Ethiopia is home to about a dozen species of tropical grain legumes that are extensively grown in many parts of the country contributing the major diet both in rural and urban population. Grain legumes have a nitrogen fixing symbiosis with soil root nodule bacteria. Among these, Faba bean (Vicia faba L.) is an annual grain legume widely cultivated, which serves as foods for human and animal nutrition in many countries, since it is rich in protein, minerals and vitamins (Tekle et al., 2016). Cultivation of faba bean plays important roles in maintaining sustainable agriculture system in many marginal areas; due to its high nutritional value, multiple uses and ability to grow over a wide range of climatic and soil conditions.
Faba bean is a dominant pulse crop in Ethiopia in terms of area coverage and amount of production (CSA, 2014), however its average yield under small holder farmers is not more than 1.6 t ha-1 (CSA, 2013) due to lack of improved varieties, insects/pests and diseases. Historically, Ethiopia is considered as the secondary center of diversity and also one of the nine major agro-geographical production regions of faba bean. The
world’s foremost producing countries for faba bean are China, Ethiopia, Egypt and the United Kingdom. Ethiopia is the leading producer of faba bean in Africa accounting for 56% of the production (FAOSTAT, 2014). Faba bean popularity has increased recently as its high yield makes it attractive to producers while its high protein content and low-priced makes it attractive to consumers (Stagnari et al., 2017).
The productivity of faba bean in Ethiopia is low. Most farmers in Ethiopia cultivate local faba bean varieties which are susceptible to biotic and abiotic factors (Link et al., 2010). The Central Statistical Agency (CSA, 2013) reported that faba bean is planted in to 4.34% of the grain crop area with an annual production of about 9.917 million quintals, 3.94% of the total grain production and yield of 18.42 q/ha in Ethiopia.
Legumes are able to establish nitrogen-fixing symbioses with bacterial microsymbionts (rhizobia), thus reducing the need for chemical fertilizers. This reduction may help to minimize greenhouse- gas emissions and to avoid contamination of ecosystems. This association further provides a nitrogen supplement for the subsequent crops. Faba bean like other legumes crops has the ability to form symbiotic association with root nodulating bacteria (rhizobia) group called Rhizobium leguminosarum bv. Viciae.
Polyphasic characterization approach and selection of potential R. leguminosarum bv. viciae that show better nitrogen fixing capacity are still insufficient. Hence, the present study aimed to identify efficient Rhizobium isolates and their effect on nodulation from soil samples collected from major faba bean producing areas of North and South Gondar, Ethiopia.
Collection of soil samples
Three representative faba bean growing woredas were selected from North Gondar and South Gondar zone (Lay Gayint and Farta woreda from South Gondar; Chilga from North Gondar) based on their productivity of faba bean in the last few years. From each woreda, the productive areas were selected based on their productivity of faba bean. From each woreda of productive area, representative farmer fields were selected based on the previous history of cultivating faba bean crop. Thus, 20 farmer fields from each of Chilga and Farta woredas and 17 farmer fields from Lay Gayint were selected. About 3 Kg of soil sample was collected from each selected farmer fields at a depth of 20 cm using sterile (fresh) plastic bags.
The presumptive test, Rhizobium isolation, identifications and pot experiments were carried out at the Department of Biology Laboratory, University of Gondar. Plant total nitrogen and Soil chemical analysis were done at Adet Agriculture Research Center, Ethiopia.
Colony morphological and biochemical characterization
Colony morphology of rhizobial isolates was studied on Yeast Extract Mannitol Agar (YEMA) according to Jordan (1984). Gram staining and acid/base reactions were evaluated on YEMA containing 25 µg ml-1 bromothymol blue (BTB) (Lupwayi and Haque, 1994).
Utilization of carbon source
Carbon source utilization of isolates was determined following the method of Somasegaran and Hoben (1994) on 15 carbohydrates prepared as 10% (w/v) in sterile distilled water. Each 10 ml of the carbohydrate stock solutions was added to 90 ml of the carbohydrate free basal medium and their growth was observed after 2-3 days of incubation and YEMA medium plates incubated at 28°C were used as controls.
Acidity, alkalinity, salinity and temperature tolerance
All experiments on tolerance to acidity, alkalinity, temperature and salinity were performed according to McVicar et al, (2005). Tolerance to acidity and alkalinity of each isolate was evaluated on YEMA with pH adjusted between 4.0 to 9.0. For salt tolerance, the isolates were transferred to YEMA plates supplemented with NaCl at concentrations of 0.1, 0.5, 1, 2, 3, 4, 5% (w/v). The ability of bacterial strains to grow at high and low temperatures was monitored at incubation temperatures of 5, 10, 15, 35, 40 and 45°C and YEMA medium plates incubated at 28oC were used as controls. Strains were considered salt tolerant, resistant to acidity and temperature resistant when growth was similar to the growth in the control plates.
Intrinsic antibiotic resistance
The intrinsic resistance of isolates was determined by inoculating 10 µl of each culture (109 cells/ml)) on solid YEMA medium containing four filter sterilized with Millipore filters antibiotics at different concentrations of water and ethanol according to Taye (2010).
Authentication of the strains (Symbiotic effectiveness)
In order to determine the isolates performance (effectiveness) in nitrogen fixation, authentication test was done as described by Vincent (1970). Out of 57 isolates, 40 best isolates were selected from the presumptive test based on nodule color, nodule number, nodule fresh weight and shoot height. For each isolate three surface sterilized pots were filled with approximately 3 kg acid washed and heat sterilized river sand. In each pot four to five healthy surface sterilized Adet faba bean variety seeds obtained from Adet Agricultural Research Centre were germinated and planted according to the methods of Somasegarian and Hoben (1994). A total of 120 pots were sown for this experiment. As a starter, 20 ppm of nitrogen was included in each pot before planting. Each seedling was inoculated with 1 ml of each isolate with an inoculum size of 109 cells/ml. After a week, the seedlings were reduced into three per pot. Two treatments were used as control: one without nitrogen supply and an uninoculated (i.e. negative control) and the other an uninoculated but with provision of 0.05% (W/V) KNO3 per week (i.e. positive control). This experiment was conducted in triplicates and the plants were grown under glasshouse condition. The pots were arranged in Completely Randomized Design (CRD) and plants were also fertilized with the quarter strength Broughton and Dilworth nitrogen-free nutrient solution once a week and received water every three days. After 45 days of planting, the plants were carefully uprooted and nodule color, nodule number, nodule fresh weight, shoot length were counted and measured, nodule dry weight, root biomass and shoot dry weight were also estimated after drying at 70°C for 24 h in under dry oven, and total nitrogen was analyzed by modified Kjeldahl method after Sahelemedihin and Taye (2000). The Relative symbiotic effectiveness of the isolates was calculated according to the equation proposed by Date et al, (1993) cited in Purchino et al, (2000) [100 X inoculated plant dry matter (DM)/ N-fertilized plant DM) with Nitrogen fixing effectiveness classified as ineffective <35%; poorly-effective, 35 to 50%; effective, 50 to 80%; and highly effective, >80%.
Nodulation status survey
The general survey was conducted during the identification and collection of soil samples. In most cases, attempts were made to meet the owner of the field to establish cropping history.
Data analysis
Statistical data analysis was done by using SAS software version 9.2. Analysis of variance (ANOVA) was done for the comparison of means for all treatments and Duncan’s multiple range test was used to detect the significant difference among treatment means at p≤ 0.05. Correlation coefficients were calculated to study the association among the measurement traits using Pearson correlations. Data from all physiological studies was also used for cluster analysis and similarities between isolates and a dendrogram was constructed based on average linkage hierarchical cluster analysis between groups using SPSS version 20.0 software statistical program.
Morphological and Biochemical characteristics
All the 57 isolates recovered from the root nodule fulfilled the characteristics of rhizobia (Lupwayi and Haque, 1994): as gram-negative, rod-shaped, the color of colonies was milky-white opaque with a circular shape, regular borders and raised, showing intermediate to high production of mucus after 2 to 3 days of growth on YEMA medium at 28°C. Furthermore, the colony diameter of all isolates of Rhizobium leguminosarum bv. viciae was within the range of 1.5-4.5 mm after 2 to 3 days of incubation at 28°C (data not shown). According to the report of Jordan (1984), R. leguminosarum isolates usually show colony size between 2-4mm in diameter and 95 % of our isolates fall to this group (data not shown).
All the isolates changed the YEMA-BTB medium to yellow color and did not absorb Congo red on YEMA-CR medium, indicating that all the isolates are acid producer and fast growing rhizobia. Similar classification has been done by Ondieki et al. (2017). The color formation is due to the utilization of the sugar component of the medium for their growth. This finding is similar with the previous work of Mulisa and Fassil (2011) indicating that many Rhizobium strains isolated from each sampling field of Northern Ethiopia were fast growing and acid producing. All the strains were gram negative and rod shaped as revealed by Gram’s staining technique. These results confirmed the finding obtained by Tagelsir and Mohamed (2015).
Hierarchal cluster analysis
Hierarchal cluster analysis for 57 isolates of Rhizobium based on their physiological characteristics including carbon source utilization, pH tolerance, salinity tolerance, temperature tolerance and different antibiotic tolerance at different concentrations (data not shown) were used to construct a dendrogram using average linkage clustering method between groups. Soil temperature, physical and chemical composition, moisture content in soil varies within small areas and these variations affect the populations of the soil inhabitants. Therefore, differences in response towards salinity, pH and temperature are expected. Based on the above parameters, Rhizobium isolates from different North and South Gondar locations essentially grouped in to six main clusters and the individual main cluster contains sub clusters to analyze their similarity using Pearson’s coefficient on the dendrogram (Figure 1).
The highest similarity was computed between isolate (KD-4) from Kimir Dingay and (LG-3) from Lay Gayint in cluster 4 and isolate (KD-12) from Kimir Dingay and (LG-9) from Lay Gayint in cluster 5 which were nearly 100% similar. This 100% similarity indicates that the isolates were retrieved from the same soil pH, temperature, salinity, and showed resistance of different antibiotics at different concentrations and carbon source utilization (data not shown). On the other hand, CHI-6 and CHI-19 isolates from Chilga in cluster 2, CHI-14 and CHI-20 isolates from Chilga in cluster 1 and KD-6 and KD-10 isolates from Kimir Dingay in cluster 6 were revealed 88% similarity on the dendrogram. Whereas, the lowest similarity was computed between isolate CHI-15 from Chilga and isolate KD-6 from Kimir Dingay which presented in to two different clusters (that is CHI-15 in cluster 1 and KD-6 in cluster 6). This lowest similarity shows that the isolates were retrieved from different soil pH (CHI-15 ranging 5-9 and KD-6 between 5.5-8), temperature (CHI-15 between 15-400Cand KD-6 ranging from 5-350C, salt concentration (CHI-15 at 0.5 % w/v and KD-6 at 1% w/v), carbon utilization (CHI-15 uses 87% and KD-6 uses 100% of tested carbon sources) and different antibiotics at different concentrations (data not shown). Similarity increases as we read from right to left on the dendrogram, based on Pearson’s correlation coefficient value (Figure 1).

Analysis of variance
Forty isolates obtained from presumptive test of faba bean nodules were assessed for their infectiveness and effectiveness of nitrogen fixation on Adet faba bean on sterile and acid treated sand in a pot experiment conducted under Greenhouse. Out of these, only fifteen isolates formed nodules on the test faba bean root authenticating as Rhizobium (Table 1). The ability to form nodules along with the subsequent nitrogen fixing capacity is widely used as means of assessing the association between rhizobia and respective hosts (Anteneh, 2012a).
The results of analyses of variance showed that Rhizobium inoculation significantly (P<0.05) increased at all investigated parameters such as, number of nodules per plant, nodule fresh weight, nodule dry mass, shoot dry weight, shoot length, root biomass, plant total nitrogen and symbiotic effectiveness as compared to the standard and control treatments (Table 1). This finding is similar with Dereje et al. (2015) showing symbiotic effectiveness of faba bean nodulating on sand culture.
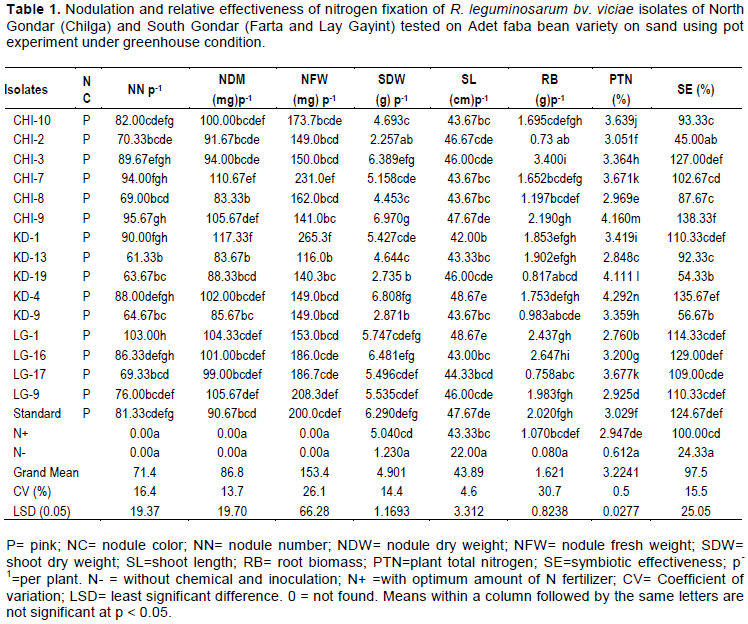
The nodulating isolates showed difference with nodule number of 61.33 per plant for (KD-13) and 103 for (LG-1) p-1 (Table 1) which were less than 67 and 168 nodule number p-1 of faba bean on sand culture reported by Zerihun and Fassil (2011), but higher than 17 p-1 for isolate AUFR-11 (Semema) to 91 p -1 for isolate AUFR-5 (Koyetsa) of degaga variety of faba bean on sand culture reported by Solomon and Fassil (2014). The mean nodule number p -1 recorded in this study was 71.4 and which was less than both 88 nodule number p -1 for degage and 92 nodule number p -1 for dosha faba beans on sand culture studied by Dereje et al, (2015) and 98 nodule number p -1 of faba bean on acidic soil reported by Girmaye et al. (2014). Fifty three percent of isolates showed higher number of nodules p-1 as compared to standard Rhizobium isolate from national soil laboratory, Addis Ababa.
The highest plant shoot length 48.67cm was recorded for both isolate (KD-4) and (LG-1) inoculated with Adet faba bean variety (Table 1). These improvements in shoot length were equivalent to 54.8% over the negative control (without inoculation and nitrogen supplement), 11% over the positive control (nitrogen treated plants) and over the standard Rhizobium 2.1% with Adet faba bean. This result was the best compared with the results of Dereje et al.(2015) study on faba bean inoculation with Degaga variety which was measured shoot height of 43.3 cm of isolate HUGAVf1 collected from acidic soils of Ethiopia, which was 32.94% over the negative control and 16.9% over the positive controls. Our study found better result than previously reported by Anteneh (2012a). Their study on Degaga variety displayed shoot length of of 49.7cm for isolate NSFBR-48 and 51cm over negative control. This improvement of shoot length could be regard as; the rhizobia may increase plant growth by providing products through nitrogen fixation (Kumar et al, 2014).
Plant total nitrogen ranges from 2.76 % for isolate (LG-1) to 4.29 % for isolate (KD-4) and the mean plant total nitrogen of isolates was 3.22% (Table 1). In general, inoculation of Rhizobium isolates resulted in a significant difference at (p<0.05) in plant total nitrogen over negative treatments. Analysis of variance shows that inoculation increased the plant total nitrogen at 31.3, 29.4 and 85.7% over nitrogen treated plants, standard Rhizobium and negative treatment (plants without nitrogen sources and inoculation) respectively. The application of nitrogen had negatively affected nodule color (could not be pink), and nodule number, nodule dry weight and nodule fresh weight (0.00 mg plant-1) (Table 1) and this indicates the negative effect of nitrogen fertilizer application on nodulation of the legume plants. In this experiment, application of mineral nitrogen fertilizer did not improve growth and development of plants; instead it delayed and inhibited nodulation and effectiveness of nitrogen fixation potential of Rhizobium isolates. Similarly, Ouslim et al. (2015) reported that addition of nitrogen fertilizer had a negative effect on the nodulation and nitrogen fixation of Rhizobium isolates.
Based on the percentage differences of shoot dry weight of inoculated and nitrogen-fertilized plants as a measure of effectiveness, more than 80% of the isolates were found to be highly effective, 13 % were effective and 7 % were poorly effective nitrogen fixers (Table 1). The highest scores of 87.67 % to 138.33 % effectiveness were displayed by isolates CHI-3, CHI-7, CHI-8, CHI-9 and CHI-10 from Chilga, KD-4 and KD-13 from Kimir Dingay and LG-1, LG-9, LG-16 and LG-17 from Lay Gayint areas (Table 1). The data showed that, more than 93% of the rhizobial isolates from North and South Gondar were highly effective and effective Rhizobium on the sand culture. This result reveals that, the effectiveness of our isolates was relatively high compared with the finding of Zerihun and Fassil (2011) where 80% of the isolates from North Gondar were effective indicating variation in effectiveness of isolates was found to be widespread in Ethiopia.
The first parameter for a Rhizobium strain used as inoculant or biofertilizer is it must be superior and highly effective in nitrogen fixing ability forming symbiotic association with the host legume. Nine isolates identified with Adet faba bean variety showed effectiveness ranging from 102.7% to 138.3% as compared to the nitrogen treated plants (Table 1). Mainly the best four isolates (CHI-3, CHI-9, KD-4, and LG-16) showed effectiveness of 127, 138.33, 135.67 and 129% respectively as compared to inoculants of standard Rhizobium which showed effectiveness of 124.67%. The standard Rhizobium inoculated plants also showed effectiveness over nitrogen treated plants (Table 1).
In this study, more highly effective isolates were obtained compared to other investigator reports. Dereje et al, (2015) observed that 56% of the isolates were highly effective in both Degaga and Dosha varieties collected from acidic soils of Ethiopia. Girmaye et al. (2014) reported that 16% of the isolates of faba bean were highly effective collected from acidic soils of Wollega, Western Ethiopia and Anteneh (2012b) result showed that 20.9% of isolates were very effective collected from major lentil growing areas of Ethiopia. Generally, the results of this study indicates that, screening of local Rhizobium isolates gives paramount importance for enhancement of dinitrogen fixation in faba bean.
Correlation analysis
Nodule number was found to be positively correlated with nodule fresh weight (r = 0.469 P <0.01) and strongly positive correlated with nodule dry mass (r =0.677, P <0.01), shoot dry weight (r = 0.591, P <0.01), root biomass (r = 0.561, P <0.01) and symbiotic effectiveness (r =0.586, P <0.01) (Table 2). Shoot dry weight was found to be positively correlated with nodule dry mass (r = 0.393, P <0.01), strongly positive correlated with root biomass(r =0.614, P <0.01) and symbiotic effectiveness(r = 0.994, P <0.01) (Table 2). Shoot dry weight and nodule dry weights are usually positively correlated revealing that shoot dry weight can be used as an indicator of relative symbiotic effectiveness.
Shoot length was found to be positively correlated with nodule dry mass (r = 0.396, P <0.01) and with nodule fresh weight (r = 0.391, P <0.01) (Table 2). Nodule fresh weight was negatively correlated with soil nitrogen content (r =-0.318, P <0.05) and there was also strongly positive correlation among nodule fresh weight (r =0.775, P <0.01), root biomass (r =0.610, P <0.01) (Table 2). A similar result was reported on lentil, using growth pot experiment by Anteneh (2012b). Many research findings reveal that nodulation status positively correlated with plant tissue nitrogen, symbiotic effectiveness and shoot biomass or dry weight (Ashenafi and Mekuria, 2015; Tekle et al, 2016).
Conclusion and recommendation
In the present study, most of our isolates displayed abundant diversity with respect to their response to morphological and physiological characteristics. Inoculation of isolates significantly increased at all investigated parameters such as, number of nodules per plant, nodule fresh weight, nodule dry mass, shoot dry weight, shoot length, root biomass, plant total nitrogen and symbiotic effectiveness as compared to the standard and control treatments. About 80% of the isolates collected from major faba bean growing areas of North and South Gondar, Ethiopia were found to be highly effective, 13 % were effective, only one isolate (7 %) from Chilga area categorized as poorly effective and none of isolates grouped as ineffective.
The best nine effective isolates were selected over nitrogen treated plants with Adet faba bean variety from Chilga (3), Kimir Dingay (2) and Lay Gayint (4) study areas. The best four effective isolates, two of them from Chilga, one from Kimir Dingay and one from Lay Gayint were also selected as compared to the standard Rhizobium isolate from National Soil Laboratory, Addis Ababa.

Inoculation of selected Rhizobium isolates revealed shoot dry weight enhancement over nitrogen treated plants of Adet faba bean on sand culture using pot experiment under controlled greenhouse condition. Finally, further investigations of very effective isolates need to be tested under greenhouse and field condition on soil culture to assess their competitiveness ability, adaptability to the wide edaphic condition and survival and colonization within soil. Further research work with various molecular approaches should be conducted to investigate the protein and DNA pattern for better classification of the Rhizobium strains.