernatant was also filtered through a Whatman No.1 paper, and to the filtrate 0.15 ml n-hexane was added and mixed. β-Carotene was extracted twice in the hexane phase and the collected extract was dried under a stream of liquid nitrogen. Dried extract was solubilized in 0.2 ml methanol for high-performance liquid chromatography (HPLC). Injections were made in duplicate for each sample. The quantification utilized absorption spectra of 436 nm for β-carotene. HPLC separations were accomplished at room temperature with a Perkin-Elmer liquid chromatograph system (Series 1100), consisting of a sample injection valve (Cotati 7125) with a 20 ml sample loop, an ultraviolet (UV) spectrophotometric detector (Cecil 68174), integrator (HP 3395) and a Techsphere ODS-2 packed (5 mm particle and 80 A? pore size) column (250_4.6 i.d.) with a methanol: acetonitrile: chloroform (47:42:11, v/v) mobile phase at 1 ml/min flow rate.
b) Measure vitamin E was applied from the method of Qian et al. (1998). An initial extraction procedure was developed as follows: Sample was ground in a warring blender and screened through an 80 mesh sieve. One gram (1 g) of the sample was precisely weighed and transferred to a 10-ml screw-capped extraction tube. 4ml of n-hexane was added to the tube and the tube was flushed with a steam of N2 to protect vitamins from air exposure before capping. The mixture was shaken on a vortex mixer for 0.5 min, rested for 5 min, and shaken another half minute. After centrifugation at 4000 rpm for 5 min, 1 ml of supernatant was transferred to a 1.5-ml vial and evaporated under nitrogen to remove the solvent. The residue was re-dissolved in 0.3 ml n-butanol before being injected into the HPLC system.
Chromatographic separations were performed on a 150 × 3.9 mm Novapak C column (Waters). Methanol was used as mobile phase at a flow-rate of 1.5 ml/min and a pressure of 1000 p.s.i. (1 p.s.i. = 6894.76 Pa) All injections were 50 ml loop injections on a M710B autosampler (Waters). A Model M510 Waters pump and a Model M490 Waters variable Wavelength UV-visible detector set at 290 nm were used. All quantitation was by peak area using a Waters M740 integrator. Based on the established chromatographic conditions, repeated injections of 0.1, 0.5, 1, 5 and 10 mg of the standard vitamin E was made duplicated onto the HPLC system. The retention time for vitamins E was 4.1 min. A Shimadzu MPS-2000 universal spectrophotometric scanner was used to determine the spectrograms of vitamin E in n-butanol.
c) Measure vitamin C was applied from the method of Sanchez-Moreno et al. (2003). Total vitamin C (ascorbic acid plus dehydroascorbic acid) were determined by HPLC. The procedure employed to determine total vitamin C was the reduction of dehydroascorbic acid to ascorbic acid, using DL-dithiothreitol as reductant reagent. A volume of 50 ml of each orange juice was homogenized with 40 ml of an extraction solution (3% metaphosphoric acid plus 8% acetic acid). The resulting mixture was centrifuged, filtered, and adjusted to 100 ml with distilled water. Samples were filtered through a 0.45 μm membrane filter, and duplicates of 20 μl for each extract were analyzed by HPLC. Results were expressed as milligrams of ascorbic acid per 100 ml. An aliquot of the mixture was taken to react with 2.0 ml of a solution 20 mg/ml DL-dithiothreitol for 2 h at room temperature and in darkness. During this time the reduction of dehydroascorbic acid to ascorbic acid has been placed. Samples were filtered through a 0.45-μm membrane filter, and duplicates of 20 μl for each extract were analyzed by HPLC. Results were expressed as milligrams of total vitamin C per 100 ml. A Hewlett-Packard model 1050 quaternary solvent delivery controller pump was used for analysis. Samples was introduced onto the column via a manual injector (Rheodyne) equipped with a sample loop (20 μl). Separation of ascorbic acid was performed by HPLC using a reversed-phase C18 Hypersil ODS (5 μm) stainless steel column (250 × 4.6 i.d. mm) (Technochroma). The solvent system used was an isocratic gradient of a 0.01% solution of H2SO4, adjusted to pH 2.5 to 2.6. The flow rate was fixed at 1.0 ml/min. A Hewlett-Packard 1040A UV-visible photodiode array detector was set at 245 nm; chromatographic data and UV-visible spectra were collected, stored, and integrated using a Hewlett-Packard Chem Station and related software. Identification of the ascorbic acid was carried out by HPLC by comparing the retention time and UV-visible absorption spectrum with those of the standard previously referred to. Calibration curves were built with a minimum of four concentration levels of ascorbic acid standard.
Test of biological activities
The pepper samples were dried by hot air and ground. Bring 100 g of ground dried pepper for continuous extraction, then, extract with hexane and ethanol using Soxhlet apparatus. Finally, get the solvent evaporated through rotary evaporation apparatus under vacuum.
Total phenolic content (TPC)
Measurement using Folin-Ciocalteu reagent (Singleton et al., 1999) was done by comparing it with standard solvent, that is, gallic acid at 1 to 0.125 mg/ml concentration; then, calculating TPC of gallic acid in mg/g of the extracts.
Antioxidant activity measurement
a) 2,2-Diphenylpicrylhydrazyl (DPPH) radical scavenging assay to measure the decreasing light absorbance of DPPH radical (Yen and Duh, 1994) using negative control by DPPH radical (6 × 10-5 M), promptly measure at nm and positive control using vitamin C.
b) 2, 2-Azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) cation radical scavenging assay similar to the 1st method but using ABTS radical instead (Re et al., 1999) and also using Trolox (soluble vitamin E) as standard substance to create standard graph (0.5 to 5.0 mg/ml concentration). The antioxidant activity of the Bang Chang’s Cayenne peppers would be shown in Trolox equivalent antioxidant capacity (TEAC)/gm of the pepper extracts.
c) Oxygen radical absorbance capacity (ORAC) to measure ability of extract to scavenge oxygen radical (Prior et al., 2003) and the fluorescent signal generated by fluorescene sodium salt (Sigma-aldrich, Inc.) was measured by FLUOstar OPTIMA microplate reader (BMG) for 1 h. The antioxidant activity of the Bang Chang’s Cayenne peppers would be also shown in TEAC/gm of the pepper extracts.
Cytotoxic activity screening test
Test for cytotoxic activity on primate cell line (Vero cell) using green fluorescent protein (GFP)-based assay (Hunt et al., 1999) by ellipticine as a positive control and 0.5% dimethyl sulfoxide (DMSO) as a negative control.
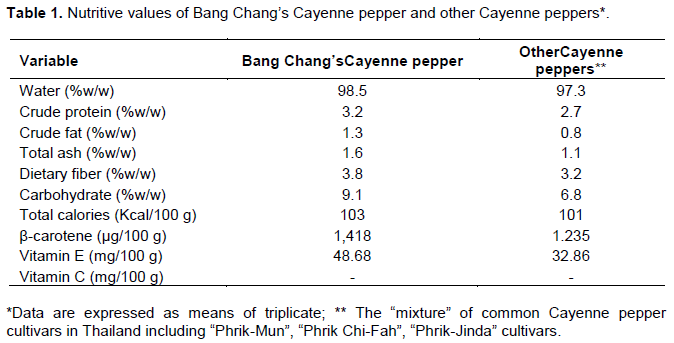
Nutritional value of Bang Chang’s Cayenne peppers
It was found that there were higher nutritive values of Bang Chang’s Cayenne pepper than other Cayenne pepper including β-carotene, vitamins E and C as shown in Table 1. Both samples were lack of vitamin C contained that may be affected by sundried preparation.
Biological properties of Bang Chang’s Cayenne peppers
As a result, it was found that Bang Chang’s Cayenne pepper extracted with ethanol exhibited the higher TPC and antioxidant activities than hexane extract (TPC of 256.4 ± 18.9 mg GAE/100 g, DPPH values of 1751.8 ± 119.1 μmole TEAC/100 g, ABTS+· values of 2663.2 ± 79.0 μmole TEAC/100 g and ORAC values of 4166.3 ± 103.8 μmole TEAC/100 g), followed (Table 2). The test for the cytotoxic activity on cell showed that hexane and ethanol extract yielded no toxic on Vero cell at the concentration of 50 μg/ml (Table 3). The antioxidant activity values from all methods were related to amount of phenolic content.
1mg GAE/100 g DW; 2μmole TEAC/100 g DW; 350% of inhibitory concentration (IC50); 4μ0% e TEAC/g of polyphenols. GAE = Gallic acid equivalent, TEAC = Trolox equivalent antioxidant capacity, DW = dried weight.
These results corresponded to the previous research (Sun et al., 2007), which found that methanol could extract higher quantity of flavonoids such as quercetin and luteolin from red pepper (Sun et al., 2007). Our ethanol extract had higher polarity, however, its polarity closed to methanol, thus ethanol extract may contain high amount of phenolic compounds that correlated to TPC as well. Additionally, red pepper was also reported to contain higher quantity of flavonoids than green pepper (Materska and Perucka, 2005) and when compared to our study, Bang Chang’s Cayenne pepper had attractive red color (Figure 1), which may also contain high amount of flavonoids. On the previous studies, Loizzo et al (2014) were evaluated antioxidant activities of C. annum (two cultivars) by wide range methods (Table 2) including DPPH., ABTS+., β-carotene bleaching test and Fe2+ chelating activity test. Ethanol extract of Bang Chang’s Cayenne pepper was possessed ABTS+· radical scavenging values higher than all extracts (Table 2) from previous study (Loizzo et al., 2014; Hernández et al., 2010). It may also have higher antioxidant activity than Cayenne peppers on previous studies according to DPPH. radical scavenging assay, however, incomparable for DPPH. radical scavenging assay, because of different on reported value (between μmole TEAC/100 g DW and 50% of inhibitory concentration, IC50). Moreover, antioxidant activities of Cayenne peppers were preferable on other antioxidant assay also (Loizzo et al., 2014; Hernández et al., 2010) and different on temperatures and assays may affect antioxidant values (Yazdizadeh et al., 2013).

These results suggested that TPC and antioxidative agents might possess hydrophilic properties more than lipophilic properties, and were corresponded to the previous research, which reported that flavonoids are commonly extracted with methanol or ethanol solvent systems (Bae et al., 2012). Bang Chang’s Cayenne pepper contained higher nutritive values rather than other Cayenne pepper, because amount of fleshy pulp was thicker than other Cayenne pepper when compared in the same weight and ease to ground for cooking as spices. Polyphenolic compounds may have the major bioactive components in Bang Chang’s Cayenne pepper, which are responsible for antioxidation and antiproliferation. Natural antioxidants have been proved to inhibit tumor growth selectively, because of different redox status between normal cells and cancer cell (Nair et al., 2007). In case of this study, both of extracts lack cytotoxicity against a noncancerous cell line, Vero cells and this results was corresponded to previous study, which was reported to lack of cytotoxicity on C. annum against Vero cell line and an adenocarcinoma cervical cancer, HeLa cell line study (Berrington and Lall, 2012). However, C. annum was exhibited cytotoxicity against hepatocellular cancer cell, Hep-G2 cell line and the cytotoxicity of pepper was depended on red color peppers with small size and capsaicin content (Popovich et al., 2014).