ABSTRACT
Bio-preservation of Nigerian soft-white cheese (wara) in submerged consortium of bacteriocinogenic lactic acid bacteria (LAB) culture was investigated. Lactobacillus acidophilus PIT17 and Lactococcus lactis PIT30 were isolated from pito using the pour plate technique on MRS medium. The selection of L. acidophilus PIT17 and L. lactis PIT30 for the bio-preservation studies were based on their ability to produce acidophilin and nisin to inhibit the growth of the test isolates. The ‘wara’ were submerged in consortia of the L. acidophilus PIT17 and L. lactis PIT30 culture and were kept/stored at 2, 4, 6, 8, and 10°C and at room temperature. The shelf-life of the wara alongside control was determined. Physico-chemical and proximate analysis, microbial counts and organoleptic characteristics of the wara were also carried out before and after the bio-preservation. The shelf life of the wara were observed with a significant difference (p < 0.05) at storage temperatures of 2°C (5 days), 4°C (6 days), 6°C (4 days), 8°C (3 days), 10°C (6 days) and 24±1°C (3 days). The proximate analysis of wara showed significant difference (p < 0.05) at different storage temperatures employed. The total viable bacterial count (TVBC) of the cheese (wara) decreased significantly (p < 0.05) after 24 h of submerged bio-preservation of wara from 9.8 × 105 cfu/ml, 1.3 × 106 cfu/g to between 6.2 × 105 and 7.5 × 105 cfu/g. The organoleptic characteristics of wara revealed that there were significant difference (p < 0.05) in colour, texture, aroma, taste, and general acceptability.
Key words: Organoleptic characteristics, proximate, physico-chemical, cheese, consortium.
Lactic acid bacteria (LAB) are a diverse group of microorganisms with different metabolic activities. This diversity makes them very adaptable to a range of conditions and is largely responsible for their success in acid food fermentation (Beuchat, 1995). LAB have no strict taxonomic significance although they had been shown by serological technique and 16S ribosomal RNA cataloguing to be phylogenetically related. They share a number of common features (Adams and Moss, 2008). Historically, bacteria from the genera Lactococcus, Lactobacillus, Streptococcus, Leuconostoc and Pediococcus are the main species involved. Several more have been identified but with minor significance or role in lactic acid fermentations (Food and Agriculture Organization, FAO, 2013). LAB produce various compounds such as organic acids and bacteriocin during lactic acid fermentation (Lindgren and Dobrogosz, 1990). Bacteriocins are naturally occurring antibiotic peptides produced by Gram positive bacteria and may contain as much as 24 amino acids. Some bacteriocins are lantibiotics, which means that they are post translationally modified so as to encompass the amino acid lanthionine or “Lan” (Chatterjee et al., 2005).
In recent years, the interest increased in bacteriocin-like inhibitory substances (BLIS) producing LAB because of their potential use as natural antimicrobial agents to enhance the safety of food products. Bacteriocins from LAB are described as “natural” inhibitors and as a result LAB had acquired generally recognized as safe (GRAS) status. The BLIS from LAB are antimicrobial compounds that possess bacteriocin requisites but that have not yet been characterized for their amino acid sequence (Jack et al., 1995). Bacteriocins from the GRAS-LAB have received significant attention as a novel approach to the control of pathogens in foods (Settani et al., 2005). Pito is one of the indigenous alcoholic beverages. Mainly, pito is produced from the grains of guinea corn (Sorghum vulgare and Sorghum bicolor). Sorghum is one of the cereals cultivated in the tropical regions of Africa and is about the largest cultivated crop in the Northern Guinea Savanna areas of Nigeria (Okoro et al., 2011). The process of pito production is similar to burukutu production which involves malting, mashing, fermentation, and maturation as described by Okoro et al. (2011).
Geotrichum candidum and Lacobacillus species have been described to be responsible for souring pito (Okoro et al., 2011). From plant extracts of tea leaf (Camelia species), cashew tree bark (Anacardium occidenttale) and the bark of mango tree (Magnifera indica), pito can be produced. Steeping and boiling are the process involved in production of unfermented pit (Kolawole et al., 2007). Most of the bacterial cultures found during the production of pito include LAB, which include those bacteria capable of metabolising fructose, galactose, lactose lactic acid that lowers pH of product. The LAB have optimum pH range between 3 and 6.8. Many bacteria species had been found in fermented pito. They included Leuconostoc mesenteriodes, Bacillus subtitis, Staphylococcus species, G. candidum and Lactobacillus spp. The species are responsible for the souring of pito. Due to consumers demand for the locally fermented beverages such as pito, the bacteriocin producing organisms are considered a potential source of biological preservatives for such local drinks (Okoro et al., 2011).
Soft white-cheese (wara) can be defined as consolidated curd milk solid in which fat is entrapped by coagulated casein. The physical characteristics of cheese are far removed from milk, this is because protein coagulation proceeds to a greater extent as a result of the use of proteolytic enzymes and much of the water content of the milk separates and it is removed in the form of whey (Taylor et al., 1997). Some examples of cheese include soft ripened cheeses which include camemberti and blue cheeses. The cheese starter culture is the combination of Streptococcus cremoris and Lactobacillus lactis (O’ Sullivan et al., 2002). Bio-preservation has gained increasing attention as natural means for controlling the shelf-life and safety of food products. The application of bio protective cultures to ensure the hygienic quality is a promising tool although, it should be considered only as an additional measure to good manufacturing, processing, storage and distribution practices (Amani, 2012).
The application of bacteriocins as natural antimicrobial substances in biopreservation (the use of living cells and/or their products for preservation purposes) has focused mainly on foods and foodstuffs from animal origin (Cleveland et al., 2001; Devlieghere et al., 2004; Stiles, 2004). Consequently, bio-preservation systems such as bacteriocinogenic LAB cultures and/or their bacteriocins have received increasing attention and new approaches to control pathogenic and spoilage microorganisms have been developed (Ross et al., 1999). This study is aimed at bio-preservation, physicochemical, proximate, microbial analysis and evaluation of organoleptic characteristics of Nigerian soft-cheese in submerged consortium of bacteriocinogenic LAB culture.
Collection of study samples
Pito samples were purchased from Unguwan Kaje, Minna in sterile bottles and were taken to the laboratory for LAB isolation. Samples of soft-white cheese (wara) were purchased from Bosso Market and deposited in sterile conical flask for the bio-preservation and organoleptic characteristic studies.
Culture media
The standard laboratory methods as prescribed by Cheesebrough (2003) were used to prepare the culture media. The media used in this study include nutrient agar (NA) (Oxoid), urea agar base (Analar), mannitol salt agar (MSA) (Oxoid), Simon’s citrate agar (Oxoid), De Man Rogosa Sharpe (MRS) broth (Oxoid) and De Man Rogosa sharpe (MRS) medium (Oxoid). The MRS is a selective medium for the growth of LAB.
Isolation of LAB
One milliliter of pito was aseptically transferred into 9 ml buffered peptone water, Bpw (Oxoid) to obtain 1:10 dilution. In 0.1% peptone water, serial dilution of the pito was carried out. The serially diluted samples of pito were plated on MRS medium and were incubated at 37°C for 24 h. Colonies/Growth that appeared on the culture plates were counted using the colony counter (Stuart, 6339, Co. Ltd. Great Britain). The result of the count was recorded as colony forming units per milliliter (cfu/ml). Repeated sub-culturing of the isolates on fresh media was used to obtain pure cultures. The pure culture was maintained on agar slant for further characterization and identification (Bromberg, 2004; Oyeleke and Manga, 2008).
Characterization and identification of microbial isolates
The microbial isolates were identified based on colony morphology, cell morphology and biochemical tests (Fawole and Oso, 1998; Cheesbrough, 2003; Manga, 2008). The biochemical tests include Gram’s reaction, motility, oxidase, ammonia from arginine, coagulase, catalase, citrate utilization, indole test, gelatine liquefaction, carbohydrate utilization profiles, and mannitol activity. The LAB were characterized and identified as Lactobacillus acidopholus PIT 17 and Lactococcus lactis PIT 30 using standard scheme.
Selection of LAB for bio-preservation studies
The L. acidopholus PIT 17 and L. lactis PIT 30 were selected from other LAB after vigorous screening with reference amount of bacteriocin produced using the methods described by Kacem et al. (2005) and Mohammed et al. (2013).
Inoculum preparation of LAB
The bacteriocinogenic LAB (L. acidophilus PIT17 and L. lactis PIT30) were inoculated into nutrient broth medium and then incubated at 37°C overnight, serial dilutions was carried out thereafter. The total count of microorganisms per milliliter (ml) of the stock suspension was determined by means of the surface viable count (SVC) technique. The McFarland standard was prepared by mixing 0.85% of 1% sodium chloride and 9.95 ml of 1% sulphuric acid in a separate test tube. While Microbial cell dilutions of the L. acidophilus PIT17 and L. lactis PIT30 in normal saline initially prepared were compared with the turbidity that matches that of the 0.5 (108 cells/ml) McFarland standard prepared. Thus, standard inoculums for the culture consortia of L. acidophilus PIT17 and L. lactis PIT30 were prepared. The 0.5 McFarland standards is comparable to a bacterial suspension of 108 cells/ml. From the inoculums, wara were preserved/submerged in 108 cells/ml (w/v) of the consortia of L. acidophilus PIT17 and L. lactis PIT30 cultures employed in this study (McFarland, 1907; Sanaa et al., 2008).
Bio-preservation studies of soft-white cheese using consortium of bacteriocinogenic LAB
From the inoculum preparations, the cheese (wara) were submerged and preserved in 108 cells/ml (w/v) of the consortia of L. acidophilus PIT17 and L. lactis PIT30 cultures. The preserved wara were kept/stored at refrigeration temperature (2, 4, 6, 8 and 10°C) and room temperature (24±1°C) to determine the shelf life of the wara under study. The experimental control (wara without consortia bacteriocinogenic LAB) were set aside (Mcfarland, 1907; Techno serve, 1994; Food Storage Time Guide Line, FSTGL, 2003; Food Safety Authority of Ireland, FSAI, 2005; Sanaa et al., 2008).
Proximate analysis of soft-white cheese (wara)
Percentage moisture content
In an oven at 80°C, the metallic dishes were dried for 20 min and were allowed to cool in desiccators and weighed. About 5 g of wara were placed in the dishes and were weighed. The dishes with the wara samples were then dried at 80°C in an oven for 24 h to achieve a constant weight. These were quickly transferred to desiccators to cool. It was then weighed immediately with minimum exposure to the atmosphere. The loss in weight of the wara sample during drying is the moisture content (AOAC, 2005).
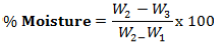
Where, W1 = Initial weight of empty crucible, W2 = weight of crucible + food before drying, W3 = final weight of crucible + food after drying.
% Total solid (dry matter) = 100 - % Moisture
Percentage protein content
The concentrated H2SO4, concentrated NaOH (40%), K2SO4 and CUSO4 were used to digest the wara sample. About 5 ml of the digested wara samples each were placed into a micro-Kjeldahl distillation apparatus with excess concentrated NaOH to make the solution strongly alkaline. Ammonia were distilled into 5ml of boric acid indicator in a titrating flask separately. About 45 ml of the distillates were collected. Titrations were done with 0.01M HCL. The end points of titration were light green (AOAC, 2005).
% Protein = %N × F
Where, F = Conversion factor = 100 / (%N in food protein) and % Nitrogen (N) = (VSVB ×Nacid × 0.01401 / W) × 100
Where, VS = vol. (ml) of acid required to titrate sample, VB =vol. (ml) of acid required to titrate blank, N acid = Normality of acid (0.1N), and W = weight of sample in grams. The common factor used for most food and food mixture is 6.25
Percentage total ash content
Ten grams of wara samples were weighed into a small dry crucible of known weight separately. The wara samples in the separate crucible were charred on a low furnace. At 550°C, the charred samples of wara were ashed in a muffle furnace for 2 h. The ashed materials were removed from the furnace and cooled. The materials were placed in the desiccators and were weighed (AOAC, 2005).
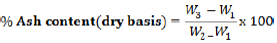
Where, W1=Weight of empty crucible, W2=weight of crucible + food before drying and/or ashing, and W3=weight of crucible + ash.
Percentage total fat contents
The percentage fat content of wara was determined by direct Soxhlet extraction using petroleum either (bp = 40 to 60°C) as solvent. The 0.5 g of wara samples were measured into separate filter papers and were placed in the extractor. The set-up was placed on a heating mantle separately. The heat source was adjusted such that the solvent was boiled gently and refluxed several times for 6 h until the ether had siphoned over and the barrel of the extractor was empty. On removal, the filter paper was placed in an oven at 50°C and dried to constant weight. The percentage of fat was then calculated (AOAC, 2005).
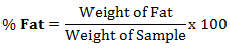
Total carbohydrate content (%)
The total percentage of carbohydrate content of wara was calculated by adding percentage moisture, ash, protein, fat, and fibre contents of the wara and subtracting it from 100% (AOAC, 2005).
Microbial counts
The pour plate method was used. Serially, diluted sample of the bio-peserved wara was inoculated into nutrient agar and incubated at 37°C for 24 h for the presence of aerobic viable bacteria. Colonies that appeared on the plates were counted using colony counting chamber and were recorded as colony forming unit per millilitre (cfu/ml) or (cfu/g) of samples (Cheesbrough, 2003; Oyeleke and Manga, 2008). Microbial counts were taken before biopreservation of products, every 24 h after first preservation of dairy products in consortia of L. acidophilus PIT17 and L. lactis PIT30 cultures and at the expiration of shelf life of the wara.
Organoleptic characteristics of soft-white cheese (wara)
The method of Ranganna (2008) was employed. Sensory quality attributes such as colour, aroma, texture, taste, and general acceptability of the biopreserved wara were evaluated using six-point Hedonic scale. For this purpose, the wara samples were served to ten panellists for rating on Six-point scale as score 1 (dislike very much/most undesirable), 2 (dislike much), 3 (dislike), 4 (like), 5 (like much), and 6 (liked very much/most desirable) compared with the control samples of wara. The organoleptic scores generated were analyzed statistically.
Statistical analysis of data
Data generated in this study were subjected to statistical analysis using analysis of variance (ANOVA), that is, one way analysis of variance (ANOVA), SPSS 19.0 version package and Pearson’s correlation with MINITAB 14 package to determine the level of significance between variables.
Isolation, characterization, identification of bacteriocinogenic LAB and it selection for bio-preservation studies
The pito analyzed had varying species of LAB in them. L. acidophilus PIT 17 and L. lactis PIT 30 were isolated, characterized and identified (Table 1). The L. acidophilus PIT 17 and L. lactis PIT 30 were selected after vigorous screening based on its ability to grow in MRS broth to produce acidophilin and nisin. Also, through spectrophotometric analysis at the 580 nm wavelength, bacteriocin activity (AU/mL), pH and potentials for use as food preservative. It was observed that L. acidophilus PIT 17 and L. lactis PIT 30 had growth ability of 0.89, at pH of 5.00 and 0.91 at pH of 5.80 and bacteriocin activity of 8200 and 9400 AU/mL, respectively with significant differences (p < 0.05) (Table 2).
Table 1. Morphological and biochemical characteristics of bacteriocinogenic LAB isolated from fermented food product.
Proximate analysis of bio-preserved soft-white cheese (wara)
The proximate analysis of the wara such as %moisture, protein, total ash, fat and carbohydrate showed significant difference (p < 0.05) at different storage temperatures employed. The variation in the proximate compositions could be attributed to effects of the LAB consortium used and/or differences in storage temperatures employed in this study (Table 3).
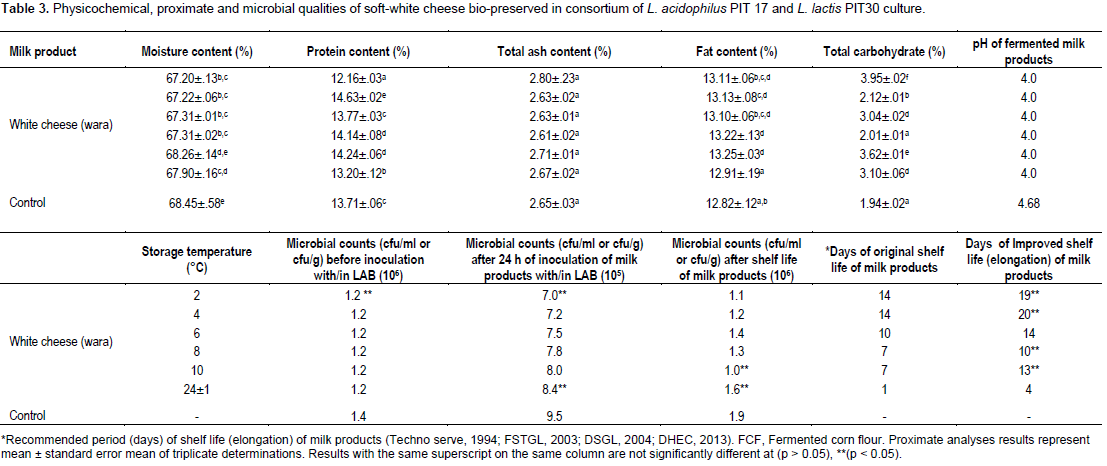
Microbial count of bio-preserved soft-white cheese (wara)
The total viable bacterial count (TVBC) of the cheese (wara) decreased significantly (p < 0.05) after 24 h of submerged technique of bio-preservation of wara from 9.8 × 105 cfu/ml, 1.3 × 106 cfu/g to b 6.2 × 105 and 7.5 × 105 cfu/g. Shelf life extension days of wara were observed with a significant difference (p < 0.05) at storage temperatures of 2°C (5 days), 4°C (6 days), 6°C (4 days), 8°C (3 days), 10°C (6 days) and 24±1°C (3 days) (Table 3).
Organoleptic characteristics of bio-preserved soft-white cheese (wara)
The organoleptic characteristics of the wara revealed that there were no significant difference (p > 0.05) in colour, texture, aroma, taste and general acceptability compared with the wara not submerged in the consortia culture of the bacteriocinogenic LAB. This could be as a result of the effects of the bacteriocinogenic LAB and/or storage temperatures employed when compared with the wara not submerged in the consortia culture of the bacteriocinogenic LAB (control) (Table 4).
The pito analysed showed the presence of LAB. Similarly, the occurrence of LAB in locally fermented foods were also reported by Oyeleke et al. (2006) who reported frequent isolation of L. bulgaricus and L. acidophilus with 29% each of occurrence, followed by Streptococcus thermophilus (25%), S. cremoris (10.6%) and L. lactis (6.4%) products. This is in conformity with the report of Mohammed and Ijah (2013) who isolated and characterized LAB from fermented milk (nono), cheese (wara) and yoghurt and revealed that 13 (86.6%) out of 15 samples analysed, harboured LAB. Nono had the highest LAB counts (9.8 × 106 cfu/ml), while yoghurt had the lowest LAB counts (3.1 × 106 cfu/ml). The LAB were identified as Lactobacillus bulgaricus (31.6%), L. lactis (15.8%), L. acidophilus (10.5%), S. thermophilus (15.8%), S. cremoris (10.5%), Pediococcus halophilus (5.3%) and Saccharomyces cerevisiae (5.3%). The bio-preservation of wara (w/v) in consortia of L. acidophilus PIT17 and L. lactis PIT30 culture revealed that pH, storage temperature and microbial load played significant roles in shelf life determination. Similarly, FSAI (2005) reported that the shelf life of many food products is dependent on storage temperature and microbial load.
At refrigeration, storage temperatures of 4 and 10°C, fermented milk products in this study were also better preserved than other storage temperatures (2, 6, 8 and 24±1°C) employed in this study. This could be due to the inability of some the spoilage pathogenic organisms to grow at those temperatures and/or the presence of consortia culture of LAB employed. This is not the same but similar with the report of Mohammed et al. (2013) worked on bio-shelf life extension of fresh beef in Lactobacillus plantarum FALB 33 culture at different storage temperatures and revealed that at refrigeration storage temperatures of 4 and 10°C, fresh beef were best preserved than other storage temperatures (2, 6, 8 and 24±1°C) employed in their study. This finding is similar to the report of Techno serve (1994) that most commercial products, like milk products are refrigerated at 10°C which also encourages the growth of many psychrophiles like Pseudomonas, Alkaligenes, Flavobacterium and Micrococcus species at room storage temperature (24±1°C). This is similar to the result of the present study where the preserved wara with the consortium proved effective and extended the shelf life by 2 to6 days at different storage temperatures.
This agrees with the report of O’Sullivan et al. (2002) that as an alternative to using bacteriocin itself for bio-preservation of foods, direct introduction of live bacteriocin-producing culture of LAB as a protection starter has been investigated extensively and has achieved favourable results in some food systems. For example, the nisin- producing starter has been shown to have the potential to inhibit L. monocytogenes in Camembert cheese manufacture. Furthermore, it was reported that Lactobacillus or Pediococcus strains producing an antilisterial class IIa bacteriocin could inhibit L. monocytogenes growth in meats and meat products. The lacticin 481-producing and lacticin 3147-producing cultures have been used successfully to improve the quality of Cheddar cheese through the inhibition of NSLAB (Ryan et al., 1996; O`Sullivan et al., 2003). O`Sullivan et al. (2003) reported a reduction of 4 log units in the number of NSLAB after 4 months of ripening in experimental Cheddar cheese with lacticin 481-producing strain L. lactis CNRZ 481 used as an adjunct to the lactococcal starter culture L. lactis HP, compared with the same number of bacteria in the control cheese (obtained with the standard starter culture only).
The recorded decrease in the number of NSLAB was 2 log units achieved at the end of the ripening period (after 6 months). Nisin Z-producing strain L. lactis IPLA 729 has been successfully applied on the inhibition of the spoilage strain of Clostridium tyrobutiricum CECT 4011, a late blowing agent, in semi-hard Vidiago cheese making as reported by Rilla et al. (2003). The results of the proximate analysis of the bio-preserved wara showed major differences at different storage temperatures employed. The variation in the proximate compositions could be attributed to presence of the biopreservation culture or differences in storage temperatures employed in this study. This is similar to the report of Elewa (2009) who revealed that the proximate analysis of cheese samples produced using combined culture of Lactobacillus bulgaricus and L. plantarum had the highest protein content (29.5%), while the sample produced by natural fermentation had the lowest protein content (26.66%). The moisture content of the samples ranged between 26.64 and 32.09%. This is also similar to the report of Ramzi and Ahmed (2013) who worked on soft-white cheese and revealed that the total solids, fat, crude protein, titratable acidity, pH, and volatile fatty acids were affected by the storage period, while there was no significant difference (p>0.05) in ash contents.
The organoleptic characteristics observed in this present study on the wara revealed that there were significant difference (p < 0.05) in colour, texture, aroma, taste and general acceptability compared with the control. This is similar to the findings of Papetti and Carelli (2013) who worked on composition and sensory analysis for quality evaluation of a typical Italian cheese and revealed that cheese were evaluated for various sensory attributes (taste, flavour, texture, and overall acceptability) during storage. In the affective tests, the panellists evaluated the samples for overall quality. The results proved that months of production had significant effects on the sensory quality of the cheese. This is also similar to the report of Katikou et al. (2005) who worked on sensory changes in colour and odour of sliced-bio preserved-refrigerated beef with bacteriocin and revealed that instrumental colour measurements changed with storage time, but no treatment effects were observed during the whole 28-day storage period.
This is not the same but similar to the report of Mohammed et al. (2014) who worked on sensory evaluation of African Catfish (Clarias gariepinus) bio-preserved in culture of Lactobacillus sake FMB 9 and revealed that the shelf life of the African catfish was extended significantly (p < 0.05) between 2 and 5 days at the different storage temperatures employed. The implication of these research findings is that bio-preservation of wara using consortia culture can extend the shelf life of the products particularly at refrigeration temperatures of 4 and 10°C, respectively. In conclusion, the findings of this study demonstrate that the use of consortium of bactriocinogenic LAB improved the nutritional quality, shelf life and acceptability of the wara.
It is therefore recommended that this method of biopreservation be used in food and dairy industries.
The authors have not declared any conflict of interests.
REFERENCES
|
Adams MR, Moss MO (2008). Food Microbiology. Cambridge CB4 0WF, UK: Royal Society of Chemistry.
|
|
|
|
Amani MS (2012). Bio-preservation Challenge for Shelf-Life and Safety Improvement of Minced Beef. Glob. J. Biotechnol. Biochem. 7(2):50-60.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (2005). Official methods of Analysis.18th Edition. Washington, D.C, USA.
|
|
|
|
|
Beuchat LR (1995). Application of biotechnology to fermented foods. Food Technol. 49(1):97-99.
|
|
|
|
|
Bromberg M (2004). Isolation of Bacteriocin-producing Lactic acid bacteria from meat products and it spectrum of inhibitory activity. Braz. J. Microbiol. 35:1-2.
Crossref
|
|
|
|
|
Chatterjee C, Paul M, Xie L, Vander Donk WA (2005). Biosynthesis and mode of action of Lantibiotics. Chem. Rev. 105:633-683.
Crossref
|
|
|
|
|
Cheesebrough M (2003).District Laboratory Practices in Tropical Countries. Cambridge University Press, Edinburgh, UK. pp. 382-407.
|
|
|
|
|
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001). Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71(1):1-20.
Crossref
|
|
|
|
|
Devlieghere F, Vermeiren L Debevere J (2004). New preservation technologies: possibilities and limitations. Int. Dairy J. 14:273-285.
Crossref
|
|
|
|
|
Department of Health and Environmental Control (DHEC) (2013). South Carolina Department of Health and Environmental Control, Food Safety for Home Cooks: Shelf life of foods in fridge. Columbia, SC 29201 (803) 898-DHEC (3432).
|
|
|
|
|
Elewa M (2009). Influence of Lactic Starters on Sensory Properties and Shelf-Life of 'Wara'-a Nigerian (Unripened) Soft Cheese. J. Appl. Biosci. 13:714-719.
|
|
|
|
|
Fawole MO, Oso BA (1998) Laboratory Manual of Microbiology. Spectrum book limited, Ibadan, Nigeria. pp. 16-35.
|
|
|
|
|
Food and Agriculture Organization (FAO) (2013). Fermented fruits and vegetables: A global perspective (bacteria fermentations). FAO
|
|
|
|
|
Food Safety Authority of Ireland (FSAI) (2005). Determination of Product Shelf life: Guidance Note No. 18 FSAI, Dublin. pp. 19-46.
|
|
|
|
|
Food Storage and Time Guidelines (FSTGL) (2003). Food Service of America.
|
|
|
|
|
Jack RW, Tagg JR, Ray B (1995). Bacteriocins of Gram-positive bacteria. Microbiol. Rev. 59:171-200.
|
|
|
|
|
Kacem M, Zadi-Karam H, Karam N (2005). Detection and activity of plantaricin OL15 a bacteriocin produced by Lactobacillus plantarum OL15 isolated from Algerian fermented olives. Gras. Aceites 56(3):192-197.
|
|
|
|
|
Katikou P, Ambrosiadis I, Georgantelis D, Koidis P, Georgakis SA (2005). Effect of Lactobacillus-protective cultures with bacteriocin-like inhibitory substances_ producing ability on microbiological, chemical and sensory changes during storage of refrigerated vacuum-packaged sliced beef. J. Appl. Microbiol. 99:1303-1313.
Crossref
|
|
|
|
|
Kolawole OM, Kayode RMO, Akinduro B (2007) Proximate and microbial analyses of burukutu and pito produced in Ilorin, Nigeria. Afr. J. Biotechnol. 6(5):287-590.
|
|
|
|
|
Lindgren SW, Dobrogeosz WL (1990). Antagonistic activities of lactic acid bacteria in food and food fermentation. Microbiol. Rev. 87:49-160.
Crossref
|
|
|
|
|
McFarland JL (1907). McFarland Standard: Barium Sulfate Turbidity. J. Am. Med. Assoc. 49:1176.
Crossref
|
|
|
|
|
Mohammed SSD, Damisa D, AbdulRahman AA, Balogu TV, Niranjan K (2014). Sensory Evaluation of African Catfish (Clarias gariepinus) Biopreserved in Culture of Lactobacillus sake FMB 9 isolated from fermented Beef. Lap. J. Sci. Technol. 2(1):11-28.
|
|
|
|
|
Mohammed SSD, Ijah UJJ (2013). Isolation and Screening of Lactic Acid Bacteria from Fermented Milk products for Bacteriocin Production. Ann. Food Sci. Technol. 14(1):122-128.
|
|
|
|
|
Mohammed SSD, Ijah UJJ, Damisa D, Muhammad IL, Bala E (2013). Bio-shelf life Extension of fresh Beef using Lactobacillus plantarum FALB33 Culture at different storage temperatures. Jew. J. Sci. Res. 1(1):16-23.
|
|
|
|
|
O`Sullivan L, Ross R, Hill C (2003). Alacticin 481-producing adjunct culture increases starter lysis while inhibiting nonstarter lactic acid bacteria proliferation. J. Appl. Microbiol. 95:1235-1241.
Crossref
|
|
|
|
|
O'Sullivan L, Ross RP, Hill C (2002). Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 84:593-604.
Crossref
|
|
|
|
|
Okoro IA, Ojimelukwe PC, Ekwenye UN, Akaerue B, Atuonwu AC (2011). Quality Characteristics of Indigenous Fermented Beverage; Pito using Lactobacillus sake as a starter Culture. Cont. J. Appl. Sci. 6(1):15-20.
|
|
|
|
|
Oyeleke SB, Faruk AK, Oyewole OA, Nabara HY (2006). Occurence of Lactic acid bacteria in some locally fermented food products sold in Minna markets. Niger. J. Microbiol. 20(2):927-930.
|
|
|
|
|
Oyeleke SB, Manga BS (2008). Essentials of Laboratory practicals in Microbiology (first edition).Tobest publishers, Minna, Nigeria. pp. 28-62.
|
|
|
|
|
Papetti P, Carelli A (2013). Composition and sensory analysis for quality evaluation of a typical Italian cheese: Influence of ripening period. Czech J. Food Sci. 31:438-444.
|
|
|
|
|
Ramzi DKR, Ahmed HOI (2013). Physicochemical and sensory characteristics of white soft cheese made from different levels of Cassava powder (Manihot esculenta). Int. J. Curr. Res. Acad. Rev. 1(4):1-12.
|
|
|
|
|
Ranganna S (2008). Hand book of Analysis and Quality Control for fruit and Vegetable products. 2nd ed. McGraw Hill, New Delhi. pp. 979-1070.
|
|
|
|
|
Rilla N, Martinez B, Deldago T, Rodriguez A (2003). Inhibition of Clostridium tyrobutyricum in Vidiago cheese by Lactococcus lactis ssp. lactis IPLA 729, a nisin Z producer. Int. J. Food Microbiol. 85:23-33.
Crossref
|
|
|
|
|
Ross PR, Galvin M, McAuliffe O, Morgan SM, Ryan MP, Twomey DP, Meaney WJ, Hill C (1999). Developing applications for lactococcal bacteriocins. Antonie van Leeuwenhoek 76:337-346.
Crossref
|
|
|
|
|
Ryan MP, Rea MC, Hill C, Ross RP (1996). An application in cheddar cheese manufacture for a strain of Lactococcal lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62:612-619.
|
|
|
|
|
Sanaa OX, Fadous AB, Ibtisarn EE (2008). Effects of temperature and storage period on the constituents of milk inoculated with Pseudomonas aeruginosa. Res. J. Microbiol. 3(1):30-34.
Crossref
|
|
|
|
|
Settani L, Massitti O, Van Sinderen D, Corsetti A (2005). In situ activity of a bacteriocin – producing Lactococcus lactis strain. Influence on the interactions between lactic acid bacteria during sourdough fermentation. J. Appl. Microbiol. 99:670-681.
Crossref
|
|
|
|
|
Stiles ME (2004). Biopreservation by lactic acid bacteria. Antonie van Leeuwenhoek 70:331-345.
Crossref
|
|
|
|
|
Taylor DJ, Green NPO, Stout GW (1997). Biological Science. 3rd Ed. Cambridge University Press Cambridge, pp. 401-402.
|
|
|
|
|
Techno serve (1994). Male milk manual, A guide for establishing and operating small scale enterprises for production of culture milk. Techno. Nairobi. P 55.
|
|