Ozone is active against a broad spectrum of microorganisms. Ozone treatment can enhance safety and increase shelf life with limited impact on product quality. Ozone is known to be one of the strongest oxidizers that can have applications in foods. In the gaseous state, ozone is denser than air, colorless at lower concentrations and possesses a distinct odor. Ozone can be generated using a few methods, by photochemical procedures specifically UV light, electrolysis of water, with corona discharge being the most common method. In the food processing industry, ozone acts as a powerful sterilizer against both Gram-positive and Gram-negative bacteria, bacterial spores, fungi, viruses, and protozoa. Ozone affects the unsaturated lipids in the cell membrane causing leakage of cellular components that can lead to cell death. There are numerous examples to show that ozone has been successfully applied in food processing, specifically in sanitation by disinfecting food plant equipment and contact surfaces, packaging materials, water, air in storage and refrigeration systems, and for foods such as dried and fresh fruits and vegetables. The shelf life and quality of different food products can be maintained using ozone through reduction of spoilage microorganisms.
Ozone has been widely used in numerous industries including the food industry for many years (Fundo et al., 2018). Recently, there has been a renewed interest in ozone and its application in the food processing industry for use as a sanitizer (Lelieveld et al., 2016). Ozone or triatomic oxygen (O3) is an unstable allotrope of oxygen. It is an effective alternative to chlorine compounds in the food industry (Ziyaina and Al Zogne, 2010). This is due to the advantage that, when decomposed into free radicals, ozone leaves no residual components on the food product when decomposition is complete, liberating the main product of oxygen (Segat et al., 2014). Ozone use was approved by the US Food and Drug Administration US in 1982 for use in water treatment. In 2001, the FDA officially approved ozone for applications in the food industry and for direct contact with food products, including use as an antimicrobial and surface sanitizer in CIP (Clean-In-Place) systems and in fish, meat, and poultry processing plants (Al-Qadiri et al., 2019; Fundo et al., 2018; Najafi and Khodaparast, 2009; Zhang et al., 2016). There are several other applications of ozone in food processing, such as plant and equipment sanitation at food factories, surface hygiene, drinking water purification, and treatment for reuse of wastewater. Ozone can be applied to dried and fresh fruits and vegetables as a disinfectant (Rojas-Valencia, 2011). In food processing, ozone is used as a sterilizer against a wide range of microbes. Ozone is a triatomic allotrope of oxygen that is characterized by a high oxidation potential (Khadre et al., 2001; Lelieveld et al., 2016). Due to its antimicrobial properties, ozone conveys bactericidal properties that kill microorganisms such as viruses, bacteria, fungi and protozoa, which are causes of foodborne illness and food spoilage. Ozone actively destroys bacterial and fungal spores (Guzel-Seydim et al., 2004; Öztekin et al., 2006). Ozone treatment can remove up to 99% of bacteria and viruses at a concentration of 10 mg/l within 10 min.
Ozone is more stable in its gaseous phase than its liquid phase. Gaseous ozone dissolves easily in water and has higher solubility than nitrogen (N2) and oxygen (O2) gases. Ozone solubility in cool water is 13 times more than that of oxygen at 0–30°C. Gaseous ozone is more soluble in water compared to oxygen and nitrogen, but less soluble than chlorine and carbon dioxide (CO2) (Guzel-Seydim et al., 2004). Solubility and stability of ozone in water depends upon several physical parameters, such as water temperature (the solubility of ozone in water is higher at lower temperatures), ozone concentration, other materials in the water, pH, metal ions, radical scavengers, and use of mechanical stirring (Kim et al., 2003; O'Donnell et al., 2012).
The use of ozone in food plants has led researchers to further explore other applications within their facilities and to study its effect on microbes. Continued research is needed for relevant and concise information about this sanitizer. This review surveys information about this important topic.
There are two methods commonly used to produce ozone for food applications, corona discharge (CD) commercially and photochemically with ultraviolet (UV) light. Electrochemical, chemical, biochemical, thermal, and chemonuclear are other possibilities (Cullen et al., 2011; Guzel-Seydim et al., 2004; O'Donnell et al., 2012).
To generate ozone by the corona discharge method, oxygen molecules are passed through the electrical field where they are split, resulting in oxygen free radicals. The free radicals can react with diatomic oxygen to form a triatomic ozone molecule (O3). To break the bond between the oxygen molecules (O–O) requires a great deal of energy. Formation of ozone can be represented in the following equations:
UV wavelength (188 µm) can be coupled with corona discharge to initiate the formation of oxygen free radicals through photo disassociation. In the photo disassociation process, a small percentage of oxygen molecules are split by UV rays, into unstable oxygen radicals. To become more chemically stable, O1 radicals readily attach to surrounding O2 molecules forming ozone shown in Figure 1 (Guzel-Seydim et al., 2004).
ADVANTAGES OF OZONE USAGE
One of the primary advantages of ozone is that it is a non-thermal process. It is a potent sanitizer and relatively safe and reliable for food processing applications. It has an oxidation potential of 2.07 V, which is much higher than chlorine (1.36 V) (Aguayo et al., 2006)while leaving no residue on food (Lelieveld et al., 2016; O'Donnell et al., 2012). Ozone half-life in water at room temperature is about 20 min, with it decomposing into molecular oxygen and posing few safety concerns about the consumption of residual ozone in a treated food. Because it rapidly decomposes into oxygen, there are no traces of toxic halogenated compounds present as residues (Isikber and Athanassiou, 2015). Ozone gas can be produced on-site, and it does not need to be stored or transported from another location; however, it must be used in a well-ventilated area since it is toxic when inhaled. However, a major disadvantage involves monitoring since as the lack of stable residue limits the ability for online testing (Cullen et al., 2011; Lelieveld et al., 2016; O'Donnell et al., 2012).
Recent research points out that ozone is a powerful broad-spectrum antimicrobial agent, active against bacteria (Gram-positive and Gram-negative bacteria, bacterial spores, fungi, viruses, and protozoa.
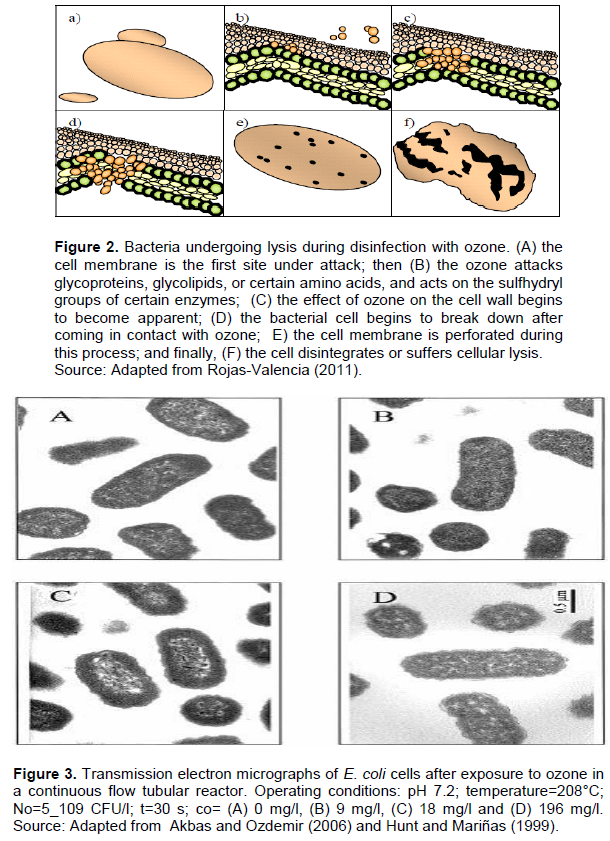
A deactivation involves destroying biological activities of a microbial cell such as inducing changes to structural components of the cell causing cell death through a change in cell permeability and cell lysis and by altering the ability of a cell to divide and thereby reproduce. Ozone damage results in breakage of the cellular membrane, inhibiting cellular reactivation mechanisms, and oxidizing unsaturated fatty acids, lipid fatty acids, glycoproteins, glycolipids, amino acids, sulfhydryl groups of certain enzymes, phenolic rings, and nucleic acids (Khadre et al., 2001).
The efficacy of ozone has been demonstrated against Gram positive Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, Enterococcus, and Gram-negative Pseudomonas aeruginosa and Yersinia enterocolitica; Escherichia coli is one of the most sensitive to ozone damage, while Gram-positive cocci (Staphylococcus and Streptococcus) and Gram-positive bacilli (Bacillus) and mycobacteria are among the most resistant to ozone damage (Le Chevallier, Au, and World Health, 2004).
Ozone is a potent oxidant, as shown in Figure 2, and destroys microorganisms by reacting with oxidizable cellular components, particularly those containing double bonds.
The rate and degree of bacterial inactivation resulting from ozone treatment will depend upon the species. Findings from studies on ozone microbial inactivation are subsequently presented in the paper.
Effectiveness of ozone on various food products
The effect of zone on bacteria depends on various factors, including the type of food product, microbial load, concentration of ozone and a suitable ozone treatment system, and treatment time. Optimizing these factors can decrease damage to susceptible food products and lead to favorable outcomes in the majority of commodities. A 2007 study by Bialka and Demirci, documented treatment of blueberries and spinach with 5% (wt/wt) gaseous ozone (Bialka et al., 2008). They reported that ozone resulted in a 2.2 and 1.9 log, respectively, reduction of Escherichia coli O157:H7. Perry and Yousef (2011) conducted a research study exposing whole apples to 23–30 mg/L aqueous ozone, finding a reduction of about 3.7-log in E. coli O157:H7. Also, they noted that treatment of blueberries with 5% (wt/wt) gaseous ozone for one hour caused a 2.2-log reduction of E. coli O157:H7 (Perry and Yousef, 2011). Akbas and Ozdemir (2006) reported cell damage to E. coli exposed to 0.167/mg/min/L ozone, at different times, caused deformation, surface roughness and surface destruction within 60 min. A 90-min treatment caused cell rupture and cellular lysis as shown in Figure 3.
The pH plays a significant role in ozone inactivation. For example, lower pH values provide a higher inactivation rate. Based on this factor, it was revealed that ozone treatment concentration 0.048 mg/min/mL and pH 3 and pH 5 reduced about 5-log of E. coli in apple juice in 4- and 18-min treatments.
Ozone has significant results in preservation efficacy and has been evaluated in a variety of liquid food products, including milk and water (Kim et al., 1999; Kim et al., 2003). Selma et al. (2007) found that ozone treatment by 1.6 and 2.2 ppm for 1 min decreased Shigella sonnei populations in water by 3.7- and 5.6-log CFU/ml, respectively; moreover, ozone applied to skim milk effectively decreased psychrotrophic counts by 2.4- logs (5-35 mg/l, 5-25 min (Rojek, 1996; Selma et al., 2007). Treatment of whey and apple juice also produced favorable microbial reduction. Greene et al. (1993) reported that ozone treatment at 0.5 ppm for 10 min eliminated more than 99% of the population of milk spoilage bacteria, such as Pseudomonas fluorescens and Alcaligenes faecalis.
A study reported ozone inactivation of Gram-positive bacteria such as Streptococcus faecalis, S. aureus and the yeast Candida albicans. One study found the S. aureus isolate to be more resistant than S. faecalis or C. albicans, and the longest time required to achieve total inactivation was 10 min. Exposure of P. fluorescens, E. coli O157:H7, L. mesenteroides, and L. monocytogenes to ozone at 2.5 ppm within less than a minute resulted in 5- to 6-log decrease for those bacteria (Cullen et al., 2011; Zhang et al., 1993).
Exposure to ozone (0.23–0.26 mg/L; 1.67 min; 24°C) achieved a 4.3 log inactivation of Salmonella Typhimurium. Ozone plus heat treatment is effective in causing greater inactivation at higher temperatures (50°C). According to a 2012 study, a higher process temperature (50°C) resulted in slightly higher inactivation (4.8 log in apple cider and orange juice) of Salmonella compared to 4.5 logs at 4°C due to higher ozone reactivity (Mukhopadhyay and Ramaswamy, 2012). Similarly, another study observed that apple cider treated with ozone can result in a 6-log reduction of E. coli O157:H7 following a 45 min ozone treatment (9 g h−1) to apple cider held at 50°C. The same conditions produced a 4.8-log reduction of Salmonella within 30 min (Perry and Yousef, 2011).
Ozone gas could also be used in activating Salmonella on food surfaces. The experimental process involves applying ozone gas to inoculated fruits, resulting in maximum reductions of 1.5 and 0.9 log CFU/g. Treatment of ozone gas under pressure yielded greater reductions in Salmonella sp. in raspberries (1.9 CFU/g for Salmonella and E. coli O157:H7 2.8 log CFU/g) (Sung et al., 2014; Williams et al., 2005).
Viruses
Generally, viruses are more resistant to ozone compared to vegetative bacteria (Rojas-Valencia, 2011). Burleson et al. (1975) found that ozone killed viruses. In their waste-water treatment study, they found that ozone treatment successfully inactivated vesicular stomatitis virus, encephalomyocarditis virus, and GDVII virus after 15 s of treatment (Burleson et al., 1975). O'Donnell (2012) reported that viruses that contained lipid encirclement (lipid bodies) were more resistant to ozone than those that did not possess this feature. Also, he indicated that hepatitis A virus could be inactivated within 5 s by 0.4 ppm aqueous ozone treatment dose. Ozone can be effective in controlling Norwalk virus found in drinking water, with ozone (0.37 ppm) at pH 7 for 5 min at 5°C being effective reducing concentrations by greater than 3 logs in 10 s (O'Donnell et al., 2012).
The mechanic effect of ozone on viruses breaks the protein capsid into subunits, liberating RNA and disrupting virus adsorption to the host pili. In addition, ozone can randomly destroy the head, collar, contractile sheath, endplate, and tail fibers and liberate the DNA from the head.
Fungi
Many fungi produce toxins that can cause foodborne illness. One genus, with the members Aspergillus flavus and Aspergillus parasiticus, produce aflatoxin that can cause illness in humans and animals’ while other fungi can cause food spoilage. Ozone can deactivate fungi by causing irreversible cellular damage (Valencia, 2011) and by oxidizing mold toxins. Zorlugenç et al. (2008) found that when dried figs were exposed to gaseous ozone, aflatoxin B1 content in the figs was reduced by exposure to 13.8 mg L-1 ozone gas at 15 and 30 min, and ozone treatment at 15 min was sufficient for deactivation of A. flavus and A. parasiticus cells. Also, they noted that the samples of dried figs that were artificially contaminated with aflatoxin when treated with gaseous ozone and ozonized water for 30, 60 and 180 min, experienced a degradation of aflatoxin B1 that increased with increasing ozonation time (Öztekin et al., 2006; Zorlugenç et al., 2008).
APPLICATIONS OF OZONE IN THE FOOD INDUSTRY
Ozone improves food preservation in an aqueous solution or as a gas because of its ability to disinfect food, food surfaces and contact surfaces within a food processing facility. Ozone can be used to sanitize process water and to treat the atmosphere within a food processing facility; for example, a storage unit. Usually, gaseous ozone is used inside an enclosed treatment chamber because inhalation is dangerous. Ozone has been used in sanitizing eggs, fresh fruits and vegetables, fresh fish, and in cold storage applications. Ozone can also be used for sanitizing packaging materials (Khadre et al., 2001; Kim et al., 2003).
Ozone applications in the dairy industry
Recent studies have examined using ozone to remove biofilms from food plant equipment. Biofilms are a source of microbial contamination in addition to causing reduced heat transfer (O’Donnell et al., 2012). Concerns with E. coli and L. monocytogenes contamination in processed dairy food products (O'Donnell et al., 2012) resulting from contaminated food contact surfaces has led to a number of interesting studies. In a study from Baumann et al. (2009), L. monocytogenes biofilms formed on stainless steel chips were deactivated by ozone treatment, and higher concentrations of ozone had a synergistic effect when used with sonication for a 60-s exposure time(Baumann et al., 2009). Another research study from Dosti et al. (2005) showed the effectiveness of ozone (0.6 ppm at 10 min) on sessile cells in a bacterial biofilm.
They observed that ozone significantly reduced biofilms formation by Pseudomonas spp. on stainless steel and recommended the use of ozone as an alternative to chlorine when sanitizing dairy processing equipment (Dosti et al., 2005). A common problem in cheese processing plants is mold growth that occurs when cheese is ripening in storage rooms. Thus, cheese becomes moldy and could become contaminated with Aflatoxin M1. Ozone has been used to control mold growth associated with cheese ripening rooms (O'Donnell et al., 2012).
Ozone applications in raw poultry and meats
A significant number of cases of food poisoning in humans occur from contaminated raw poultry and meat products with pathogenic bacteria such L. monocytogenes, Campylobacter spp., Salmonella spp. and other enteric bacteria. Contamination of meat and poultry also dictates its shelf-life (Khubaib, 2019). Ozone is useful for decontamination of beef. Kim et al. (2003) reported that gaseous ozone was effective in preventing growth of microorganisms on meat surfaces. In addition, ozone has demonstrated microbiocidal efficacy and safety when washing poultry carcasses (Kim et al., 2003).
Another application of ozone is to control Salmonella enterica in shell eggs, where ozone can be used at low temperatures and under mild pressure for cold sanitization treatments. Yousef and Rodriguez-Romo (2008) noted that treating Salmonella in eggs with gaseous ozone for 10 min at 22-25°C and 15 psi led to decreased growth of Salmonella populations by more than 5 log (Perry et al., 2008).
Ozone applications for vegetables
Vegetables prior to harvest can become contaminated for a number of reasons from exposure to dirty irrigation water, lack of employee hygiene, improper handling, and after-harvest storage, use of contaminated water when washing or cleaning, dirty processing equipment, or from insanitary handling at the transportation facilities. In addition, when cutting fresh vegetables, the loss of surface integrity could lead to penetration and rapid growth of microorganisms within plant tissue. Using ozone to sanitize fresh produce is known to work very well. In one study on contaminants in fresh lettuce treated by ozone (1.3 mM) mesophilic and psychrotrophic bacterial counts were reduced 3.9 and 4.6 log units, respectively, during 5 min of ozone treatment. Ozone treatment of alfalfa sprouts for 5 min decreased their microbial load by 1.2 log units.
In another study on treatment of alfalfa sprouts, sprouts were placed in ozonized water (30-32 ppm) and stirred for 20 min; the average count decreased to 4.8 × 107CFU g-1 and the sprouts had better quality and texture (Kim et al., 2003). Other applications of ozone are described as follows:
i) Removal of pesticide residues from drinking and wastewater
ii) Disinfection of drinking and wastewater
iii) Control of air pollution
iv) Sterilization of containers and food storage tanks
v) Disinfection of aquaculture farms
vi) Sterilization of bottles and cans
vii) Disinfection of drinking and wastewater
viii) Organic oxidation of drinking and wastewater.
Ozone may cause headaches and irritation of eyes, upper respiratory tract, and lungs at concentration used for food treatment, but usually these effects disappear after breathing fresh air. According to the American Council of Industrial Hygienists, the short-term exposure limit is 0.3 ppm for 15 min. However, Occupational Safety and Health Administration (OSHA) regulations state that ozone concentration of 0.1 ppm inhaled during an eight-hour workday is the maximum limit (O'Donnell et al., 2012).
OZONE AND REGULATORY RESTRICTIONS
Ozone has been ‘Generally Recognized as Safe’ (GRAS) in food processing since 1997 in the U.S. (Patil et al., 2009). Furthermore, in 2001, the Food and Drug Administration (FDA) approved ozone as an antimicrobial agent for food additive applications (O'Donnell et al., 2012; Rice, 2011).
Studies have been proved that ozone has effects significantly on a wide range of microorganisms including bacterial and spores, viruses, fungi, and parasites, with different concentrations and times. The application and effect of ozone on food decontamination are summarized in Table 1.