Use of microbial systems for functional foods supplementation can be defined into fermentative process by starter cultures, use of probiotics microorganisms and use of microbial biomass. Scientific and technical aspects as related to use of microorganisms in functional foods supplementation are highly diverse and complex, since they have to deal with the viability of probiotic strains as well as with the safe production, nutritional composition and acceptability of the new product. However, limited information is available on the microorganisms which can be used in functional food supplements, as well as how to use these microorganisms and generation of products. This review reports details of this information. The data from this review can be useful to support the development of new functional products, with microbial supplementation, for the market.
Functional foods are a trend for industry for healthy eating and consumers’ interest as a new alternative to prevent diseases such as allergies, intestinal problems and cardiovascular diseases among others (Ahmed et al., 2013; Altay et al., 2013). These functional foods are based on microorganisms’ use that affect consumers’ health positively, in the form of fermentation process and/or in the use of microbial biomass (Corona et al., 2016).
The functional food microorganisms include lactic acid bacteria such as Lactobacillus, Lactococcus, Leuconostoc and Staphylococcus (Duarte et al., 2010; Magalhães et al., 2010) for fermented foods of plant or animal origin and Saccharomyces yeasts for alcohol production (Dhaliwal et al., 2011). Kefir grains has also been used for fermentation of milk and fruits (Magalhães et al., 2010; Nalbantoglu et al., 2014). Kefir is a beverage prepared by kefir grains fermentation in different substrates and is a combination of yeasts and bacteria's in a symbiotic polysaccharide matrix (Puerari et al., 2012;Guedes et al., 2014). The microorganisms present in kefir grains are non-pathogenic bacteria and yeasts (Magalhães et al., 2010; Nalbantoglu et al., 2014).
In general, fermentation process has been shown to preserve food with modification of physicochemical properties of foods but also provide impact on the raw material functional quality (Ahmed et al., 2013; Altay et al., 2013). Microorganisms are also used for the production of enzymes, pigments, vitamins, poly-saccharides and prebiotics (non-digestible ingredients that benefits the host health by stimulating the bacteria in the gut) (Magalhães et al., 2010; Rodrigues et al., 2016).
Microalgae have also been used in the functional foods supplementation (Mata et al., 2010; Hoseini et al., 2013; Vaz et al., 2016). Microalgae are generally used in the lyophilized biomass form. Spirulina microalgae are the most used in food supplementation (Hoseini et al., 2013; Vaz et al., 2016). Nutritional bioactive compounds produced by microalgae’s can meet the energy needs of the consumer and prevent chronic disease (Christaki et al., 2011; Hoseini et al., 2013; Vaz et al., 2016).
This review reports detail of the use of microorganisms for functional food supplements in the form of fermentation of the substrate and/or addition of lyophilized microbial biomass. The data from this review can be useful to support the development of new functional products for the market.
Lactic acid bacteria
A crucial question for the manufacture of fortified fermented food by lactic acid bacteria: is increased nutraceutical compounds formed by these micro-organisms? (Corona et al., 2016). Lactic acid bacteria lactic produce various products, such as dairy beverage, fermented milk and yogurt (Table 1). Lactic acid bacteria, Streptococcus thermophilus and Lactobacillus bulgaricus produce yogurt (Ahmed et al., 2013; Altay et al., 2013). The fermented milk is also produced by other lactic acid bacteria such as Lactobacillus casei and Bifidobacterium animalis. The fermented milk products have been produced since around 10,000 BC (Corona et al., 2016).
Lactic acid bacteria's are industrially important all over the world in industrial food fermentations (Ahmed et al., 2013; Altay et al., 2013). Their contribution primarily consists of formation of lactic acid from the lactic fermentation resulting in an acidification of the formed products, which is a factor in the preservation of these products. Lactic acid bacteria also have the ability to contribute to flavour, texture and formed product nutrition (Ahmed et al., 2013; Altay et al., 2013). Lactic acid bacteria are ideal for the production of important nutraceuticals in fermented foods. In this review, the nutraceuticals produced by these microorganisms as prebiotics (vitamins, low-calorie sugars) and components that improve the health of consumers, through growth stimulation of probiotics were described (Table 2) (Abd-Rabou et al., 2010).
Low-calorie sugars body-weight control is a concern in all countries and a new food products elaboration containing low-calorie sugars is required (Ahmed et al., 2013; Altay et al., 2013). Sorbitol and mannitol are low-calorie sugars that replace sucrose, glucose, fructose, lactose and glucose in food (Ahmed et al., 2013; Corona et al., 2016). Mannitol also serves as antioxidant for consumers (Corona et al., 2016).
Heterofermentative lactic acid bacteria are known for their ability to produce mannitol in fermentative process (Marsh et al., 2013). Production of sorbitol can be induced in these bacteria in fructose addiction in fermentation substrate (Ahmed et al., 2013).
Tagatose is another carbohydrate that is considered as sucrose replacement. It has much lower caloric value because the human body poorly degrades it. It has recently been launched as lower caloric sugar (Marsh et al., 2013). Tagatose is formed in lactose degradation by lactic acid bacteria (Marsh et al., 2013).
Trehalose is another low-calorie sugar. It is partially digested by humans, and is considered a dietetic sugar. It is metabolised by lactic acid bacteria such as S. mutans and S. salivarius (Corona et al., 2016). Other biological
activities than is attributed to trehalose are different stress conditions decrease (Marsh et al., 2013).
Lactic acid bacteria produce vitamins such as folate (vitamin B11) and cyanocobalamine (vitamin B12) (Marsh et al., 2013; Corona et al., 2016).
Folate or folic acid (chemically synthesized vitamin) is an essential vitamin for human diet. It is involved in the biosynthesis of deoxyribonucleic acid (DNA) and Ribonucleic Acid (RNA). For pregnant women, folic acid is recommended to prevent neural-tube defect in new-borns (Corona et al., 2016). It is even reported against some forms of cancer (Marsh et al., 2013; Corona et al., 2016). Folic acid is produced by lactic acid bacteria (Marsh et al., 2013).
Some lactic acid bacteria have long been known for their production capacity of vitamin B12. These microorganisms produce coenzyme B12 or deoxyadeno-sylcobalamin via a pathway involving the starting precursor’s uroporphyrinogen III (precursor for heme, F430 and cobalamin), dimethylbenzimidazole and adenosyl-moiety. Many fermentative processes have been described for the production of vitamin B12 from lactic acid bacteria varying the nutrient composition of the substrate, such as amino acid composition including cobalt ions (Abd-Rabou et al., 2010).
Examples have been presented where significant production of nutraceuticals fermented products can be induced by lactic acid bacteria. These microorganisms, undoubtedly, is a new way of supplementation functional fermented foods.
Saccharomyces cerevisiae yeast
S. cerevisiae is the most thoroughly known eukaryotic microorganism, which aids our understanding of biology. This yeast has been used in the production of alcoholic beverages, and today this microorganism is used in functional food industries processes (Dias et al., 2007; Dhaliwal et al., 2011; Andrade et al., 2017). S. cerevisiae is a non-pathogenic microorganism, and due to its long application in the production of consumable products, it has been classified as a generally regarded as safe (GRAS) organism (Dhaliwal et al., 2011). In addition, the well-established fermentation technology for large-scale production with S. cerevisiae makes this organism attractive for several biotechnological purposes, as the production of fruits and vegetables beverages containing nutritional values (Table 1).
The substrates metabolized by S. cerevisiae serves as an important option in utilization of new raw materials, e.g., a variety of fruits and vegetables (Dias et al., 2007; Dhaliwal et al., 2011). The utilization of molasses (glucose, fructose, sucrose and trisaccharide raffinose) expands the range of substrates used by S. cerevisiae. Molasses, which is used for ethanol and fermented beverages production, contains mainly glucose, fructose, sucrose and disaccharide raffinose (Dias et al., 2007; Dhaliwal et al., 2011; Andrade et al., 2017).
Several compounds are produced by S. cerevisiae in fermentation, including alcohols, ethyl esters, acetates, monoterpenic alcohols, volatile fatty acids and aldehydes, providing pleasant and nutritional characteristics of fermented beverages (Dias et al., 2007; Dhaliwal et al., 2011; Andrade et al., 2017). Propionic and citric acid is metabolized by S. cerevisiae. Propionic acid is an important odor-active compound in fruits and vegetables pulp, and citric and malic acids are in fruit and vegetables fermented beverages, acting as preservatives with antimicrobial properties (Dias et al., 2007; Duarte et al., 2010). The organic acids produced by S. cerevisiae species, contribute to the refreshing flavour and fermented beverages aroma (Duarte et al., 2010). The alcohols produced by S. cerevisiae provide pleasant taste in beverages besides presenting role in the conservation of beverages (Dias et al., 2007; Duarte et al., 2010; Dhaliwal et al., 2011).
Fruits and vegetables pulp-based fermentation by S. cerevisiae can produce novel beverages of acceptable organoleptic character. This is significant because new processing fruits can be used to minimize production losses, to generate more profits and introduce new products of nutritional value to the market.
Kefir is fermented milk beverage, widely known as an excellent source of probiotics with potential health benefits (Magalhães et al., 2010; Nalbantoglu et al., 2014). Kefir beverage is a traditional to Middle Eastern. It originated in the Caucasus in Asia thousands of years ago (Magalhães et al., 2010; Nalbantoglu et al., 2014). Kefir is a symbiosis between yeast and bacteria (Figure 1). A vast variety of different species of microorganisms forms the kefir grains (Magalhães et al., 2010; Nalbantoglu et al., 2014). The Lactobacillus genera are the most frequent in kefir. Other lactic bacteria including Lactococcus and Leuconostoc genera are also common in kefir (Magalhães et al., 2010; Nalbantoglu et al., 2014). Acetobacter genera represent acetic bacteria and the yeast isolates are Kluyveromyces, Candida and Saccharomyces genera (Leite et al., 2013; Marsh et al., 2013).
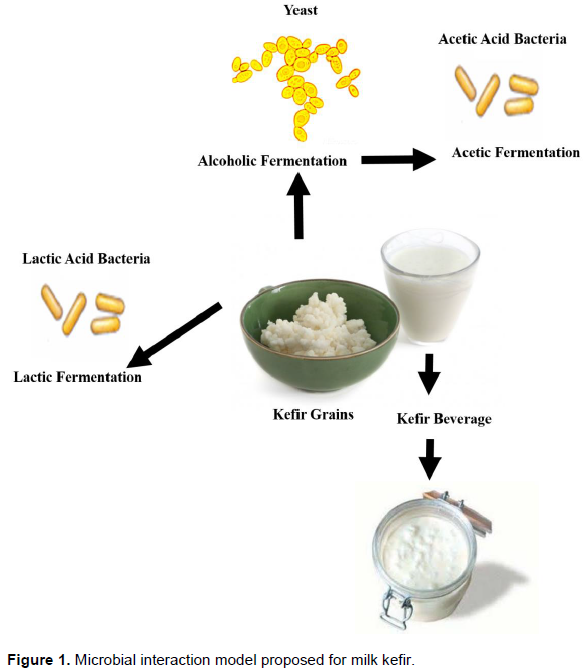
The microbial species from kefir grains carry out three types of fermentation during the process: lactic, alcoholic and acetic. The kefir grains can easily adapt to different substrates and lead to production of new probiotic products (Magalhães et al., 2010; Nalbantoglu et al., 2014). Recent studies show new functional kefir beverages such as whey-based beverages (Magalhães et al., 2010), cocoa pulp-based beverages (Puerari et al., 2012), walnut milk beverage (Cui et al., 2013) and vinegar kefir (Viana et al., 2017).
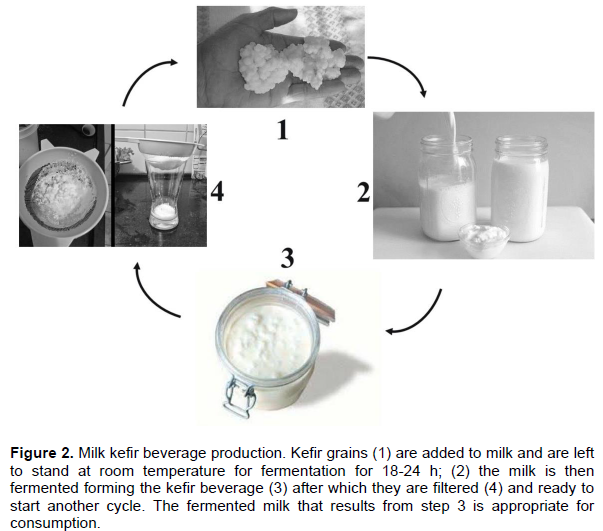
“Traditional” way of kefir beverage production is using pasteurized or Ultra-high-temperature processing (UHT) treated milk (Figure 2). The milk is mixed with the kefir grains. The mixture is left to stand at room temperature for approximately 24 to 48 h. The fermented kefir is filtered to obtain the kefir grains. This kefir beverage is appropriate for consumption. Kefir grains are added to new milk, and the process is repeated.
Kefir beverage is mainly considered a probiotic resource (Leite et al., 2013). Kefir may help bridge the gap between the health benefits and consumption of non-dairy foods and provide the benefits of probiotic without milk (Leite et al., 2013; Garofalo et al., 2015). Kefir can ferment non-dairy substrates such as fruits, cereals and vegetables (Magalhães et al., 2010; Leite et al., 2013; Nalbantoglu et al., 2014; Garofalo et al., 2015).
The microorganisms in the kefir grains produce lactic acid and antibiotics, which inhibit the development of pathogenic microorganisms in kefir (Leite et al., 2013; Garofalo et al., 2015). Furthermore, kefir beverage ingestion provides decreased levels of Enterbacteriaceae in the intestinal tract (De Oliveira Leite et al., 2013). Other important bioactivities have been tested in kefir beverage. Anti-inflammatory effect (Diniz et al., 2003), anti-ulcerogenic (Rodrigues et al., 2016), antioxidant (Alsayadi et al., 2013), cicatrizing (Moreira et al., 2008), antimicrobial (Fiorda et al., 2016) and healing activity activities (Rodrigues et al., 2016) have been reported.
Recently, Kefir grains fermented in honey produced a kefir beverage with health characteristics, such as antioxidant capacity and DNA protection effect (Fiorda et al., 2016). Kefir contributes to defence system against oxidative damage reactions, avoiding formation of free radicals and/or repairing the damage caused by them (Fiorda et al., 2016).
Due to the numerous positive effects of kefir on the human health, alternative substrates may be used for kefir grains fermentation. Fruits, molasses or vegetable are used as medium of fermentation for kefir grains (Magalhães et al., 2010; Nalbantoglu et al., 2014). Fruits are the most diverse group of alternative substrates for kefir fermentation. Typical fruits, such as apple, strawberry, pear and grape, as well as region-specific fruits (pomegranate and quince) can be used. This allows for production of new functional and probiotic beverages, with characteristics similar to traditional kefir beverage (Grønnevik et al., 2011).
The adaptation of kefir grains into different substrates has shown potential for production of kefir beverages with distinct sensory characteristics and functional proprieties.
Spirulina microalgae biomass
Applications of microalgae biomass for food supplementation have developed significantly over the last years (Mata et al., 2010; Hoseini et al., 2013; Vaz et al., 2016). Spirulina microalgae biomass is important for consumer providing several biological benefits, such as anti-inflammatory, antioxidant, anti-obesity and anti-carcinogen (Guedes et al., 2011; Pangestuti and Kim, 2011). Spirulina biomass supplements various products.
These microalgae are integrated into the nutritional formula of cheese, cookie, cake and fruits beverages (Vílchez et al., 2011) (Figure 3).
Spirulina biomass (60% dry weight) protein percentage exhibits high digestibility and contains all essential amino acids (Vaz et al., 2016). Spirulina biomass is protein sources rich (Guedes et al., 2011; Pangestuti and Kim, 2011; Vaz et al., 2016). Spirulina microalgae biomasses are also sources of antioxidant and vitamins. These microorganisms are a source ascorbic acid, B1, B2, B3, B6, B9, B12 vitamins, folic acid and biotin (Christaki et al., 2011). Spirulina is also rich in vitamin B12 and β-carotene and their consumption facilitates the vitamin B1 absorption from foods (Vaz et al., 2016).
Spirulina microalgae pigments also are important for consumer providing health. The major pigment in microalgae is the carotenoids. Currently, carotenoids are mainly used for fortified foods and food dyes (Guedes et al., 2011; Pangestuti and Kim, 2011; Vaz et al., 2016). Chlorophyll, present in Spirulina microalgae, is also used as a nutritional source (Vaz et al., 2016). In addition to chlorophyll, Spirulina microalgae biomass contains phycocyanin, which is an antioxidant blue pigment (Guedes et al., 2011; Pangestuti and Kim, 2011; Vaz et al., 2016).
Rabelo et al. (2013) found that adding Spirulina microalgae increased the nutritional quality of the produced foods. The authors observed an increase in the contents protein, lipids, fibers and minerals. Figueira et al. (2011) developed gluten-free bread for consumers with celiac syndrome. The authors found that the breads produced supplemented with Spirulina microalgae biomass showed higher protein content and better amino acid composition when compared with the control (gluten-free bread without Spirulina microalgae).
Therefore, Spirulina microalgae biomass is a promising food source. These microalgae are active ingredients source for functional food supplements. Thereby, new functional foods supplemented with Spirulina microalgae biomass promote market future prospects.
Considerations
The importance of foods supplementation with microorganisms in food industry is a good alternative of further research. Scientific study should be performed on the relations between different microorganisms and human consumers and how these relations can result in nutritional and therapeutic benefits, as curing and/or preventing human diseases.