ABSTRACT
Physico-chemical analyses and sensory evaluation were carried out on commercially and freshly prepared orange juice (100%) in the laboratory and its shelf-life after the storage period of 90 days using various storage methods. At the end of the study, the result showed that the laboratory processed orange juice in terms of the nutritional composition when compared with the commercially processed orange juice had a better quality considering the parameters assessed. The results of the laboratory prepared orange juice showed that the chemically treated, pasteurized, concentrated and carbonated orange juice had pH 1.6 to 4.5, TTA 0.03 to 0.31%, maturity ratio 1.16 to 8.55, total soluble solids (TSS) ranging from 26 to 33°Brix. The vitamin C content for the untreated juice (fresh) and the commercially produced were 43 and 2.67 mg/100 ml, respectively. The experimental samples of chemically treated, pasteurized, concentrated and carbonated had vitamin C content ranging from 22-29, 27-32, 28-30 and 17-29 mg/100 ml, respectively. Sodium and phosphorus were found in small amounts ranging from 0.01-0.12 and 0.001-0.02%, respectively in the orange juice. Among the various processing methods concentrated orange juice ranked first followed by chemically treated, pasteurized and carbonated in terms of the sensory evaluation as assessed by the panelists.
Key words: Orange juice, physico-chemical properties, processing techniques, sensory evaluation.
Fruit juices are well recognized for their nutritive value, mineral and vitamin contents (Wardlaw, 2004; Mannay and Shadaksharaswany, 2005). They are important source of bioactive compounds such as phenolics (such as flavanone glycosides, hydroxycinnamic acids), vitamin C and carotenoid which is an excellent source of bio-available antioxidant phytochemicals and it improves blood lipid profiles especially for people affected with hypercholesterolemia (Franke et al., 2005). Juices are the aqueous liquids expressed or otherwise extracted usually from one or more fruits or vegetables, purees of the edible portion of one or more fruits or vegetables, or any concentrates of such liquids or purees (Fraternale et al., 2011).
Orange fruits have been reported as a rich source of these phytochemicals such as flavonoids, especially flavanones, which have been shown to possess several physiological properties which can help inhibit cell proliferation and cell differentiation (Vanamala et al., 2006; Ndife and Abbo, 2009). However, due to the perishable nature of fruits and vegetables, high post harvest losses occur immediately after harvest, during distribution and marketing (Manny and Shadaksharaswany, 2005; Potter and Hotchkiss, 2006) resulting from lack of cold storage facilities on the farms, improper handling and inadequate processing facilities (Landon, 2007: Adubofuor et al., 2010). Reports have shown that the post harvest losses for oranges range between 31 and 50% (Alaka et al., 2003; Landon, 2007). One of the ways of preserving these fruits and vegetables from deterioration and subsequent loss is to process them into fruit juices (Vanamala et al., 2006). The processing of fruit juices such as orange juice presents the problem of storage, an unstable product for periods of 12 months or more. These juices are susceptible to three types of deterioration such as microbial spoilage, colour changes and off flavours due to enzymatic reactions (Obire et al., 2008; Lawlor et al., 2009: Sospedra et al., 2012). Juices are no exception, and there is, therefore, a strong tendency towards consumption of premium quality juices. These juices are directly obtained from fruits (not from concentrate), are distributed through the refrigerated chain and have a relatively short shelf life (Esteve et al., 2005). Traditionally, thermal pasteurization is used to inactivate micro-organisms to prolong shelf life on the one hand, and to inactivate heat-stable pectinmethylesterase (PME) for preventing cloud loss on the other hand (Chen and Wu, 1998). Typically, for shelf stable juices, processing times for thermal pasteurization are equivalent to 90°C for 1 min (Eagerman and Rouse, 1976). Thermal processing has a negative impact on the quality of orange juice such as loss of fresh flavor (Braddock, 1999), degradation of ascorbic acid (Chen et al., 1993), and discoloration (Arreola et al., 1991; Li et al., 1988; Tatum et al., 1975). To avoid quality degradation of orange juice by thermal processing, non-thermal preservation techniques such as high pressure and pulsed electric fields have been studied during the last decade.
Various methods which can be used for the processing and preservation of orange juice include; the traditional thermal processing method (pasteurization) also called high temperature short time (HTST), evaporation (concentrated juice), pressurized carbon dioxide process (carbon dioxide saturation), high pressure processing (HPP), pulse power submerged electrical arc technology method, sterile filtration and spray drying (Andres et al., 2001; Deliza et al., 2005). The pasteurization method kills bacteria and extend the shelf life of juices, but affects the taste of the product (Andres et al., 2000). Orange juice that has been extracted and pasteurized for few seconds to inactivate yeasts and moulds will have a shelf life of up to 21 days when stored at 4°C or less.
A shelf life of up to three months at chill temperatures (4.4 to 7.2°C) can be attained with orange juice treated with chemical preservatives. Preservatives used are typically sulphur dioxide at 100 ppm and a combination of sorbic and benzoic acid at up to 400 ppm (Mehmood et al., 2008).
Concentrated juices produced through the evaporation method are another method of storing juices, but this when reconstituted could not approach the quality of the original. The reason is because most of the compounds which give a fruit juice its flavour and aroma are volatile, and lost in the usual evaporation process (Moshonas and Shaw, 1998). The carbon dioxide saturation method has a high degree of stability in juices and it prevents the development of yeasts, probably by retarding the conversion of pyruvic acid into acetaldehyde and carbon dioxide. Moulds and bacteria are less affected and could cause spoilage pressurized carbon dioxide process, according to (Giovanna et,al; 2009) showed that the process is as effective as heat pasteurization but does not change the taste and preserves more of the vitamins found in fresh squeezed juices.
Today’s society is characterized by an increasing health consciousness and growing interest in the role of food for maintaining and improving human well-being and consumer health. Orange juice is a heterogeneous, two-phase system consisting of the serum, a clear aqueous phase containing soluble compounds, and a water insoluble phase made up of particles ranging from 0.05 µm to a few hundred micrometers in size. These insoluble particles enhance the colour, flavour, aroma and body of the juice; as such they are highly desirable in commercial product (Rega et al., 2004). However, like most fruit juices, orange juice undergoes changes in chemical, nutritional and flavour characteristics during processing. Studies by Dauda and Adegoke (2014) showed that non-enzymatic browning during processing or storage of juices may affect the flavour, colour or other quality factors of the product. Kampen (1976) stored freeze-dried orange juice crystals and a synthetic mixture for 40 days at 50°C and monitored losses of total amino acids (75%), ascorbic acid (100%), citric acid (5.1%) and sucrose (4.4%). The orange juice crystals were discoloured from Maillard browning and several carbonyl compounds and furfural derivatives were identified as products of reactions. Several attempts have been made to examine the influence of different processing techniques on the quality and shelf life stability of orange juice. Nagy and Smoot (1977), Roig et al. (1999), Kaanane et al. (1988), and Kennedy et al. (1990) studied the kinetics of ascorbic acid degradation in pasteurized orange juice during storage as a possible marker for the end of shelf life.
During the processing of fruit juices, a large part of the quality characteristics of the fresh fruits undergo remarkable changes which could reduce the nutritional value of the products. Moreover, the fruit juices may be stored for several months in unfavourable conditions before consumption, thus leading to undesirable quality changes due to the influence of temperature, time, oxygen content, light exposure and packaging material (Averbeck and Schieberle, 2010). Products obtained from orange juice are frozen concentrated orange juice (FCOJ) in which about 85% of the water content is removed, freshly- squeezed or not from concentrate (NFC) also known as single strength orange juice (SSOJ) and powdered juice (Anonymous, 2003). Processed products are expected to be essentially uniform in composition and sensory characteristics from one batch to another. Infact, compositional limits for many processed foods such as orange juice are set out in official regulations and purchasing specifications. To obtain the required degree of uniform quality in these products, processors often have one option that is, to use carefully selected raw materials (Board and Woods, 1983).
Sensory quality attributes and nutritive value of fruit play an important role in consumer satisfaction and they influence further consumption. Sensory ratings of fruit juice by products and physical measurements of fruit juice properties are useful methods in the evaluation of fruit juice quality (Colaric et al., 2005). Sensory quality is a difficult concept to define; it should be comprehended as interaction between the product and the consumer. It is necessary to establish a relationship between the physical and chemical composition of the product and its sensory attributes such as colour, texture, aroma (volatile compounds) and taste (sweet, sour, salty and bitter sensations) as well as between the sensory perceptions and the acceptability for the consumer (Escribano et al., 2010).
Several factors need to be considered when assessing for quality of fruit juice. The composition of a fruit juice depends on the variety, origin and growing conditions of the fruit, its quality and the processing and storage procedures (Ndife et al., 2013). Apart from nutritive value of the juice, it should have acceptable organoleptic and physicochemical characteristics as well as free from microbial and chemical contaminants. The juice organoleptic features of interest include colour, aroma, taste/flavour, texture, degree of spreadiblity and overall acceptability by the consumers (Iwe, 2010). The juice must have the characteristic colour, flavour and taste typical of the fruit from which it comes.
The physicochemical characteristics of juices considered in quality assessment include pH, titratable acidity (TA), total soluble solids (°Brix), dry matter contents, ash content, crude protein, ascorbic acid, total sugar, reducing sugar and °Brix (sugar)/acid ratio. The constituent of juice predominantly is water and also contains carbohydrate, sucrose, fructose, glucose, sorbitol and small amount of protein (Pao et al., 2001). Fruit juices have a low pH (2-5) because they are comparatively rich in organic acid (Tasnim et al., 2010). The total acidity of fruit juices is due to presence of a mixture of organic acids, whose composition varies depending on fruit nature and maturity. The total soluble solids (TSS) content is significantly influenced by the combined effect of stages of maturity and ripening conditions (Tasnim et al., 2010). Consumption of fruit juices is popular in Nigeria because of their health and invigorating benefits. Though some fruit juices are produced locally, most of the fruit juices and drinks found in Nigerian markets are imported (Okorie et al., 2009).
There are many studies on the effect of mild preservation techniques on micro-organisms, quality and shelf life of food products. Variance in initial quality of the food, caused by use of different species, maturity and storage conditions, and the use of different process conditions make it difficult to compare the results obtained. In view of the growing demand for processed products with the guarantees of better quality and quantity, researchers has to focus most of their efforts on studying ways of extending the shelf life of processed products so as to deliver specific benefits in terms of health, safety and environmental quality.
The objectives of our study were to examine the physico-chemical analyses and sensory evaluation of commercially and freshly prepared orange juice (100%) in the laboratory and its shelf-life after the storage period of 90 days using various storage methods.
Sample collection
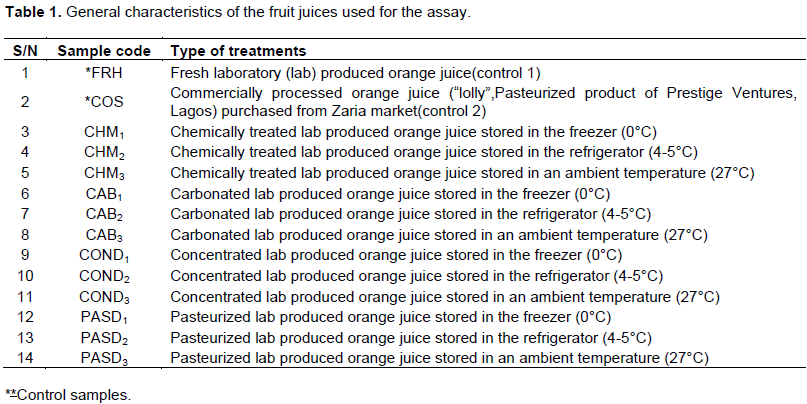
Matured, firm and ripe sweet intact oranges (Citrus sinensis), variety-Valencia and “lolly” commercially processed orange juice were purchased from the market in Zaria, Kaduna State, Nigeria.
Preparation of Juice and treatment
Six hundred fresh intact juicy sweet oranges were processed on a laboratory-scale into juice samples using a modified method of Akpapunam et al. (1993) with some modifications was adopted for the study and the flow chart is as shown in (Figure 1). The fruits were washed, manually peeled, cut into halves with sterile knife using hand gloves and their seeds removed. Sweet oranges were washed, peeled and sliced into halves with sterile knife using hand gloves during processing. The cut oranges (mesocarp) were pressed with a hand juicer squeezer to extract the juice. The juice and pulp obtained were homogenized (blended) in a sterile hand Monilex blender ®. The homogenate was clarified manually using a sterile muslin cloth to obtain a clear juice. The juice was subjected to different treatments: Four hundred millimeters (400 ml) each of the extracted juice was carbonated (carbondioxide Saturation) with1.5 kg/100 ml of juice at a pressure of about seven atmospheres at 15°C, concentrated by heating to boiling with distillation apparatus and treated with a preservative (sodium metabisulphite at the rate of 0.035 gm). As a guideline FDA recommends a minimum temperature-time equivalent for juice of 71.1°C for 3 s for products with a pH in the range of 3.6 to 4.0. However, this specific temperature–time is insufficient to inactivate spoilage organisms (Standard Organization of Nigeria (SON), 1976).
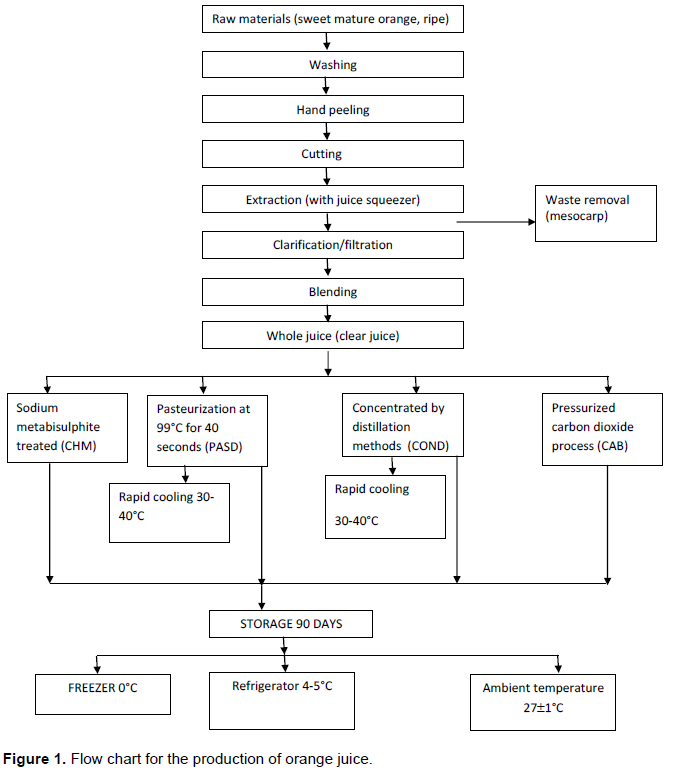
The pasteurized and concentrated samples were allowed to cool to 30 to 40°C. The freshly laboratory prepared and commercially processed samples served as the control. Individual aliquots were kept in the ambient temperature (27°C), the refrigerator (4-5°C) and the freezer (0°C), respectively as described in Table 1. Orange juice was bottled under hygienic conditions in 250 mL PET bottles and sealed with PE caps. The experimental setup were disposed in a factorial completely randomized design 4×3+2, where 4 traditional types (chemically, carbonated, concentrated and pasteurized), 3 storage mode (freezer, refrigerator and ambient temperature) with 2 controls. In all treatment XX replicates was used.
This was divided into five batches of 400 ml each in a plastic container and given the following different treatments:
1. Fresh orange juice was treated with sulphur dioxide, that is, 0.035 gm of sodium metabisulphite was added into each 100 ml of
the fresh juice sample (for 400ml 0.14 gm was added).
2. Orange juice was pasteurized at 99°C for 40 s.
3. Orange juice was concentrated by heating to boiling and 10 ml of distillate was collected from every 100 ml of orange juice using distillation apparatus.
4. Pressurized carbon dioxide (carbon dioxide saturation) was introduced into the juice at the coca-cola bottling plant in Kaduna. The concentration of gas used was 1.5 kg/100 ml of orange juice with a pressure of about seven atmospheres at 15°C.
5. The freshly laboratory prepared orange juice without any treatment serving as control
6. A second control sample, that is a commercially prepared orange juice, a product of “lolly” was purchased from Zaria market Kaduna State, Nigeria and assessed along with the treated samples for their physico-chemical and sensory evaluation qualities. The pasteurized and the concentrated samples were allowed to cool to 30 to 40°C. All the samples with different processing techniques were stored for 90 days under freezer, refrigeration and room temperature, respectively.
Storage stability
This test is carried out in order to maintain quality in storage. Sensory tests for storage stability involve:
i. Difference tests to establish that stored lot is not different from the control. If no difference is found, product stability is good.
ii. Descriptive and scalar tests to describe the changes that may have occurred or to grade the product as acceptable or not acceptable.
A group of about 3 to 10 trained panelist and 1 to 18 experimental samples are used to determine if difference exist in the samples quality at storage with that of the control.
Sensory evaluation (Product rating method)
The organoleptic attributes evaluated for were colour/appearance, taste, aroma, and general acceptability using a five point hedonic scale, varying from “dislike extremely” (score 1) to “like extremely” (score 5) according to the method of Stone and Sidel (1992). Five highly trained panel members carried out the sensory evaluation. The juices were served in a coded and transparent white glass cups for proper assessment. Five samples were coded and presented to each of the panelists.
Physico-chemical analyses
The proximate analysis (quantitative) of the fresh samples of orange juice (FRH), concentrated (COND), carbonated (CAB), pasteurized (PASD), chemically treated (CHEM) and the commercially processed (COS) was carried out according to the
methods of Association of Official Chemists (AOAC, 1995).
pH determination
The pH of orange juice samples was measured using a glass electrode connected to a standard pH-Meter (HB-652359,Hanna, USA) at 20°C. pH meter was standardized using a phosphate buffer solution of 4, 14 and 7. Aliquot of 5 ml each of the orange juice samples was used to determine the pH using pH meter (HB-652359, Hanna, USA) at 20°C.
Total titratable acidity
The test of maturity for oranges is based on the ratio of total solids to acid present in the juice. Since the major part of the total solids is sugar, this is often termed the sugar: acid ratio for oranges and the minimum ratio are 8:1. The ratio is determined by dividing the degrees Brix of a sample by the percentage of titratable acidity of a sample (AOAC, 1995). Aliquot of 5 ml of each sample was titrated with 0.1 M of NaOH using 1% phenolphthalein as the indicator. The percentage acidity was expressed as citric acid.
Maturity ratio (MR)
Citric acid of 0.8% was added to 5 ml of each sample, to obtain the degree brix which is determined by ratio. For oranges the minimum ratio is 8:1.
Total soluble solids
The total soluble solids content was determined using an Abbé 60 refractometer (HB-6959980, Hanna, USA.) at 20°C calibrated against sucrose (AOAC, 1995).
Total solids, moisture and ash content
Total solids, moisture and ash content of the samples were determined using the weight reduction method (AOAC, 1995).
Vitamin C (ascorbic acid) content
The ascorbic acid content (vitamin C) of the orange juice samples were determined by the method described by Nielsen (2003). Aliquot of 10 ml of each sample was added, 10 ml of 3% metaphosphoric acid solution and titrated with the standard dye solution of sodium 2.6-dichlorophenol dye to a faint pink end point. The ascorbic acid content was expressed as milligram per 100 ml (mg/100 ml).
Estimation of Amino Acids (free amino nitrogen)
Five milliliters (5 ml) of each sample and 10 ml of 40% formaldehyde solution with distilled water was titrated against 0.1 M NaOH according to the method of (AOAC, 1995).
Mineral assay
The orange juice samples were digested by the wet ashing method prior to mineral content determination using flame photometer (400, Corning Sherwood,United Kingdom.) for potassium (k) and sodium (Na) (Abulude, 2005). While the phosphorus content was determined colorimetrically with spectrophotometer (6100, Jenway Beckman, England.) using the method described by Nielson (2003).
Statistical analysis
The sensory evaluation data was analysed using analysis of variance (ANOVA) and the post Hoc test using multiple Duncan range procedure (DMRT) with significance level at P≤0.05 in order to determine the levels of significance between the different methods and the control (Ihekoronye and Ngoddy, 1985). A correlation test was used to analyse the different variables of the physico-chemical properties at significance level of P≤0.05 using SAS computer software.
Sensory analysis
The results for the sensory evaluation and overall acceptability of the various samples of orange juice before and after the period of storage (90 days) are shown in Table 2a and b. There were no significant differences (P≥0.05) between colour and viscosity, but there was significant difference (P≤0.05) between odour (aroma) and flavour when compared with their rating with the control samples for general acceptability (Table 2a). The differences obtained in flavour and aroma could be attributed to the different compounds formation based on the different methods of processing and storage of the tested samples of orange juices (Adubofuor et al., 2010). Moreover, orange juice can lose its flavour, colour or other quality factors due to non-enzymatic browning reactions during processing or storage of the juices (Dauda and Adegoke, 2014). The result of this study which showed off-flavour and aroma is also in support of the reports of Moshonas and Shaw (1998), they reported that orange juice can lose some of its fresh flavour during storage because of decreased levels of volatile flavour constituents, thus developing a stable flavour. They also reported that for juices packaged in plastic containers, a major contributor to this flavour lose is often caused by absorption of flavour constituents by the plastic containers.
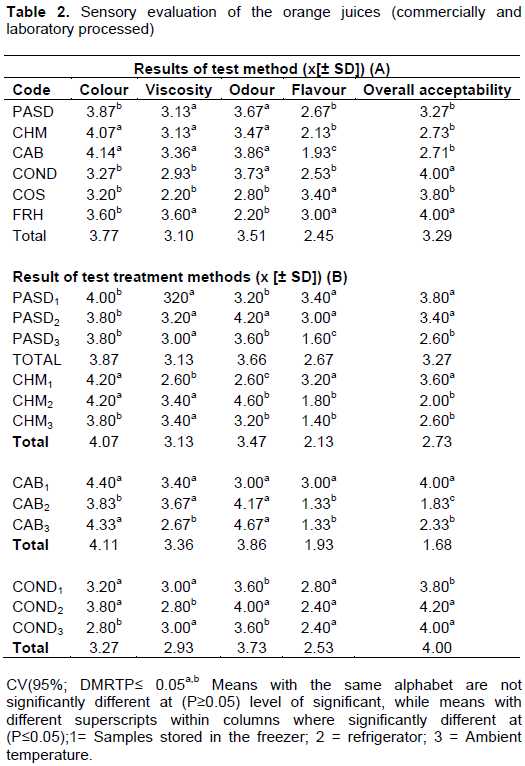
At the end of storage for 90 days, the panelist had preference for concentrated juice stored under the refrigeration temperature and the room temperature; pasteurized and carbonated both were stored under the freezer and room temperature. Shelf life of the orange juice on sensory evaluation after the period of storage was determined. A mean value equal to 3 was chosen as the acceptance limit to determine the end of the shelf life of juices. The hedonic rating showed that samples of pasteurised (PASD), chemically tested (CHM), concentrated (COND), stored in the freezer and refrigerator with the values (3.80 and 3.40; 3.60 and 3.80 and 4.20), respectively was highly accepted by the consumers preference. These treated samples were judged superior organoleptically in quality than the commercially processed one, retaining more of its colour, viscosity and flavour.
Samples of PASD, CHM and carbonated (CAB) stored at ambient temperature was least generally accepted. The samples of CAB, PASD stored in the freezer with values (4.00 and 3.80) and COND stored in the refrigerator and ambient temperature with values of 4.20 and 4.00 had the best in overall acceptability. The result of this study is in agreement with the report of other researchers (Ploydera et al., 2003; Ndife et al., 2013). The deterioration of orange juice during storage comprises changes in organoleptic quality or sensory evaluation, nutritional value and appearance. The main parameters that influence the deterioration of orange juice is the temperature and the presence of oxygen (Dauda and Adegoke, 2014).
A shelf-life study was carried on mango, orange and pineapple juices by Obeta and Ugwuanyi (1997) and these juices were inoculated with ascospores of Neosartorya sp. The result showed that only fruit juices at 4 and 5°C storage and those containing sodium benzoate stored at room temperature were protected from spoilage by these fungi for 64 days.
Physico-chemical analyses
Table 3 shows the physico-chemical properties of the laboratory prepared orange juice fresh, pasteurized, pressurized carbon dioxide (Carbonated), chemically treated, concentrated and commercially processed orange juice. For all the samples the pH ranged from 1.6 to 4.5 and the pH values for the commercially processed sample showed the lowest value compared with the laboratory prepared orange juice with different treatments. The titratable acidity values ranged from 0.03 to 0.24, the Brix acid (maturity) ratio from 1.16 to 4.73, the total soluble solids (TSS) 26 to 33° Brix, the vitamin C content from 2.79 to 43.78% (mg/100 ml juice), total solids from 3.46 to 7.43%, moisture content 2.0 to 10.59%, estimation of amino acids from 0.08 to 0.25 molarity, ash content 0.29 to 1.29%, and minerals (macro-nutrients): Potassium from 0.51 to 1.73%, sodium 0.01 to 0.12% and phosphorus 0.01 to 0.02%.
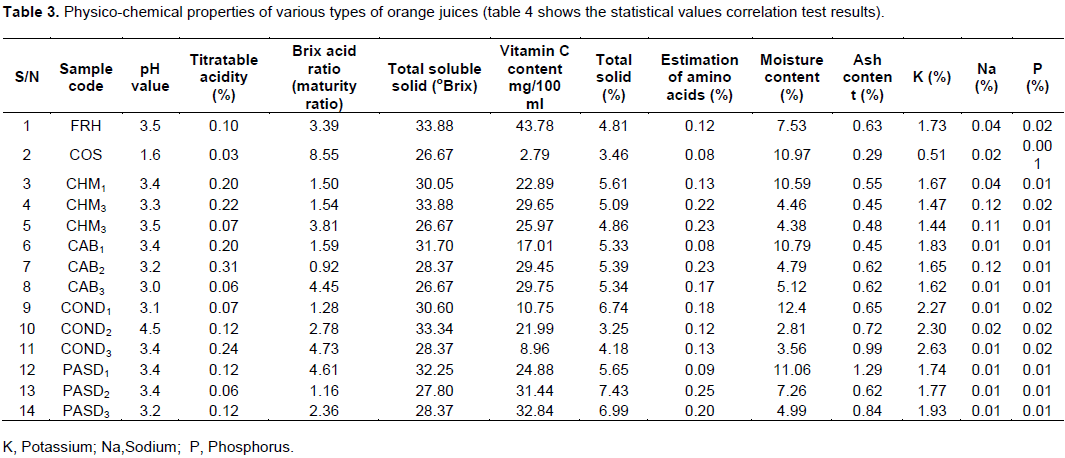
The decline in pH was in good agreement with previous work (Akpapunam et al., 1993). The increase in hydrogen concentration could be explained by the dissociation of component acids by heat. The increase in the TTA of the processed orange juices as was observed in this present study. This could be as a result of increase in temperature, oxidation of aldehydes and alcohols to acid during processing and consequently contributing to an increase in acidity (Adubofuor et al., 2010). The maturity ratio (MR) increases as fruit ripens and is used for assessing the quality of the juice (Kareem and Adebowale, 2007).
The increase in soluble solids contents and the reduction in total solids show the effect of a decrease in acidity. The reduction in acidity during ripening plays an important role in the Brix: Acid ratio and consequently in influencing the taste and flavour of the juice (Braddock and Marcy, 1985; Akpapunam et al., 1993; Cinquanta et al., 2010; Ndife et al., 2013; Dauda and Adegoke, 2014). The increased soluble solids contents obtained compares with the result of Braddock and Marcy (1985). The increase in soluble solids is explained by the evaporation of water during pasteurization and concentration of the juice (Akpapunam et al., 1993).
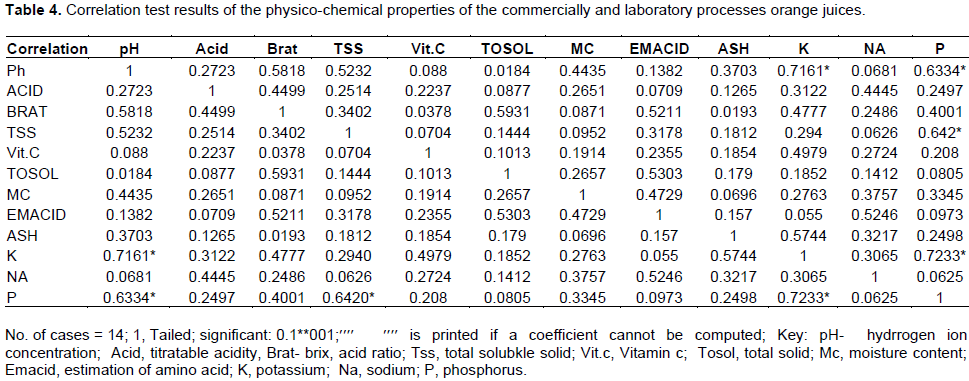
A correlation analysis of the different variables of the physico-chemical properties of the laboratory prepared orange juice is shown in Table 4. The result showed that there was a significant correlation between pH and the percent Brix:acid ratio (r = 0.58, P < 0.05), total soluble solid (r = 0.52, P < 0.05), potassium (r = 0.7, P < 0.05), and phosphorus (r = 0.63, P < 0.05). There was a significant correlation between percent Brix:acid ratio and total solid ( r = 0.59, P < 0.05), and level of amino acid (r =0.52, P < 0.05). Also there was correlation between level of amino acid and sodium (r = 0.05, P < 0.05), percent ash and potassium (r = 0.57, P < 0.05), total soluble solid and potassium (r= 0.64, P < 0.05), potassium and phosphorus (r = 0.72, P < 0.05). There was no significant correlation between other variables.
Vitamin C (Ascorbic acid) content
The vitamin C of the orange juice studied under different processing conditions compared favourably with freshly prepared juice. More of the vitamin C content was conserved in the pasteurized, chemically treated, and carbonated. The results showed that the chemically treated and carbonated orange juice is as effective as pasteurized because it does not change the taste and it preserves more of its vitamins found in fresh-squeezed orange juice. Thus this result agrees previous studies (Murat, 2000; Polydera et al., 2003; Dauda and Adegoke, 2014). According to the Association of the Industry of Juices and Nectars from fruits and Vegetables of the European Union and the report by Kimball (1999), they stated that orange juice should contain at least 20 to 25 mg/100 ml of vitamin C (ascorbic acid) at the time of expiration of storage, which is about 50% of the initial amount. The loss in vitamin C which was observed drastically in the commercially processed sample as stated by Nagy and Smoot (1999) and Dauda and Adegoke (2014) could be attributed to the oxidation by residual air layer within the container during processing. According to Nagy and Smoot (1999) temperature and storage affects the presence of vitamin C content in orange juice and orange fruit. This also accounts for the low level of vitamin C observed in the juice stored at the freezer and ambient temperature for carbonated and concentrated juice.
Considering other parameters like, amino-acids, ash content and mineral elements, potassium was a major macro mineral in orange juice, followed closely by phosphorus, and the result compared favourably with the work of Parichart et al. (1999), and Nnam and Njoku (2005). The result of amino acid showed that the treatments did not show any significant difference in the nutrient compositions during the period of storage and it was consistent with the data obtained from Mears and Shenton (1973). The amino acids also affect sensorial quality attributes, including taste, aroma, and color (Ames, 1998; Kirimura et al., 1969).
The kinetics of reactions involving those amino acids in pineapple juice during heat treatments such as pasteurization, need to be assessed because of their role in the Maillard reaction, which can be of importance for human nutrition.
Statistically, a correlation test shown in Table 4 was applied to assess the nutritional components in order to determine possible significant relationships between the parameters analyzed. In the test, pH value was significantly related to total soluble solids, Brix:acid ratio, potassium and phosphorus (P < 0.05). No significant relationship was found between pH and the other parameters. Brix:acid ratio was negatively correlated with pH, total solids and estimation of amino acids (P<0.05). Total soluble solids were significantly related to pH and phosphorus. There was no significant relationship between vitamin C, acidity, moisture and ash among the other selected parameter (P >0.05).
CONCLUSION AND RECOMMENDATION
Nigerian processors of fruit juices employ techniques similar to the ones that was adopted in this study. These techniques are pasteurization, carbonation, concentration and use of chemical preservatives and their products being stored under similar methods applied in this work; freezer, refrigeration and ambient temperature. The processes were designed among other things to prolong shelf-life of the products by controlling microbial contamination and growth, maintain nutritional value and the chemical composition. From this study, it was observed that the different methods employed, showed that orange juice can store in any of these methods when suitable conditions are being applied in terms of satisfactory cleaning/sanitary conditions of handling and processing methods based on the result obtained, it can be said that the product under study can still be acceptable and fit for human consumption since the result of the nutritional values that is the chemical composition when compared with the Standard Organization of Nigerian (SON) (1976) and the International Standards. Therefore, orange juice under proper quality control and application of good manu-facturing practices will still maintain its nutritional values, organoleptic qualities, not exceeding its microbiological limits as provided by Nigerian Agency for Food and Drug Administration Committee (NAFDAC) still serves as a most invigorating fruit drink. The technologies for commercial production have been revolutionized with the fruit juices with the introduction of ultra-high pressurized packaging such as hydrostatic pressure (HHP), high pressure homogenization, pulsed electric field (PEF) and ultrasound (US) (Quek et al., 2012; Vasantha and Li, 2012). These emerging non-thermal techniques should be encouraged since they have the potential to provide “fresh-like” and safe fruit juices with prolonged shelf life.
The authors have not declared any conflict of interests.
REFERENCES
|
AOAC (1995). Official methods of Analysis, Association of Official Analytical Chemists, 16th edition Washington DC, U.S.A. Pp. 362- 363, 373-378, 507-508.
|
|
|
|
Abulude FO (2005).Distribution of selected mineral in some Nigeria white bread. Niger. Food J. 23:139-147.
|
|
|
|
Adubofuor JA, Amankwash EA, Arthur BS, Appiah F (2010). Comparative study related to physcio-chemical properties and sensory qualities of tomato juice and cocktail juice produced from oranges, tomatoes and carrots. Afr. J. Food Sci. 4(7):427-433.
|
|
|
|
Akpapunam MA, Mepba HD, Wokoma AL (1993). Effects of pasturing time on the quality of processed pineapple juice. Niger. Food J. 11:9- 11.
|
|
|
|
Alaka O, Aina J, Falade K (2003).Effect of storage conditions on the chemical attributes of Ogbomoso mango juice. Euro. Food Res. Technol. 218:79-82.
Crossref
|
|
|
|
Ames JM (1998). Application of the Maillard reaction in the food industry. Food Chem. 62:431-439.
Crossref
|
|
|
|
Andres SC, Giannuzzi L, Zaritzky NE (2000). New gas process keeps orange juice on shelf longer. In Technology 47(8):22.
|
|
|
|
Andres SC, Giannuzzi L, Zaritzky NE (2001). Mathematical modeling of microbial growth with chemical preservatives. J. Food Sci. 66(5):724-728.
Crossref
|
|
|
|
Anonymous (2003). Making Orange Juice, Southern Gardens Citrus, Clewiston Florida. pp. 1-3.
|
|
|
|
Arreola AG, Balaban MO, Marshall MR, Peplow AJ, Wei CI, Cornell JA (1991). Supercritical carbon dioxide effects on some quality attributes of single strength orange juice. J. Food Sci. 56(4):10300-1033.
Crossref
|
|
|
|
Averbeck M, Schieberle P (2010). Influence of different storage conditions on changes in the key aroma compounds of orange juice reconstituted from concentrate. Euro. Food Res. Technol. 217:1366- 1380.
|
|
|
|
Board PW, Woods HJ (1983). Compositional variations and sensory acceptability of apple juice drink. J. Food Technol. 18(6):763-769.
Crossref
|
|
|
|
Braddock RJ (1999). Handbook of citrus by-products and processing technology. New York: John Wiley and Sons Inc.
|
|
|
|
Braddock RJ, Marcy JE (1985). Freeze – Concentration of Pineapple juice. J. Food Sci. 50(6):1681-1683.
Crossref
|
|
|
|
Chen CS, Wu MC (1998). Kinetic models for thermal inactivation of multiple pectinesterases in citrus juices. J. Food Sci. 63(5):1-4.
Crossref
|
|
|
|
Chen CS, Shaw PE, Parish ME (1993). Orange and tangerine juices. In. S. Nagy, C. S.Chen, & P. E. Shaw (Eds.), Fruit juice processing technology, Auburndale. pp. 110-165.
|
|
|
|
Cinquanta L, Albanese D, Cuccurulla G, Dimatteo M (2010). Effect on ornage juice of batch pasteurisation in an improved pilot-scale microwave oven. J. Food Sci. 10:1750-3841.
|
|
|
|
Colaric M, Veberic R, Stampar F, Hudina M (2005). Evaluation of peach and nectarine fruit quality and correlation between sensory and chemical attributes. J. Sci. Food Agric. 85:2611-2616.
Crossref
|
|
|
|
Dauda A, Adegoke GO (2014). Preservation of Some Physico-chemical properties of Soymilk-Based juice with Aframoum Daielli Spice powder. Am. J. Food Sci. Technol. 2(4):116-121.
Crossref
|
|
|
|
Deliza R, Rosenthal A, Abadio FBD, Silva CHO, Castillo C (2005). Application of the high pressure technology in the fruit juice processing: benefits perceived by consumers. J. Food Eng. 67:241- 246.
Crossref
|
|
|
|
Eagerman BA, Rouse AH (1976). Heat inactivation temperature–time relationships for pectinesterase inactivation in citrus juices. J. Food Sci. 41(6):1396-1397
Crossref
|
|
|
|
Escribano S, Sanchez FJ, Lazaro A (2010). Establishment of sensory characterization protocol for melon (cucumis melo L.) and its correlation with physical chemical attributes: indications for future genetic improvements. Eur. Food Res. Technol. 231:611-621.
Crossref
|
|
|
|
Esteve MJ, Frígola A, Rodrigo C, Rodrigo D (2005). Effect of storage period under variable conditions on the chemical and physical composition and colour of Spanish refrigerated orange juices. Food Chem. Toxicol. 43(9):1413-1422.
Crossref
|
|
|
|
Franke AA, Cooney RV, Henning SM, Custer LJ (2005). Bioavailability and antioxidant effects of orange juice components in humans. J. Agric. Food Chem. 53:5170-5178.
Crossref
|
|
|
|
Fraternale D, Ricci D, Flamini G, Giomaro G (2011). Volatile profiles of red apple from Marche Region (Italy). Rec. Nat. Prod. 5:202-207.
|
|
|
|
Giovanna, F., Mariacarmela, B., Giovanna, F., Massimo, P. and Murat, O. B, (2009). Microbial inactivation and shelf life of apple juice treated with high pressure carbon dioxide; J. of Biological Engineering, 3:3 DOI: 10.1186/1754-1611-3-3
Crossref
|
|
|
|
Ihekoronye AI, Nogddy PO (1985). Integrated Food Science and. Technology for the Tropics. (2nd ed.) Macmillan Publishes Ltd. London pp. 137-140
|
|
|
|
Iwe MO (2010). Handbook of Sensory Methods and Analysis. Rojoint Communication Services Ltd. Enugu. pp. 75-78.
|
|
|
|
Kaanane A, Kane D, Labuza TP (1988). Time and temperature effect on stability of Moroccan processed orange juice during storage. J. Food Sci. 53(5):1470-1473.
Crossref
|
|
|
|
Kampen WH (1976). Polytechnology. Tijadschr. Procetech. 31(8):491, 85, 175-785a.
|
|
|
|
Kareem SO, Adebowale AA (2007). Clarification of orange juice by crude fungal pectinase from citrus peel. Niger. Food J. 25(1):130-137.
|
|
|
|
Kennedy JF, Rivera ZS, Lloyd LL, Warner FP, Jumel K (1990). Studies on non-enzymatic browning using a model system based on freshly squeezed orange juice. J. Agric. Food Chem. 52:85-95.
Crossref
|
|
|
|
Kimball D (1991). Citrus Processing: Quality control and technology. Van Nostrand Reinhold: New York.
Crossref
|
|
|
|
Kirimura J, Shimizu A, Kimizuka A, Ninomiya T, Katsuya N (1969). The contribution of peptides and amino acids to the taste of foodstuff. J. Agric. Chem.17:689-695.
Crossref
|
|
|
|
Landon S (2007). Fruit Juice Nutrition and Health (Review). Food Aust. 59(11):533-538.
|
|
|
|
Lawlor KA, Schuman JD, Simpson PG, Taormina PJ (2009). Microbiological spoilage of beverages in compendium of the microbiological spoilage of foods and beverage, Sperber, W.H. and Doyle, M.P. (Eds.), Food Microbiology and Food Safety, Springer, New York, U.S.A. pp. 245-284.
|
|
|
|
Li ZF, Sawamura M, Kusunose H (1988). Rapid determination of furfural and 5-hydroxymethylfurfural in processed citrus juices by HPLC. Agric. Biol. Chem. 52(9):2231-2234.
|
|
|
|
Manny S, Shadaksharaswany CM (2005).Foods :Facts and Principles.(2nd ed.).New Age International limited Publishers, New Delhi, India. pp. 60-71.
|
|
|
|
Mears RG, Shenton AJ (1973). Analytical data of various fruits juices. J. Assoc. Public Anal. 98:745.
|
|
|
|
Mehmood Z, Zeb A, Ayub M, Bibi A, Badshah A, Ihsanullah I (2008). Effect of pasteurisation and chemical preservatives on the quality and shelf stability of apple juice. Am. J. Food Technol. 3(2):147-153.
Crossref
|
|
|
|
Moshonas MG, Shaw PE (1998). Changes in volatile flavor constituents in pasteurized orange juice during storage. Agricultural Research Service. United State Department of Agriculture.
|
|
|
|
Nagy S, Smoot YM (1999). Vitamin C and Citrus Juice. J. Agric. Food Chem. 28(1):8-18.
Crossref
|
|
|
|
Nagy S, Smoot JS (1977). Temperature and storage effects on percent retention and percent U.S. recommended dietary allowance of vitamin C in canned single strength orange juice. J. Agric. Food Chem. 25:135-138.
Crossref
|
|
|
|
Ndife J, Abbo E (2009). Functional food: prospects and challenges in Nigeria. J. Sci. Technol. 1(5):1-6.
|
|
|
|
Ndife J, Awogbenja D, Zakari U (2013) Comparative evaluation of the nutritional and sensory quality of different brands of orange-juice in Nigerian market. Afr. J. Food Sci. 7(12):479-484.
Crossref
|
|
|
|
Nielsen SS (2003). Food analysis laboratory manual. (3rd ed.). Kluwer Academic Plenum Publishers, New York. pp. 101-102.
Crossref
|
|
|
|
Nnam NM, Njoku IE (2005). Production and evaluation of nutrient and sensory properties of juices made from citrus fruits. Niger. J. Nutr. Sci. 26(2):62-66.
|
|
|
|
Obeta JA, Ugwuanyi JO (1997). Shelf life study of some Nigerian fruit juices inoculated with ascospores of Neosartorya spp. Plant Foods Hum. Nutr. 50(4):1325-1331.
Crossref
|
|
|
|
Obire O, Ramash RP, Dick AA, Okigbo RW (2008). Biotechnology influence for the production of ethyl alcohol (ethanol) from waste fruits. E-Journal Sci. Technol. 3(3):17-32.
|
|
|
|
Okorie O, Enwere NJ, Udensi EA (2009). Effect of ambient storage conditions on pH and Vitamin C content of selected tetra-pak packaged fruit juice marketed in Nigeria. Niger. Food J. 27:4-10.
Crossref
|
|
|
|
Pao S, Fellers PL, Brown GE, Chambers M (2001). Formulation of Fresh Squeezed Unpasteurized Citrus Juice Blend. Fruit Process. J. 7:268-271.
|
|
|
|
Parichart C, Vongsvat K, Somsri C (1999). Nutritive values of Thai fruit juices and fruit drinks and effect of storage time on vitamin C content in orange juice (No. 839). Food chemistry division, nutritional physiology division, institute of nutrition, Mahidol University Salaya, Nakhon Paton 73170, Thailand File: HA:/orangejuice.htm.
|
|
|
|
Polydera AC, Stoforos NG, Taoukis PS (2003). Comparative shelf-life study and vitamin C loss kinetics in pasteurized and high pressure processed reconstituted orange juice. J. Food Eng. 60:21-29.
Crossref
|
|
|
|
Potter H, Hotchkiss I (2006) Food Science. (5th ed.). CBS Publishers and Distributions. New Delhi, India. pp. 50-56.
|
|
|
|
Quek MC, Chin NL, Yus YA (2012). Optimization and comparative study on extraction methods of soursop juice. J. Food Agric. Environ. 10:245-251.
|
|
|
|
Rega B, Fournier N, Nicklaus S, Guichard E (2004). Role of Pulp in flavor release and sensory perception in orange juice. J. Food Chem. 51:7092-7099.
Crossref
|
|
|
|
Roig MG, Bello JF, Rivera ZS, Kennedy JF (1999). Studies on the occurrence of non-enzymatic browning during storage of citrus juice. Food Res. Int. 32:609-619.
Crossref
|
|
|
|
Sospedra JR, Soriano JM, Manes J (2012). Incidence of Microorganisms from Fresh Orange Juice Processed by Squeezing Machines. Food Control 23(1):282-285.
Crossref
|
|
|
|
SON-Standard Organisation of Nigeria (1976). The permissible concentrations of additives and preservatives applicable to fruits juices. Lagos, Nigeria.
|
|
|
|
Stone H, Sidel J (1992). Sensory evaluation practices. 2nd ed. San Diego: Elsevier, P 336.
|
|
|
|
Tasnim F, Anwar HM, Nusrath S, Kamal HM, Lopa D, Formuzul HKM (2010). Quality assessment of industrially processed fruit juices available in Dhaka City, Bangladesh. Malays. J. Nutr. 16:431-438.
|
|
|
|
Tatum JH, Nagy S, Berry R (1975). Degradation products formed in canned singlestrength orange juice during storage. J. Food Sci. 40(4):707-709.
Crossref
|
|
|
|
Vanamala J, Reddivari L, Sun – Yoo K, Pike L, Patil B (2006). Variation in the content of bioactive flavonoids in different brands of orange and grapefruit juices. J. Food Compost. Anal. 19:157-166.
Crossref
|
|
|
|
Vasantha RHP, Li JY (2012). Emerging preservation methods for fruit juices and beverages, food additive, Prof. Yehia El-Samoragy (ed.). DOI: 10.5772/32148
Crossref
|
|
|
|
Wardlaw GW (2004), Perspectives in Nutrition. (6th ed.). McGraw Hill Companies, New York, U.S.A., pp. 80-83.
|