ABSTRACT
L. reuteri DSM 17938 strain was encapsulated using emulsion polymerization technique with alginate and fructooligosaccharide at five different concentrations between 0 and 1.5%. This study aimed to improve the gastrointestinal system (GIS) viability of L. reuteri. Encapsulation yield was calculated and found to be between 98.67 and 86.88%, and SEM imaging was performed for beads, and their sizes were found to range from 68.81 µm to 351.0 µm. In addition, microbial growth in GIS was indicated for 3 h at intervals of one hour. 0.75% fructooligosaccharide plus 2% alginate capsules yielded the highest viability in a simulated gastric environment. At the end of 3 h, these capsules were decreased 0.39±0.03 logarithmic cycle, but the non-encapsulated control sample was decreased 2.10±0.16 log. The control sample was decreased by 5.8 log cycle in the simulated bile environment, but capsules were decreased by 2.5-3.4 log cycle on average. The result was statistically significant and showed that the encapsulation process protected the survival of microorganisms in GIS.
Key words: Encapsulation, gastrointestinal system, lactic acid bacteria, survival rate.
Probiotic microorganisms are defined as living microorganisms that provide health benefit to the host when they are sufficiently taken by the body (FAO and WHO, 2006; Muthukumarasamy et al., 2006; Ünal and Erginkaya, 2010).
Benefits of probiotics to the host are as follows: adjustment of the immune system, synthesis of the components that provide anti-cholesterol characteristics, anti-carcinogenic effects, improvement of intestinal flora with the antimicrobial materials they produce (Muthukumarasamy et al., 2006; Schell and Beermann, 2014), treatment of some intestinal disorders, positive impact on systemic diseases like allergy or inflammatory diseases, and positive effects in the treatment of vaginitis (Martin et al., 2015). Lactobacillus reuteri is an obligate heterofermentative, gram-positive, and catalase-negative lactic acid bacterium (Ünal and Erginkaya, 2010). Being indigenous to the human intestinal system and well- colonized in intestines, this probiotic microorganism shows a preventive effect on the diseases originating from pathogens (Muthukumarasamy et al., 2006; Gu et al., 2015). L. reuteri can synthesize reuterin, known as beta-3-hidroxypropionaldehyde, from glycerol in anaerobic conditions. Reuterin is a neutral metabolite with a non-protein structure. It is resistive against various proteolytic and lipolytic enzymes and effective in a wide range of pH, and shows an antimicrobial effect against Escherichia coli O157:H7, yeast, mold, and protozoon (Mohammed et al., 2020). L. reuteri DSM 17938 is the daughter strain of L. reuteri ATCC 55730 strains that has transferable resistance traits for lincomycin and tetracycline (Urba?ska and Szajewska, 2014). It has a positive effect on the treatment of diarrhea (Dinleyici et al., 2015) and colic complaint (Szajewska et al., 2013).
Probiotic foods include 108-109 cfu/ml alive microorganism (Shori, 2017)and a minimum of 106-107 cfu/ml microorganism is colonized in intestines by passing through digestive system during the entire shelf life of the product (Ünal and Erginkaya, 2010; Martin et al., 2015; Shori, 2017). The main problem with consuming these products is that the microorganisms cannot maintain their viability during shelf life and in the GIS. Supporting probiotic microorganisms with a physical barrier such as encapsulation will positively impact their viability (Ünal and Erginkaya, 2010; Martin et al., 2015). Many of the studies show that the probiotics that are encapsulated with biopolymer matrix improve the strength of the GIS (Krasaekoopt and Watcharapoka, 2014; Rodklongtan et al., 2014; Villena et al., 2015; García-Ceja et al., 2015; Martin et al., 2015). The most suitable encapsulation methods for probiotic microorganisms are emulsion, extrusion (Muthukumarasamy et al., 2006), and spray drying (Pankasemsuk et al., 2016; Ünal and Erginkaya, 2010). Extrusion technology has advantages like easier application and more uniform capsules than emulsion (Martin et al., 2013; Muthukumarasamy et al., 2006), but it has a disadvantage in that, the larger-sized capsules cause a decrease in sensory quality. On the other hand, spray drying process conditions cannot be controlled totally (Gökmen et al., 2012).
In addition, the properties of the wall material to be selected are essential for the efficiency and reliability of the encapsulation (Naveena and Nagaraju, 2020).
De Prisco et al. (2015)encapsulated L. reuteri DSM 17938 strain by alginate and alginate-chitosan matrix and exposed to different stress conditions. Non-capsulated controls were decreased by 2.09 logarithmic phase in 3 h, which simulated gastric environment, but alginate capsules were decreased by 0.35 logarithmic phase. García-Ceja et al. (2015)capsulated Lactobacillus acidophilus and L. reuteri individually and in combination by using alginate and alginate-chitosan system. They reported that alginate-chitosan capsules had a better gastrointestinal system resistance compared to the alginate capsules, and the combined encapsulation of lactobacilli could provide more health benefits than individual encapsulation. Muthukumarasamy et al. (2006)encapsulated five different L. reuteri strain by using alginate with starch, κ-carrageenan with locust bean gum, and xanthan with gallant gum and studied the endurance in simulated gastric conditions. It was reported that all the capsulated samples had a better endurance compared to the control, and alginate and alginate plus starch yielded the best positive results.
The presence of prebiotics such as various oligosaccharides in the environment was reported to increase the viability and resistance of probiotics in the gastrointestinal tract (Burgain et al., 2011; Rajam and Anandharamakrishnan, 2015). Prebiotics are resistant in the gastric environment and partially fragmented by intestinal enzymes in the intestine.
Fragmentation products are a substrate for the fermentation of probiotic microorganisms. So, the combined use of probiotics and prebiotics shows a synergistic effect. Inulin, fructooligosaccharide, and galacto-oligosaccharide are the compounds that are used in this manner (Rajam and Anandharamakrishnan, 2015) and show a prebiotic impact(Rajam and Anandharamakrishnan, 2015; Dias et al., 2018).
Although there is much information about L. reuteri DSM 17938 strain in medical literature, there is a limited number of food studies on the use of this organism. The authors aimed to use alginate in combination with FOS as wall materials for the encapsulation of L. reuteri. The effect of wall material combination, encapsulation efficiency, the morphology of microcapsules, and viability of cells in gastrointestinal (simulated gastric and intestinal) conditions was evaluated.
Preparation of probiotic culture
Freeze-dried L. reuteri DSM 17938 (BioGaia AB, Stockholm/ Sweden) was activated twice by deManRogosaSharpe (MRS) broth (Merck, Germany) under anaerobic conditions at 37ºC. In the first activation, microorganisms were washed with sterile 0.1% peptone (Merck, Germany) water. Then, the culture was inoculated to 5 ml MRS broth medium for 24 h. The culture was transferred into MRS broth and then incubated for 18 h. After the incubation, the sample was centrifuged for 10 min at 4500 rcf and washed with peptone water two times. The culture was diluted to 1010 colony forming units per ml (cfu/ml) concentration with 0.1% peptone water.
Encapsulation of probiotic cells
L. reuteri DSM 17938 was entrapped by an emulsion technique following the method of Apichartsrangkoon et al. (2015). Sodium alginate (Carl Roth, Germany) and sodium alginate containing 0, 0.5, 0.75, 1, or 1.5% fructooligosaccharide (Sigma, America; Average degree of polymerization of >10) were used as covering material. Accordingly, 40 ml sterile covering material was mixed with 10 ml of the culture and 200 ml of sterile sunflower oil (Torku, Turkey) containing 0.2% (v/v) Tween 80 (Merck, Germany) and stirred for 20 min at 450 rpm. Tween 80 used as emulsifying agents provides a better homogenization by lowering the interfacial tension of the two immiscible phases and can be used to prepare smaller capsules (Pech-Canul et al., 2020). After that, 200 mL of sterile 0.1M CaCl2 (Merck, Germany) was gently added into the mixed solution and stirred for 10 min at 350 rpm. Formed beads were separated by 110 micron filter paper under vacuum and dried at freeze dryer (Christ Alpha 1-2 LD) for one night.
Encapsulation efficiency
To identify the number of bacteria after the encapsulation, resolution of beads was provided by mixing 100 mg lyophilized bead and 9 ml EDTA for 20 min at 450 rpm (Tsen et al., 2007). The encapsulation efficiency (EE) was calculated using the following formula based on the ratio of the number of live cells released from the beads to the initial number of cells.
N0 = Viable bacteria after encapsulation (log cfu/ml); N1 = Viable bacteria before encapsulation (log cfu/ml)
SEM Screening of capsules
After freeze-drying, measuring the size of the beads and the morphology of the microcapsules was examined using scanning electron microscopy (SEM, Zeiss EVO HD 15) at an accelerating voltage of 15.0 kV. The capsulated samples were taken from the modules of SEM screening device, and their upper surfaces were covered with gold-palladium before analysis. Representative SEM images for all beads were determined.
Simulated gastric conditions
A simulated gastric solution (SGS) was prepared according to De Prisco et al. (2015). The solution was prepared with 5 g/L NaCl (Merck, Germany) and 3 g/L pepsin enzyme (Fisher, UK), and pH was adjusted to 2.5 with concentrated HCl (Merck, Germany). The gastric solution was sterilized by filtration (0.10 µm). Aliquots of 0.1 g of encapsulated cells or 0.1 mL of free cell suspensions (109 cfu/ml) were mixed with 9 mL of SGS and incubated for 60, 120, and 180 min at 37ºC. The capsulated and free cells were inoculated on MRS agar (Merck, Germany). Before the inoculation, the SGS was removed and then 10 ml 4% EDTA (abcr, Germany) solution was added for the dissolution of capsules at 450 rpm for 20 min (Tsen et al., 2007).
Simulated bile conditions
Three different simulated bile solutions (SBS) were prepared and tested. The first bile solution was prepared as 0.05M KH2PO4 (Merck, Germany) that included 6 g/L bile salt (Sigma-Aldrich, America) at 7.5 pH (Krasaekoopt et al., 2004). The second bile solution was designed as described by De Prisco et al. (2015). 5g/L bile solution was used for this. The third bile solution was prepared, according to Rodklongtan et al. (2014), as a phosphate-buffered compound that involved 0.3% bile salt. Additionally, the bile bladder of 4-year-old Simmental cattle was used as control.
Bile solution was sterilized by filtration (0.10 µm). Aliquots of 0.1g of encapsulated cells or 0.1 mL of free cell suspensions (109 cfu/ml) were mixed with 9 mL of SBS. Capsulated and free cells were incubated in SBS for 3 h and inoculated on MRS agar at intervals of one hour by the cultural way. Before the inoculation, the capsules were dissolved by the same way applied in the Section Simulated Gastric Conditions. Simulated gastric and bile resistances were examined individually.
Statistical analysis
Statistical analysis of the data was carried out using IBM SPSS Statistics software (v.24). Differences between the results were identified using One-Way ANOVA method and Tukey multiple comparison tests. The statistical significance was set at (p≤0.05).
Efficiency of encapsulation and size of capsules
Table 1 gives the average size of the beads and the efficiency of the encapsulation. The results showed that all the encapsulated cells were able to grow, and no significant or minor damage was recorded during the encapsulation. While the highest capsulation efficiency was found to be 2% alginate with 98.63±3.30%, the lowest efficiency was observed in alginate plus 1% FOS beads with 86.88±1.12%. The results indicate that the microencapsulation in the alginate beads was more appropriate for microencapsulation of L. reuteri than the alginate plus FOS capsules. Krasaekoopt et al. (2003)reported that vitality proportion of probiotic micro-organisms was between 80 and 95% in the emulsion technique.
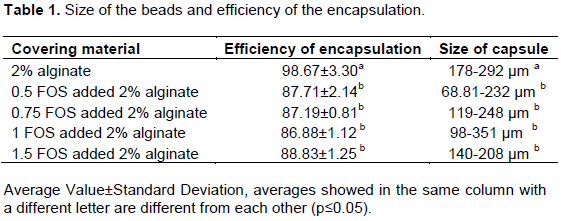
Bilenler et al. (2017)capsulated L. plantarum with alginate plus starch wall material at different proportions and found similar capsulation efficiency. A previous study examining the encapsulation efficiency for Lactobacillus fermentum CECT5716 reported less survival with the same technique in alginate (Martin et al., 2013).
The size of the beads and their surface structure were determined by SEM Screening. Thus, five particles were measured from each capsule. SEM images were illustrated in Figure 1. The beads had an almost spherical shape and a disordered structure. Moreover, it was determined that the surfaces of the beads were wrinkled during the SEM screening, and when it was zoomed, the beads showed no significant diversity in terms of surface morphology.
The average size of the beads was as follows; the alginate beads, 235±30 µm; alginate plus 0.5% FOS beads, 150±52 µm; alginate plus 0.75% FOS beads, 183±41 µm; alginate plus 1% FOS beads, 173±59 µm; and alginate plus 1.5% FOS beads, 174±25 µm. Bead sizes for each capsule were illustrated in Figure 2. 2% alginate beads were found to have a statistically significantly bigger size than the alginate with FOS beads (p<0.05). Similar results were found in a study carried out by Krasaekoopt and Watcharapoka (2014). The sizes of capsules were reported as 1.90-1.92 mm in prebiotic supplementary alginate capsules.
Similarly, Gandomi et al. (2016)reported the sizes of alginate and alginate with inulin capsules as 1.39 mm and 1.40 mm, respectively. The sizes of capsules reported by Prasanna and Charalampopoulos (2018)were bigger than those in the present study. They found the sizes of sodium alginate-cow milk and sodium alginate-goat milk capsules as 2.8±0.3 mm and 3.1±0.2 mm, respectively. Martin et al. (2015)determined the size of alginate capsules as 30-60 µm. The reason why the sizes of the capsules were smaller than those in similar studies may be the addition of CaCI2 while crushing the emulsion.
Endurance of capsules in gastric environment
Gastric environment tolerance of the capsulated cells and the control were shown in Table 2. Low viable cell counts were determined when the alginate was used alone. FOS was used in the mixture to obtain a more strong and stable wall structure. After 3 h of treatment, 0.39±0.03 log cycle decrease was observed in 0.75% FOS added alginate capsules and 0.76±0.30 log cycle decrease in 2% alginate capsules, and the other results were between these values.
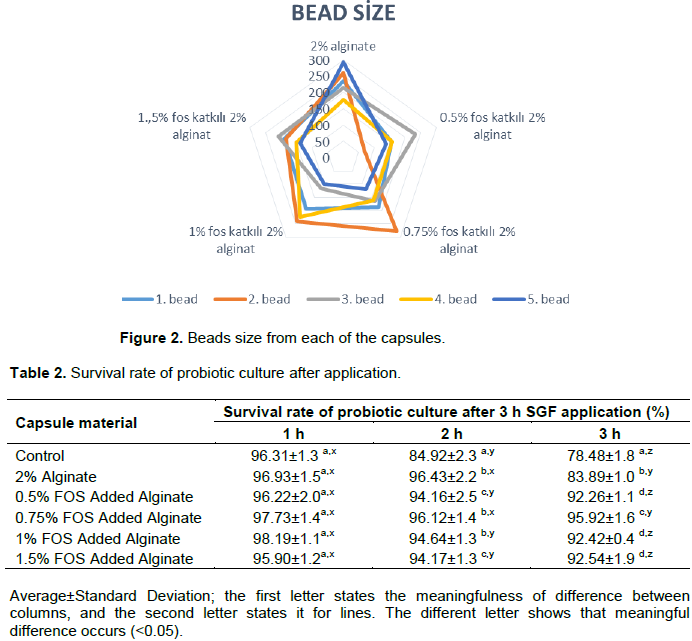
On the other hand, a 2.10±0.16 log cycle decrease was observed in the control samples. A negative correlation was found between the increase in processing time and the number of the alive microorganisms. Besides, according to the one-way ANOVA results, 0.75% FOS + 2% alginate capsule was found to be the best in terms of keeping the liveliness of microorganisms in SGS (p<0.05). There was no significant difference between the capsules of 2% alginate plus 0.5, 1, and 1.5 FOS (p>0.05). The previous studies in the literature reported results similar to ours for L. reuteri survival in SGS. Muthukumarasamy et al. (2006)examined the SGS resistance of 6 different L. reuteri strains and reported a 0.28-1.26 log cycle decrease. Similarly, Zhao et al. (2012)reported a decrease of <1 log cycle as a result of 2 h of SGS treatment on L. reuteri coated with alginate capsules. Likewise our results, numerous studies reported that the capsulation of microorganisms improved the SGS endurance (Rodklongtan et al., 2014; Apichartsrangkoon et al., 2015; De Prisco et al., 2015; Villena et al., 2015; Shori, 2017).
Endurance of capsules in bile environment
The capsules and control samples in the simulated bile environment were unable to maintain their viability. No microbial viability was observed due to the lack of nutrients in the environment or using harsh bile conditions. For bile liquid, a 4-year-old Simmental cattle’s gallbladder was taken without disintegrating, and bile was removed in aseptic conditions. It was determined that after the treatment of this bile liquid for 3 h, 64.05- 66.58% of the capsules were alive. While the capsulated samples were decreased to 2.53-3.48 logarithmic phase, the control sample was decreased to an average of 5.81 logarithmic phases. This difference was found to be statistically significant (p<0.05). It was determined that encapsulation increased the SBS resistance of L. reuteri DSM 17938. Some studies reported a better survival for L. reuteri encapsulated in alginate than our study. For example, De Prisco et al. (2015)reported a reduction of 0.82 log phase for Lactobacillus reuteri DSM 17938 in 4 h. On the other hand, Gülsüno?lu (2013)observed a reduction of 2.24 log in SBS for L. acidophilus LA5 in 4 h.
CONCLUSION AND RECOMMENDATIONS
In recent years, there is a growing interest in functional foods, especially probiotic products. However, these microorganisms lose their vitality under adverse environmental conditions. This both restricts probiotics in products such as dry food and reduces the shelf life of products that include probiotics. Scientists work on improving the endurance of microorganisms under different adverse conditions. Encapsulation can improve the resistance of microorganisms against drying, gastrointestinal system, and heat treatments like freezing and heating.
The coated material was successfully used as a wall system to encapsulate L. reuteri DSM 17938 by emulsion polymerization, and an encapsulation yield higher than 86% was obtained. In the encapsulation process, 0,5% FOS added 2% alginate capsule was found to yield the least vitality, whereas the highest vitality was observed in 2% alginate capsules. Gastrointestinal resistance of this strain was increased by alginate and FOS added alginate capsules. As a result of the statistical analysis, it was found that 0.75% FOS plus 2% alginate capsule was the best coating material and 2% alginate capsule was the worst coating material in terms of GIS viability. However, all the capsule materials protected the L. reuteri DSM 17938 strain better than the control. When the SBS resistance was examined, it was found that the encapsulated cells were better preserved than the control. FOS added capsule material was found to have a positive effect on viability. In addition, a negative correlation was found between viability and processing time in all the samples, including the control. In conclusion, the encapsulation was carried out successfully, and the gastrointestinal system resistance of L. reuteri DSM 17938 strain was improved with encapsulation.
There is an increasing interest in the studies on encapsulation with prebiotics. It is thought that this microorganism can be used as a probiotic additive in functional foods. In future studies, the authors plan to use different prebiotic or carbohydrate basic capsules and examine different probiotics together to see whether they will create a synergistic effect.
The authors have not declared any conflicts of interest.
REFERENCES
|
Apichartsrangkoon A, Chaikham P, Pankasemsuk T, Baipong S (2015). In Vitro Experiment on Lactobacillus casei 01 Colonizing the Digestive System in the Presence of Pasteurized Longan Juice. Acta Alimentaria 44(4):493-500.
Crossref
|
|
|
|
Bilenler T, Karabulut I, Candogan K (2017). Effects of Encapsulated Starter Cultures on Microbial and Physicochemical Properties of Traditionally Produced and Heat Treated Sausages (Sucuks). LWT - Food Science and Technology 75:425-433.
Crossref
|
|
|
|
|
Burgain J, Gaiani C, Linder M, Scher J (2011). Encapsulation of Probiotic Living Cells: From Laboratory Scale to Industrial Applications. Journal of Food Engineering 104(4):467-483.
Crossref
|
|
|
|
|
Dias CO, dos Santos Opuski de Almeida J, Pinto SS, de Oliveira Santana FC, Verruck S, Müller CMO, Schwinden Prudencio E, de Mello Castanho Ambone RD (2018). Development and Physico-Chemical Characterization of Microencapsulated Bifidobacteria in Passion Fruit Juice: A Functional Non-Dairy Product for Probiotic Delivery. Food Bioscience 24:26-36.
Crossref
|
|
|
|
|
Dinleyici EC, Dalgic N, Guven S, Metin O, Yasa O, Vandenplas Y (2015). Lactobacillus reuteri DSM 17938 Shortens Acute Infectious Diarrhea in a Pediatric Outpatient Setting. Jornal de Pediatria 91(4):392-396.
Crossref
|
|
|
|
|
FAO, WHO (2006). Probiotics in Food pp. 413-26. Available at:
View.
|
|
|
|
|
Gandomi H, Abbaszadeh S, Misaghi A, Bokaie S, Noori N (2016). Effect of Chitosan-Alginate Encapsulation with Inulin on Survival of Lactobacillus rhamnosus GG during Apple Juice Storage and under Simulated Gastrointestinal Conditions. LWT - Food Science and Technology 69:365-371.
Crossref
|
|
|
|
|
García-Ceja A, Mani-López E, Palou E, López-Malo A (2015). Viability during Refrigerated Storage in Selected Food Products and During Simulated Gastrointestinal Conditions of Individual and Combined Lactobacilli Encapsulated in Alginate or Alginate-Chitosan. LWT - Food Science and Technology 63(1):482-489.
Crossref
|
|
|
|
|
Gökmen S, Palamuto?lu R, Sar?çoban C (2012). G?da Endüstrisinde Enkapsülasyon Uygulamalar?. G?da Teknolojileri Elektronik Dergisi 7(1):36-50.
|
|
|
|
|
Gu Q, Zhang C, Song D, Li P, Zhu X (2015). Enhancing Vitamin B12 Content in Soy-Yogurt by Lactobacillus reuteri. International Journal of Food Microbiology 206:56-59.
Crossref
|
|
|
|
|
Gülsüno?lu Z (2013). Koaservasyon Yönteminin Model Mikroorganizmalarda Canl?l?k Korunumuna Etkisi. Thesis (M.Sc.) ?stanbul Technical University. Institute of Science and Technology 77 p.
|
|
|
|
|
Krasaekoopt W, Bhandari B, Deeth H (2003). Evaluation of Encapsulation Techniques of Probiotics for Yoghurt. International Dairy Journal 13(1):3-13.
Crossref
|
|
|
|
|
Krasaekoopt W, Bhandari B, Deeth H (2004). The Influence of Coating Materials on Some Properties of Alginate Beads and Survivability of Microencapsulated Probiotic Bacteria. International Dairy Journal 14(8):737-743.
Crossref
|
|
|
|
|
Krasaekoopt W, Watcharapoka S (2014). Effect of Addition of Inulin and Galactooligosaccharide on the Survival of Microencapsulated Probiotics in Alginate Beads Coated with Chitosan in Simulated Digestive System, Yogurt and Fruit Juice." LWT - Food Science and Technology 57(2):761-766.
Crossref
|
|
|
|
|
Martin MJ, Lara-Villoslada F, Ruiz MA, Morales ME (2013). Effect of Unmodified Starch on Viability of Alginate-Encapsulated Lactobacillus fermentum CECT5716. LWT - Food Science and Technology 53(2):480-486.
Crossref
|
|
|
|
|
Martin MJ, Lara-Villoslada F, Ruiz MA, Morales ME (2015). Microencapsulation of Bacteria: A Review of Different Technologies and Their Impact on the Probiotic Effects. Innovative Food Science and Emerging Technologies 27:15-25.
Crossref
|
|
|
|
|
Mohammed AA, Hussain NA, Niamah AK (2020). Antibacterial Spectrum of Produced Reuterin from New Isolates of Lactobacillus reuteri. Journal of Microbiology, Biotechnology and Food Sciences 10(1):134-39.
Crossref
|
|
|
|
|
Muthukumarasamy P, Allan-Wojtas P, Holley R (2006). Stability of Lactobacillus reuteri in Different Types of Microcapsules. Food Microbiology and Safety 71(1):20-24.
Crossref
|
|
|
|
|
Naveena B, Nagaraju M (2020). Microencapsulation Techniques and Its Application in Food Industry. International Journal of Chemical Studies 8(1):2560-2563.
Crossref
|
|
|
|
|
Pankasemsuk T, Apichartsrangkoon A, Worametrachanon S, Techarang J (2016). Encapsulation of Lactobacillus casei 01 by Alginate along with Hi-Maize Starch for Exposure to a Simulated Gut Model. Food Bioscience 16:32-36.
Crossref
|
|
|
|
|
Pech-Canul AC, Ortega D, García-Triana A, González-Silva N, Solis-Oviedo RL (2020). A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings 10(3):197.
Crossref
|
|
|
|
|
Prasanna PHP, Charalampopoulos D (2018). Encapsulation of Bifidobacterium longum in Alginate-Dairy Matrices and Survival in Simulated Gastrointestinal Conditions, Refrigeration, Cow Milk and Goat Milk. Food Bioscience 21:72-79.
Crossref
|
|
|
|
|
De Prisco, Maresca D, Ongeng D, Mauriello G (2015). Microencapsulation by Vibrating Technology of the Probiotic Strain Lactobacillus reuteri DSM 17938 to Enhance Its Survival in Foods and in Gastrointestinal Environment. LWT - Food Science and Technology 61(2):452-462.
Crossref
|
|
|
|
|
Rajam R, Anandharamakrishnan C (2015). Microencapsulation of Lactobacillus plantarum (MTCC 5422) with Fructooligosaccharide as Wall Material by Spray Drying. LWT - Food Science and Technology 60(2):773-780.
Crossref
|
|
|
|
|
Rodklongtan A, La-ongkham O, Nitisinprasert S, Chitprasert P (2014). Enhancement of Lactobacillus reuteri KUB-AC5 Survival in Broiler Gastrointestinal Tract by Microencapsulation with Alginate-Chitosan Semi-Interpenetrating Polymer Networks. Journal of Applied Microbiology 117(1):227-238.
Crossref
|
|
|
|
|
Schell D, Beermann C (2014). Fluidized Bed Microencapsulation of Lactobacillus reuteri with Sweet Whey and Shellac for Improved Acid Resistance and In-Vitro Gastro-Intestinal Survival. Food Research International 62:308-314.
Crossref
|
|
|
|
|
Shori AB (2017). Microencapsulation Improved Probiotics Survival During Gastric Transit. Hayati Journal of Biosciences 24(1):1-5.
Crossref
|
|
|
|
|
Szajewska H, Gyrczuk E, Horvath A (2013). Lactobacillus reuteri DSM 17938 for the Management of Infantile Colic in Breastfed Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. The Journal of Pediatrics 162(2):257-262.
Crossref
|
|
|
|
|
Tsen JH, Huang HY, Lin YP, King VAE (2007). Freezing Resistance Improvement of Lactobacillus reuteri by Using Cell Immobilization. Journal of Microbiological Methods 70(3):561-564.
Crossref
|
|
|
|
|
Ünal E, Erginkaya Z (2010). Microencapsulation of Probiotic. G?da Teknolojileri Elektronik Dergisi 35(4):297-304.
|
|
|
|
|
Urba?ska M, Szajewska H (2014). The Efficacy of Lactobacillus reuteri DSM 17938 in Infants and Children: A Review of the Current Evidence. European Journal of Pediatrics 173(10):1327-1337.
Crossref
|
|
|
|
|
Villena MJM, Lara-Villoslada F, Martínez MAR, Hernández MEM (2015). Development of Gastro-Resistant Tablets for the Protection and Intestinal Delivery of Lactobacillus fermentum CECT 5716. International Journal of Pharmaceutics 487(1-2):314-319.
Crossref
|
|
|
|
|
Zhao Q, Mutukumira A, Lee SJ, Maddox I, Shu Q (2012). Functional Properties of Free and Encapsulated Lactobacillus reuteri DPC16 during and after Passage through a Simulated Gastrointestinal Tract. World Journal of Microbiology and Biotechnology 28(1):61-70.
Crossref
|
|