ABSTRACT
The re-emergence and increase in the prevalence of tuberculosis cases and multidrug resistant strains is of public health concern. The conventional Acid Fast Bacilli detection tool is weak in the current disease trend especially in children, patients with human immunodeficiency virus (HIV) and low bacterial load. The aim of this research was to assess effectiveness of the Gene Xpert MTB/RIF assay and Microscopy in detectiing Mycobacterium tuberculosis (MTB) in sputum samples in Port Harcourt metropolis. A total of two hundred sputum samples were collected from tuberculosis (TB) suspected patients visiting the antiretroviral and directly observed short treatment clinics at Braithwaite Memorial Specialist Hospital, Port Harcourt and University of Port Harcourt Teaching Hospital, Choba. These samples were investigated to compare and evaluate the detection of M. tuberculosis using Gene Xpert MTB/RIF assay and microscopy. Results revealed that 20% (40/200) of the samples were positive for MTB. Ages 31 to 40 years had 55 samples with ten positive MTB while ages < 10 years had the least number of specimens and one MTB detected. Out of 200 samples analyzed, the percentage positive was 20% while 80% was negative for MTB. Ages < 10 years did not show any AFB positive smear whereas ages 31 to 40 years had 10 positive AFB smears. One hundred and four individuals were people living with HIV, ninety six were negative for HIV; fifteen individuals were positive for HIV as well as MTB (co-infection) and no individual that was co-infected had Rifampicin resistance. There is a significant difference in the detection of MTB across PLHIV and negative persons using AFB microscopy and Gene Xpert technique. It can be concluded that the gene xpert MTB/RIF assay is highly sensitive (98%) with a p-value of < 0.001 and specific in the detection of MTB and Rifampicin Resistance in sputum, and its use in health institutions should be encouraged to reduce exposure and spread of the disease as well correct treatment.
Key words: Gene xpert, tuberculosis, Mycobacterium tuberculosis/Rifampicin (MTB/RIF), sputum, microscopy.
All over the world, Tuberculosis (TB) control agencies often adopt sputum smear microscopy alongside chest x-ray because of its economic value and simple laboratory procedure (Small and Pai, 2010). Using acid fast bacilli (AFB) smear microscopy for the detection of tuberculosis in children, patients with low bacterial load and HIV is challenging hence the use of Xpert MTB/RIF which has great specificity and sensitivity for detecting Pulmonary tuberculosis (PTB) infection. An in vitro study when compared with approximately 10,000 colony forming units/ml with routine smear AFB microscopy showed a limit of detection of as few as 131 colony-forming units/ml(cfu/ml) of Mycobacterium tuberculosis (Dolin et al., 2010). Performing drug susceptibility testing (DST) can be done from the growth of M. tuberculosis in culture, and this can take up to six weeks and needs high biological safety level laboratory which is expensive. Isoniazid and Rifampicin are two fundamental anti-TB drugs in use until recently when high resistance set in hence performing drug susceptibility testing became very important. In many countries, multi-drug-resistant tuberculosis (MDR-TB) is greatly increasing and its treatment is very challenging as it takes longer time with the use of several anti-bacterial agents that are expensive (Palomino et al., 2007). The Xpert MTB/RIF assay technique was introduced in the United States by Cepheid Company, and was declared fit for use in December 2010 by the World Health Organization (2013) as a major tool for tuberculosis diagnosis worldwide. This declaration was made after about eighteen months of continuous effective field assessment of its use in tuberculosis multi drug resistance – TB and TB/HIV co-infection diagnosis (Van Rie et al., 2010).
MDR-TB also known as Vank’s Disease is defined as a form of TB infection caused by bacteria that are resistant to treatment with two of the most powerful first line anti-TB drugs such as isoniazid (INH) and Rifampicin (RIF). With the Xpert, MTB/RIF test MDR-TB diagnosis can be accomplished in fresh sputum samples and in prepared sediments within 2 to 3 h, microscopy cannot be used to detect tuberculosis that are resistant to drugs hence treatment is delayed. The quick detection of M. tuberculosis and Rifampicin resistance using Gene Xpert assay helps the medical personnel to make critical patient management decisions concerning treatment (Lalloo et al., 2006). This test has similar sensitivity to culture, specifically detects M. tuberculosis as well as rifampicin resistance via the rpoB gene concurrently (WHO, 2013). It can be used in low-income setting to make patients access to early and accurate diagnosis easy, hence decreasing the death rate associated with delayed diagnosis and mistreatment (Fred, 2009). The aim of this research work was to evaluate the performance of an automated system (Gene Xpert of M.MTB/RIF) and AFB smear microscopy for detection tuberculosis in sputum specimens. The specific objectives are to effectively diagnose M. tuberculosis in a significant number of individuals with negative AFB smears and to adequately diagnose patients that are resistant to TB drug Rifampicin.
Study area
This study was carried out at the Tuberculosis Reference Laboratory (TBRL) University of Port Harcourt Teaching Hospital (UPTH) located along East West Road between Rumuosi and Choba town in Obi/Akpor Local Government Area of Rivers State, Nigeria. The Hospital shares a common boundary with the University of Port Harcourt, Abuja campus in Alakahia town of Rivers State. Patient using the directly observed treatment short course (Dots) and Antiretroviral (ARV) clinics in Braithwaite Memorial Specialist Hospital (BMSH) Port Harcourt and University of Port Harcourt Teaching Hospital (UPTH) were enrolled in the study. Those enrolled for this study were subjects between ages < 10 to 70 years.
Ethical approval
Ethical approval for this research work was granted by the Rivers State Health Research Ethics Committee while the informed consent of the patients used for this study was obtained before the commencement of the research.
Sample size
Two hundred sputum samples were collected from the individuals who enrolled, and analyzed according to Leslie Kish’s formula, which was used to arrive at the sample size of 200:
N = N2 Pq/d2
Where N = sample size
Z = Z scores at 95% confidence level.
P = expected prevalence (in proportion of one: if 5% d = 0.05). For the level of confidence of 95% z value is 1.96 (Daniel 1999).
Inclusion criteria
These are individuals having cough for two weeks or more, retreatment cases of Pulmonary tuberculosis (PTB) within the last year or non-converting PTB case), persons that are in contact with individuals that have been recently treated of PTB, symptomatic presumptive TB cases with AFB negative results, to confirm MDR – TB, return after loss to follow up and people living with HIV (PLHIV) with symptomatic tuberculosis.
Patient’s exclusion criteria
Individuals producing bloody sputum (haemoptysis) and sputum specimens with obvious food particles or other solid particulates were not accepted as these could interfere with the desired result. Also excluded from the study were patients who refused to participate or submit any sputum.
Specimen collection from adults
Sputum specimens were collected in duplicate; one part was used for making smear and the other for gene xpert. Patients were given two sterile wide mouthed containers each, they were told to rinse their mouth twice with water in the morning, unscrew the lid on the sputum collection container, inhale deeply and cough vigorously, expectorate the material into the two containers. They were advised to avoid spills or soiling the outside of the container, secure the lid on the collection device sample container labeled with name, age, sex, and date of collection according to directives previously reported in literature (Fred, 2009). Sputum specimens were held at 2 to 8°C whenever possible as they got blocks of pure water and safely put the samples between the blocks in sellable bags. The bags were three for each sputum specimen, with the form being placed on the third bag to avoid being soiled in case of any spillage. Both oral and structured questionnaire helped in accessing further details about individuals enrolled.
Specimen collection from children
In children, sputum specimen collection is quite difficult since they tend to swallow the sputum rather than expectorate it; therefore induced sputum specimens were collected especially from minor patients (age 5).
Methods of collection used
The methods used are nebulization techniques using hypotonic saline: Inhaling a nebulizer 3% sodium chloride mist for 5 to 15 min, and then encouraging them to cough and expectorate the sputum into a wide mouth container. Chest or abdomen massages technique: Chest percussion, vibration and active breathing were also used for sputum collection in minors (Grant, 2012).
Specimen processing
The sputum samples were collected by expectorating into sterile wide mouthed containers and liquefied with sputolysin (N-acetyl-L-cysteine), decontaminated with 4% NaOH alkali, which was neutralized with phosphate buffer and concentrated by centrifugation at 1500 to 2000 g for 20 min, specimens collected were processed for microscopic method and Gene Xpert MTB/RIF Assay, in a biosafety cabinet. In addition, appropriate safety gadgets were worn due to the hazardous nature of such samples.
Microscopic examination (Ziehl Neelsen staining technique)
The specimens were arranged after being numbered accordingly, slides were labeled on the frosted end with graphite pencil accordingly. The recommended size is 2 x 3 cm or 1 x 2 cm (Grant 2012). The smear was placed on the flat surface with smear facing upward. It was allowed to air dry for at least 1 h, and thereafter fixed by passing 3 times through a flame, ensuring that the smear face upward. This was done for all smeared samples. Fixed slides were arranged (in batch of 12) including controls on a processing rack, making sure that smear samples were separated from each other before staining. Slides were flooded with 10% strong carbol fuchsin, and heated to steam and allowed to stain for 5 min. Heating helps to melt the wax and opens up the mycolic cell wall to take in more stains, as the heat is removed they close up to retain the primary stain. Slides were rinsed in flowing tap water until no more colour runs off. Slides were thereafter flooded with 3% acid alcohol for 3 min to decolorize, and washed with running tap water to stop decolourization. Thereafter they were flooded with methylene blue (which serves as counter stain), for 1 min and rinsed with tap water, drained and dried. Slides were viewed using oil immersion, and under X100 objective lens.
Gene xpert MTB/RIF techniques
Sputum specimens with blood, obvious food particles or other solid particulates were not accepted as these could interfere with the desired result. Sputum samples on the original tube were used to mix with the sample reagent (which constitute of NaOH and Isopropanol which breaks down the mycolic cell wall of MTB while NaOH decontaminates the sputum samples) (that is, 4mls of sample reagent to 2 mls of sputum specimen, the ratio is 2:1 v/v) except in cases where the sputum is too mucoid, more of the sample reagent can be added as this will help to liquefy the mucoid sputum specimen easily. After the addition of the sample reagent, the mixture was shaken vigorously for 10 to 20 times, and incubated for 5 min at room temperature, and then shaken again for 10 to 20 times and incubated at room temperature for 10 minutes. Each xpert MTB/RIF cartridge was labeled with the sample identification (ID) on the sides of the cartridge, and using the sterile transfer pipette provided in the package of the cartridge packs. Two millilitres (2ml) of the liquefied sample was aspirated and transferred into the open port of the cartridge after opening the lid of the cartridge, this was done slowly to minimize the risk of aerosol formation. At this point hands were on stops while the machine automatically processes the samples with result displaced at the end of 1 h 90 min on the computer screen (Fred, 2009).
Statistical analysis
Statistical Package for Social Sciences (SPSS) Version 22 was used to check if there will be significant difference or not in the detection of MTB among the different age groups and PLHIV using Gene Xpert technique and AFB microscopy.
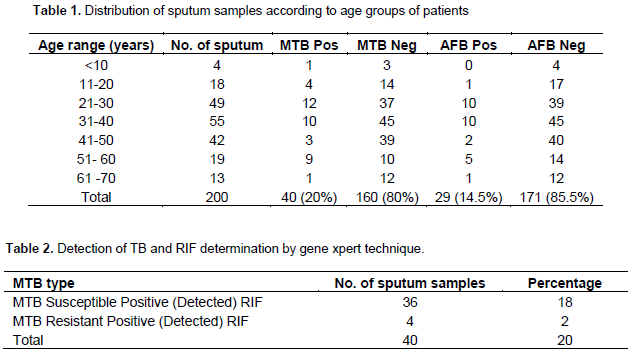
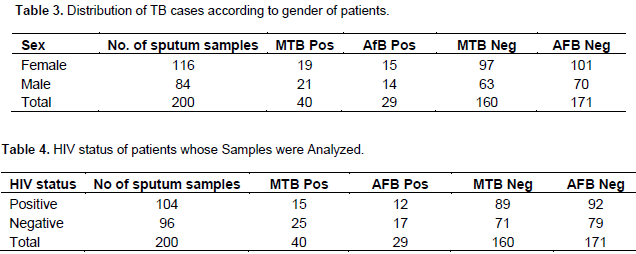
The numbers of sputum samples analyzed according to their age ranges are shown in Table 1. Most of the sputum samples 27.5% (55/200) were received or collected from patients ages 31 to 40; followed by ages 21 to 30 and 41 to 50 with 24.5 (49/200) and 21% (42/200), respectively. The least were from children under 10 with just 2% (4/200)”. Of the 200 samples analyzed, 20% (40/200) were positive while 80% were negative for MTB, and this difference was statistically significant, p = 0.4 (p>0.5). Age range of 31 to 40 years old had the highest number of samples, with ten positive cases of M. tuberculosis (MTB). Ages < 10 years old had the least number of positive samples, with one MTB positive result. The number of AFB smear positive was 29 (14.5%) while 171 (85.5%) were smear negative. Ages < 10 years did not show any smear positive, whereas ages 21 – 30 and 31 – 40 years had 10 smear positive cases each. P = 0.007 (P<0.005), also shown in Table 1. Table 2 showed the rifampicin resistant cases; out of the 40 MTB detected sputum samples analyzed, 36 (18%) persons showed MTB positive while Rifampicin susceptible 4 (2%) were MTB positive and Rifampicin resistant. Table 3 shows the gender of MTB positive and negative as well as AFB smear positive and negative cases. Maximum number of the specimens was from females (58%) (116/200), and the rest from the males. HIV status of the various persons whose specimens were analyzed is shown in Table 4. Out of the 200 sputum samples one hundred and four persons were people living with HIV, while 96 persons were negative. Amongst 104 HIV positive persons, fifteen showed MTB detected which is a case of co-infection while eighty nine showed MTB negative.
Tuberculosis is one of the deadliest public health threats today. Annually, M. tuberculosis (MTB) causes about 8 to 9 million cases of infection as well as 1.5 million deaths (Issar, 2003). These numbers are on the rise globally, especially in Eastern Europe, Africa and the former Soviet Union. Part of the problem in treating TB is the appearance of drug resistant tuberculosis strains, including strains with multidrug resistance (MDR) and more recently strains with extensive drug resistance (XDR) which are more difficult to treat (Issar, 2003). In this part or area of the country (Rivers State, Nigeria) where this study was carried out, the rate of occurrence of all forms of TB cases in 2014 were 2,279 while in 2015 it increased to 2,369. This rise could have been due to several environmental factors such as poor housing and ventilation of the surroundings, overcrowding, and congregation in schools but exposure to ultraviolet light reduces it. Then Xpert MTB/RIF assay, a non-laboratory based molecular assay according to Boehme et al. (2011) was designed specifically for use and quick asses to treatment in endemic disease settings, and it is the first diagnostic tests that have the potential to bring high sensitive nucleic acid amplification testing to peripheral sections of health system in this new generation. Besides, the microscopy only takes about 24 to 48 h, while standard cultures can take 2 to 6 weeks for MTB to grow and conventional drugs resistance test can add 3 more weeks. Infection control decision is also reached quickly since diagnosis of infection is made within 2 h and patient attended to in just one contact (Boehme et al., 2011).
This study revealed an overall infection of MTB to be 40 (20%). This number could have been due to the socioeconomic factors such as low-income people with large families, living in dense urban communities with inadequate housing conditions, people living in congregated institutions such as prisons, nursing homes for elderly people, social shelters, day nurseries and internally displaced persons camps (IDP). In addition as seen in this analysis, xpert MTB/RIF tool is highly sensitive as it was able to detect MTB positive in some persons with AFB smear negative, hence it is a highly recommended tool to be used for MTB detection than microscopy. This result was in agreement with the work by Boehme et al. (2011), with 94.4% sensitivity and 98.3% specificity for rifampicin resistance. Similarly, results from this study were also in agreement with what Small and Pai (2010) reported in relation or reference to a pooled sensitivity of 88 and 98% specificity. There is no significant difference in the detection of MTB among the different age groups in this study using gene xpert. That means using this technique, the detection rate among the groups will still be the same. Boehme and Sutherland (2009) reported a sensitivity of 98% for patients classified as smear negative; culture positive and for culture positive samples xpert gave 100%. Scott et al. (2011) in their study found out that the xpert MTB/RIF non laboratory based molecular assay has potential to improve the diagnosis of tuberculosis (TB) especially HIV infected population through increased sensitivity, reduced turnaround time and immediate identification of Rifampicin (RIF) resistance.
In a clinical study conducted, the sensitivity of MTB/RIF test on just one sputum sample was 92.2% for culture positive TB and 98.2% for smear positive TB which also agrees with the present study. It was observed that the number of positive cases could have emanated due to high rate of HIV amongst the patients, for instance out of the 200 patients screened 104 (52%) were HIV positive which is significant enough to say that it is high. There is a significant difference in the detection of MTB across people living with HIV (PLHIV), and negative persons using AFB microscopy and Gene Xpert technique. This study also revealed 14% (15 cases) prevalence of MTB positive among HIV subjects, which was similar to the report of Scott et al. (2011) (86%) and Boehme et al. (2011), which showed high level of sensitivity in HIV MTB positive patients. No co-infected individual was found to be resistant to Rifampicin. The worrisome aspect of this study is that the high positivity rate of MTB was noticed amongst ages 21 to 30 years (30%) and age 31 to 40 years (25%) which is the age of the work force of most country and owing to this, productivity will be reduced which in turn will affect the economy of the country drastically if it is not checked. Furthermore, the high MDR cases observed which if not properly managed, the dissemination rate may increase since these group of people attend the same churches, market and even join the same transport vehicles with others. Rifampicin resistant cases in this study was found to be 4 (2%), and treatment of this disease is very expensive since these patients have to be placed on second line drugs after further investigation’s using culture and drug susceptibility testing (DST) to find out the actual drug to be used.
For MDR cases, second line drugs are used, they include Amikacin, Kanamycin, Capreomycin and Etionamide. No indeterminate rate was observed (this could be attributed to the fact that the subject characteristics used in this study were well selected using inclusion and exclusion criteria) which corroborates with the work of Boehme et al. (2011) in which case the indeterminate rate was 2.4%. There is a significant difference in the detection of AFB among the different age groups used in this study using AFB microscopy with P value < 0.05 which is significant; hence it is not a sensitive tool to be used. In addition, the number of AFB smear microscopy that showed positive in this study was twenty nine 29 (14.5%) as against forty (40) (20%) for the tuberculosis detected ones. This shows that with AFB microscopy alone most patients with positive cases could actually be missed if in every 40 positive cases with xpert MTB/RIF we are missing about eleven (11) as was shown with smear microscopy in this study the AFB sensitive rate which is low in this study also confirms or tallies with the work of Ritu and Vithal (2015) which gave AFB detection to be about 22 to 43%, though the High Ventilation Air Condition system (HVAC) at the facility was down at the time of carrying out this research we would have considered performing a concentration method for AFB to still make further comparison.
The study revealed that the new automated technique is more sensitive, specific and more rapid in diagnosis of the disease in non HIV and HIV persons. This study has also shown that using this new technology will help to reduce exposure and transmission of the disease and also useful in detection of resistant strains.
The authors have not declared any conflict of interests.
REFERENCES
|
Boehme C, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirili R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Albert H, Cobelens F, Cox H, Alland D, Perkins M. (2011). Feasibility, diagnostic accuracy, and effectiveness of decentralized use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a Multicentre Implementation Study. The Lanc. 377:1495-1505.
Crossref
|
|
|
|
Boehme CC, Sutherland R (2009). Xpert, MTB/RIF Demonstrations for detection of TB and Rifampicin resistance.
|
|
|
|
Daniel WW (1999). Biostatistics, A foundation for analysis in the Health Sciences. (7th edition), New York: John Willey and Sons. 35-40.
|
|
|
|
Dolin F, Gerald L, Mandell E (2010). Principles and practice of infectious diseases. J. Infect. Dis. 250: 1124-1130.
|
|
|
|
Fred T (2009). Two-hour detection of MTB and resistance to rifampicin. Invitro diagnostic medical device. Diseases and Project-Tuberculosis-Xpert MTB/RIF Review A, 4300-7810 (Accessed 20/7/2015).
|
|
|
|
Grant LR (2012). Procedures for collection of induced sputum specimen from children. Clinic. Infect. Dis. Oxf. J. 54:140-145.
|
|
|
|
Issar S (2003). Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clinic. Microbiol. Rev. 16(3):463-496.
Crossref
|
|
|
|
Lalloo G, Naidoo R, Ambaram A (2006). Recent advances in the medical and surgical treatment of multi-drug resistant tuberculosis. Curre. Opini. Pulmon. Medic. 12:179-185.
Crossref
|
|
|
|
Palomino JC, Martin A, Portcels F (2007). Rapid drug resistance in Mycobaterium tuberculosis. Clinc. Microbio. Infect. 13:754-762.
Crossref
|
|
|
|
Ritu S, Vithal PM (2015). Microscopy as a diagnostic tool in pulmonary tuberculosis. Intern. J. Mycobact. 4(1):1-5.
Crossref
|
|
|
|
Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, Sanne I, Venter WF, Duse A, Stevens W (2011). Comparisons of Xpert MTB/RIG with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: A prospective study. J. Pub Medi. 8(7):1371-1379.
|
|
|
|
Small PM, Pai M (2010). Tuberculosis diagnosis – Time for a game change. New Engl. J. Med. 363:1070-1071.
Crossref
|
|
|
|
Van Rie A, Page-Shipp L, Scott L, Sanne I. Stevens W (2010). Expert MTB/RIF for point-of-care diagnosis of TB in high HIV burden, resource-limited countries: hype or hope? Exp. Rev. Molec. Diagn. 10:937-946.
Crossref
|
|
|
|
World Health Organization (2013). Updated WHO Recommendation for Diagnosis of Pulmonary TB, and Pediatric TB, Extra Pulmonary TB and Rifampicin resistance.
View
|