ABSTRACT
Single acid (acetic acid, lactic acid, propionic acid and phosphoric acid) and acid complex solutions at the ratio 1:1 or 2:1 at pH 3 were investigated their antimicrobial activities against three selected foodborne pathogens (Escherichia coli, Salmonella typhi and Staphylococcus aureus). The influences of the deacetylation degrees (DD) (80% and 95%), concentrations (500, 1000, and 2000 μg/mL) and contact time (10, 20, 30, 40, 50 and 60 min) on the antimicrobial activity of chitosan against three bacteria were also studied. The better condition of chitosan and acid complex solutions were selected to use as sanitizers sprayed on the broiler carcass surfaces (breast and thigh) to determined their antimicrobial activities. The results showed that acid complex solutions with the ratio 2:1 had the better inhibiting efficiency against pathogens than the single acid and acid complex solutions at the ratio 1:1. The antimicrobial activity of chitosan against bacteria significantly increased as the contact time and chitosan concentrations increased. Acetic acid+lactic acid or acetic acid+propionic acid (2:1) were dissolved with/without chitosan solution (1000 μg/mL with DD 95 %) and sprayed on the broiler carcass surfaces against pathogens. The results displayed that acetic acid + lactic acid sprayed with chitosan significantly reduced S. aureus, E. coli and S. typhi counts on the surface of the breast (2.73, 2.84 and 2.71 log CFU/cm2, respectively) and the thigh (2.56, 2.85and 2.43 log CFU/cm2, respectively). Conclusion, acid complex solutions mixed with chitosan can be used to avoid the deterioration of slaughtered meat quality.
Key words: Foodborne pathogens, chitosan, organic acid, sanitizer, broiler carcass.
During the slaughtering process for poultry and livestock, several methods, such as hot water washes, acid sprays, chemical sanitizers or flames, etc., can be used to reduce microbial contamination on the surface of the carcass before chilling or refrigeration. The use of synthetic chemical sanitizers is generally effective at reducing post- harvest microbes. Chlorine is the decontaminating agent generally used as a sanitizer to eradicate pathogenic microorganisms in the poultry slaughtering system. But, chlorine can cause severe irritation to the nose, throat and upper respiratory tract. Chlorine exposure at high concentrations results in severe respiratory tract damage, causing bronchitis and pulmonary edema and possibly be deadly (Chaiyakosa et al., 2007).
Organic acids are generally recognized as safe (GRAS) antimicrobial agents approved by USDA Food Safety and Inspection Service and they have been used as sanitizers for slaughtered carcasses with good sterilizing effects (Acuff et al., 1987; Sallam et al., 2020; FDA, 2003). Organic acids have the antimicrobial action by reducing environmental and cellular pH values and increasing anion accumulation (Carpenter and Broadbent, 2009). Moreover, the antimicrobial activities of organic acids are dependent on the pKa value and the effect is greater under acidic condition (Nguyen et al., 2020). Organic acid dilutions (1-3%) can effectively reduce the number of bacteria on an animal carcass before chilling, refrigeration or processing (Raftari et al., 2009). A high level of organic acid with low pH is highly effective in reducing microorganisms, but higher concentrations of these acids result in defects, such as bad flavor and color fading, which affect the quality of the product when applied in the poultry slaughtering system during storage or marketing (Smulders and Greer, 1998; Sohaib et al., 2016). Garbutt (1997) reported that the optimum growth pH of bacteria at neutral pH (6.8-7.2) and the minimum growth pH is nearer to 4.0-4.5. This study also found that growth of food poisoning bacteria, such as Staphylococcus aureus, Salmonella species and Listeria monocytogenes could retard when the pH adjusted lower than 4.0 with organic acids, such as lactic acid, citric acid and acetic acid. Many research found that the organic acids, such as acetic acid, citric acid and lactic acid decreased the microbial populations of Escherichia coli, Salmonella, psychrotrophic Gram-negative and Enterobacteriaceae when sprayed on pork, poultry and beef carcass or use as wash (Laury et al., 2009; Harris et al., 2012; Dan et al., 2007). Therefore, it is important to determine the optimal acidic pH for bacterial inhibition and also to meet the meat quality requirements (indicated by the least amount of discoloration, off-flavor and drip loss).
Lactic acid (2-hydroxypropanoic acid) is a natural organic acid (pKa 3.79) produced by microbial fermentation. It is commonly used in the food production as food preservative, flavor agent and acidulant (Wee et al., 2006; Lipnizki, 2010). Lactic acid is classified as GRAS for use as an antimicrobial agents for decontamination of meat carcass. It is approved for use as part of a carcass wash at level <5% acid for pre- and post-chilling, 2-3% for sub-primal cuts and 2-2.8% in washing systems for trimings and beef head and tongues (Ba et al., 2018; Mani-López et al., 2012). It can interfere with cell membrane permeability and cell functions (Chauret, 2014).
Acetic acid is a monocarboxylic and also known as vinegar, which formed naturally due to spoilage of wine. Acetic acid has a limit to use in foods due to a pungent, vinegar-like odor and sour taste. It is highly water soluble and found in pickled products (Mani-López et al., 2012).
Propionic aicd is a naturally carboxylic acid with a pungent odour, colorless and miscible with water. Propionic acid is a commonly organic acid produced through microbial fermentation (Propionibacterium species). In food industry, it is commonly used as food preservative, antimold, antirope agent and flavouring agent (Gonzalez-Garcia et al., 2017; Haque et al., 2009).
Phosphoric acid is an inorganic acid acquired by chemical reaction of phosphorous rock. It is a colorless, odourless and viscous liquid. It is an important chemical for the manufacture of fertilizers, detergents, toothpastes and alimentary supplies for cattle. In food, it is used as a sequenstrant, an antioxidant and flavor enhancer in beverages and fruit procucts (Awwad et al., 2013; Kandil et al., 2017).
Chitosan is a nontoxic natural polymer. It can be synthesized via the deacetylation of chitin which is major component of the shells of crustaceans, such as crab, shrimp and crawfish (Hong et al., 2002). The chemical structure of chitosan is a linear polysaccharide composed with β-(1-4)-linked 2-amino-2-deoxy-D-glucose and 2-acetamido-2-deoxy-D-glucose. Chitosan is a natural cationic polysaccharides and it has been applied for several purposes, including antimicrobial, food, chemical engineering, pharmaceutical, nutrition and environmental protection applications (Kahya, 2019). Many reports have shown evidence that an edible chitosan film or coating on pork, sausage or ground meat can be used to control the growth of spoilage bacteria during storage or marketing and prolong the shell life (Sagoo et al., 2002; Roller et al., 2002; Lucera et al., 2012). Chitosan has also been shown to inhibit some pathogenic bacteria, including E. coli, Pseudomonas aeruginosa, Shigella dysenteriae, Vibrio species, Salmonella Typhi and S. aureus (Sudarshan et al., 1992; Tepe et al., 2004; 1992, 1992; 1992, et al., 1992, 2011) and the reported minimum inhibitory concentrations (MIC) vary widely from 0.01 to 1.0% (Zheng and Zhu, 2003).
Although many studies have shown evidence for the antimicrobial activities of chitosan and acids, no published studies have combined chitosan with organic acids at pH 3. Thus, the aim of this study was to look for an optimum formula of the single organic/inorganic acid and their acid complex solutions at different ratios at 1:1 and 2:1 at pH 3, and the combination with chitosan on their antibacterial inhibition and the lowest amount of damage on meat quality (discoloration, off-flavor and drip loss). In this study, the single acid (acetic acid, lactic acid, propionic acid and phosphoric acid) and acid complex solutions at the ratio 1:1 or 2:1 at pH 3 investigated their antimicrobial activities against three selected foodborne pathogens including E. coli, S. Typhi and S. aureus for 1 h. Besides, the influences of the deacetylation degrees (DD) (80 and 95%), concentrations (500, 1000, and 2000 μg/mL), and the contact time (10, 20, 30, 40, 50 and 60 min) on the antimicrobial activity of chitosan against three selected foodborne pathogens were also studied. The better condition of acid complex solutions and chitosan were selected to be used as sanitizers sprayed on the broiler carcass surfaces (breast and thigh) to determine their antimicrobial activities.
Raw materials
Chitosan, with a molecular weight (MW) of 100-300 kDa and a deacetylation degree (DD) of 95%, was purchased from Lytone Enterprise Inc. (Taipei, Taiwan). Three strains of pathogenic microorganisms (E. coli BCRC 10675, S. Typhi BCRC 10746 and S. aureus BCRC 10781) were obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan).
Preparation of acid and chitosan
Propionic acid (Merck, Darmstadt, Germany), acetic acid (Union Chemical Work Ltd., Hsinchu, Taiwan), lactic acid (Wako Inc., Japan) and phosphoric acid (Union Chemical Work Ltd., Hsinchu, Taiwan) separately prepared the single acid solution at pH 3 in sterilized distilled water. For the acid complex, solutions (pH 3) were prepared by the mixtures of propionic acid + acetic acid, phosphoric acid+propionic acid, acetic acid+phosphoric acid or lactic acid+lactic acid at the ratio of 1:1 or 2:1 (v/v) in sterilized distilled water.
Preparation of chitosan
Chitosan acidic solution was prepared according to the modified method of Sudarshan et al. (1992). A 500, 1000, or 2000 μg/mL chitosan acidic solutions was prepared by dissolved chitosan powder in distilled water and adjusted to pH 5 with glacial acetic acid.
Microbial culture and growth conditions
According to the protocol of the Food Industry Research and Development Institute (Hsinchu, Taiwan), S. Typhi and E. coli were separately cultured in a nutrient broth (Acumedia, Michigan, USA) and then incubated at 37°C for 24 h. S. aureus was cultured in tryptic soy broth (Acumedia, Michigan, USA) at 37°C for 24 h. Then, S. Typhi, E. coli and S. aureus cultures were collected.
Antimicrobial activity of the acid solution
Evaluations of antimicrobial activity of acid solutions were performed as follows: 1 mL of bacterial suspension (108 CFU/mL) was mixed with 9 mL of various acid solutions and incubated at 37°C for 60 min. These mixtures were then serially diluted to 106 CFU/mL and incubated at 37°C for 24 h. Colony numbers were determined using the plate count method. The initial colonies number of S. Typhi, E. coli and S. aureus was 4.5×106, 6.1×106 and 5.4×106 CFU/mL, respectively. The inhibition efficiency was defined as: reduced count (log CFU/mL) = N1 - N2, where N1 and N2 represent the colony numbers on the plates before and after treatment.
Antimicrobial activity of the chitosan solution
Antimicrobial activity of the chitosan solution was evaluated as previously described: 1 mL of bacterial suspension was mixed with 1 mL of chitosan solutions and 8 mL of lactic acid to the final chitosan concentrations at 500, 1000 and 2000 μg/mL. Then, the suspension with chitosan was incubated at 37°C for 10, 20, 30, 40, 50 or 60 min. The mixtures were then serially diluted to 107 CFU/mL and incubated at 37°C for 24 h. Colony numbers were counted using the plate count method. The initial colony numbers of E. coli, S. Typhi and S. aureus were 7.1×107, 5.2×107 and 4.5×107 CFU/mL, respectively. The inhibition efficiency was defined in the same way as described for the acid treatments.
Preparation of sanitizing spray
Acetic acid+lactic acid and acetic acid+propionic acid solutions at pH 3 were separately prepared at the ratio 2:1 (v/v). Then, chitosan was added and dissolved completely to the final concentration at 1000 μg/mL.
Treatment of spray
A total of 15 broiler carcasses (average weight 1.67 kg) were purchased from Charoen Pokphand Enterprise (Taiwan) Co., Ltd. and divided into 3 treatment groups of 5 birds; each group was inoculated with S. aureus, E. coli or S. Typhi. The procedure was repeated three times for the experiment. Approximately, 5 log CFU/cm2 bacteria were inoculated on the surface of the breast and leg areas by cotton swab, as described by Dubal et al. (2004) and carcasses were maintained at 10°C for 2 h. The bacterial counts for S. aureus, E. coli and S. Typhi inoculated on the carcass surfaces were 3.4×105, 4.1×105 and 2.4×105 CFU/cm2, respectively. The spraying procedure was performed as follows: 100 mL sanitizer was sprayed on the whole surface of each bird, which was then maintained at 10°C for 1 h. Solutions formulated only with organic acid complexes without chitosan were used as the controls.
At the end of treatment, a sterilized albumin foil (5 × 5 cm) was placed on the breast and leg of each bird and the swab method was used to take samples to determine colony counts. The inhibition efficiency was defined in the same way as described previously.
Statistical analysis
Data were analyzed using the Statistical Analysis System’s Procedures (SAS) (Institute Inc., Cary, NC) software package with a 5% level of signiï¬cance. The GLM system was applied to determine the significance of the treatments; when significant (PË‚0.05) differences were found, the means were determined by the Duncan’s multiple range test.
Antimicrobial ability of single acids at pH 3
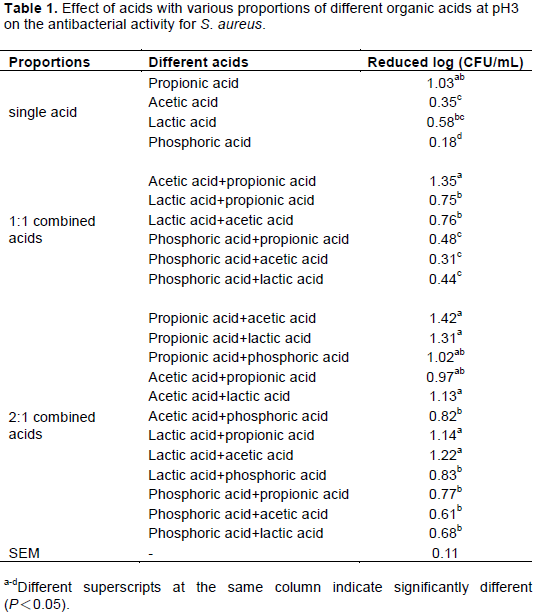
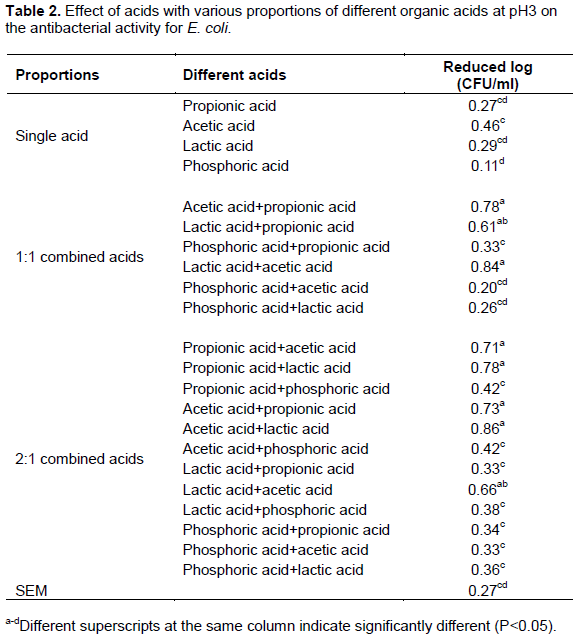
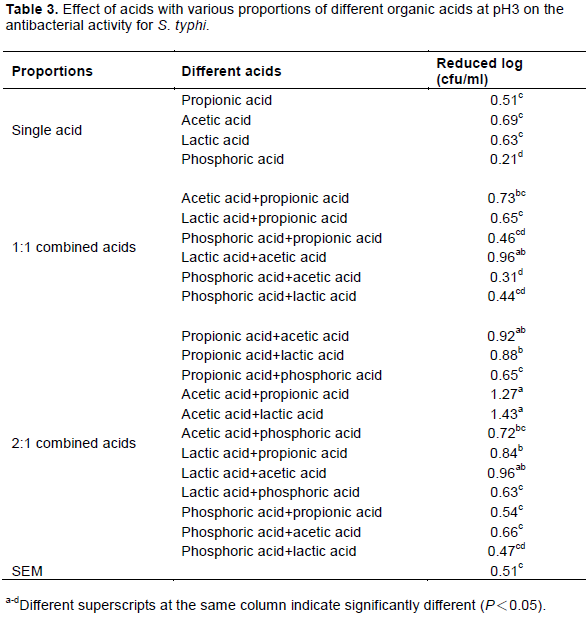
Garbutt (1997) stated that strong inorganic acids are not often included in processed foods, but hydrochloric and phosphoric acids are used in the manufacturing of carbonated drinks and non-carbonated drinks (for example, cola) contain phosphoric acid. Therefore, in this study, 3 organic acids (acetic acid, propionic acid and lactic acid) and 1 inorganic acid (phosphoric acid) were evaluated for the ability to inhibit three selected pathogens (S. aureus, E. coli and S. Typhi); the results are presented in Tables 1 to 3. For single acids at pH 3, propionic acid had the best and most highly significant inhibition (approximately reduced 1.03 log CFU/mL) against S. aureus when compared with all organic acids or the inorganic acid. Moreover, the reduced bacterial count for all organic acids was 0.35-1.03 log CFU/mL and significantly higher than that of the inorganic acid (phosphoric acid: 0.15 log CFU/mL). For E. coli, the reduction in bacterial counts for all single acids was below 0.5 CFU/mL, indicating that the antimicrobial ability of single acids was less efficacious at inhibiting E. coli regardless of whether the acid was organic or inorganic. However, acetic acid exhibited the best ability to inhibit S. Typhi, reducing growth by 0.69 CFU/mL. The data also indicated that organic acids were better than the inorganic acid on inhibit Salmonella bacteria. This result may be due to Salmonella having an inorganic acid resistance mechanism and acid tolerance response. Brenneman et al. (2013) reported that the RpoS is an essential regulator in Salmonella for the acid tolerance response. Moreover, PhoP, PhoQ and Flu also play an important role in acid response. PhoP and PhoQ protect against inorganic stress. Mani-López et al. (2012) also reported that the lethal effects of organic acid on Salmonella depended on concentration, pH of the environment and the dissociation constant of each acid. According to the data described earlier, single organic acids can be used to inhibit one specific type of bacteria; for example, propionic acid is suitable to use against S. aureus and acetic acid is suitable for S. Typhi. Acid has effect on the minimum pH for microorganism. The organic acids (acetic, lactic, citric and tartaric) have better activities than inorganic acids and the order of acids according to the level of their antimicrobial activity is as follows: propionic > acetic > lactic >citric> phosphoric > hydrochloric (Buchanan and Golden, 1994; Garbutt, 1997).
The results also signed to support this notion.
Antimicrobial abilities of acid complexes with different acids and formula ratios
The results showing the inhibitory effects of acid complex solutions (pH 3) with different acids and component proportions on three selected pathogens (S. aureus, E. coli and S. Typhi) are displayed in Tables 1 to 3. These data indicate that all acid complexes using inorganic acid (phosphoric) had the least ability to inhibit microorganisms, regardless of the ratio, when compared with organic acids. Conversely, for the microorganisms examined, acid complexes were adjusted with different acid ratios and organic acids in fact improved antibacterial ability.
For S. aureus, the result showed that all 2:1 acid complexes had better antibacterial ability than all 1:1 acid complexes and all single acids. These results also indicated that propionic acid combined with the other organic acids (lactic and acetic) had the best bacterial inhibition efficiency. Although the acid complexes using acetic acid and lactic acid were not better than propionic acid, there were no differences by statistical analysis in this study. The antimicrobial activity of organic acids is attributed with the ability of undissociated acid molecules to enter the bacteria cell and the lower pH value than the growth range of bacteria (Yu et al., 2010; Sallam et al., 2020). Dubal et al. (2004) found that spraying with the mixture of acetic acid + proionic acid (1.5 + 1.5%) on sheep/goat forequarters surfaces was completely inhibited in the inoculated pathogens, Salmonella Typhimurium (103 CFU/g). Yang et al. (1998) indicated that 2% lactic acid (pH 2.2) could reduce S. aureus by approximately 1 log CFU/mL. However, there has been some research suggesting that 2% or even 1% organic acid is responsible for the presence of detrimental effects on meat quality (Smulders and Greer, 1998). The bacterial inhibition of lactic acid (pH 3) for S. aureus in this experiment was 0.35 log CFU/mL. Moreover, better count reductions for S. aureus, 1.22-1.35 log CFU/mL, were observed in acetic acid complexes using propionic acid (1:1) and lactic acid (2:1) in this study. Thus, S. aureus count reduction can be achieved with a pH 3 acetic acid complex, which may also reduce damage to quality.
For E. coli, the results showed that all acid complexes (1:1 or 2:1) adjusted with organic acids had better antibacterial ability than all acid complexes using inorganic acids and all single acids. Moreover, these results also indicated that acid complexes using lactic and acetic acid had the best inhibition efficiency. Although acid complexes using acetic acid and lactic acid were better than propionic acid, there were no differences by statistical analysis in this study. Another study (Bracket et al., 1994) also noted that the compound use of organic acids had better inhibition effects than the use of a single organic acid against E. coli. SkÅ™ivanová and Marounek (2007) stated that the antimicrobial effect of organic acids on E. coli is depended on pH. At low pH, organic acids are undissociated. These undissociated forms are lipophilic and could permit through the cell membrane and inhibited microbial growth. Stivarius et al. (2002) applied 5% lactic acid to wash beef trimmings inoculated with a mixture of S. Typhimurium and E. coli before grinding and the results showed that higher concentration of lactic acid was effective for reducing the growth of all inoculated pathogens and increasing the shelf-life. Dorsa et al. (1997) indicated that 2% of acetic acid and lactic acid had high inhibition effects against E. coli.
However, this experiment results showed that all acids exhibited the poorest inhibition effects with E. coli and thus, these data do not agree with the results of the previous study. The reason for this discrepancy may be because a pH 3 acid solution was used in this study and the percentage of acid was significantly lower than 2%, which was used in the aforementioned review. Smulders and Greer (1998) also indicated that E. coli O157:H7 had better resistance to organic acids (lactic acid or acetic acid). When they used organic acid alone in treatment, the inhibition effect was lower than 1 log CFU/cm2.
For S. Typhi, the results showed that all acetic acid complexes (1:1 or 2:1) adjusted using lactic acid and propionic acid had better antibacterial abilities (reduced count was 1.27-1.43 log CFU/mL) than other acid complexes and all single acids. These results also indicated that acetic acid combined with lactic acid had the best inhibition efficiency. The acid complexes using acetic acid and propionic acid were not better than lactic acid and there was no difference by statistical analysis in this study. Smulders and Greer (1998) demonstrated that spraying 1-3% lactic acid or 2% acetic acid on a slaughtered body could reduce S. Typhi 1-2 log CFU/cm2. Xiong et al. (1998) also indicated that spraying 2% lactic acid or compound acids on chicken skin could reduce S. Typhi by 0.52 and 1.16 log CFU/cm2, respectively.
In this experiments, all single and complex acids displayed better antibacterial action against S. aureus (reduced count 0.18-1.42, log CFU/mL) and S. Typhi (reduced count 0.21-1.43, log CFU/mL) than E. coli (reduced count 0.11-0.86, log CFU/mL) when the results in Tables 1 and 3 are compared to those in Table 2. However, the results might be due to different microbe sensitivities to different acids and the coordination effect with organic acids. Different groups of microbes have different optimum inhibitions (Liu et al., 2001). Furthermore, the results also showed that pH 3 acetic acid complexes using propionic or lactic acid enhanced bacterial inhibition and prevented the deterioration of slaughtered animal carcasses. Therefore, the researcher decided to use 2:1 acid complexes with acetic acid + lactic acid and acetic acid + propionic acid, combined with an optimum level of chitosan, to create a sterilization solution that we could then apply in a poultry slaughtering site to evaluate antimicrobial action against E. coli, S. Typhi and S. aureus, as in the last experiment.
Antimicrobial ability of chitosan with different deacetylation degrees and concentrations
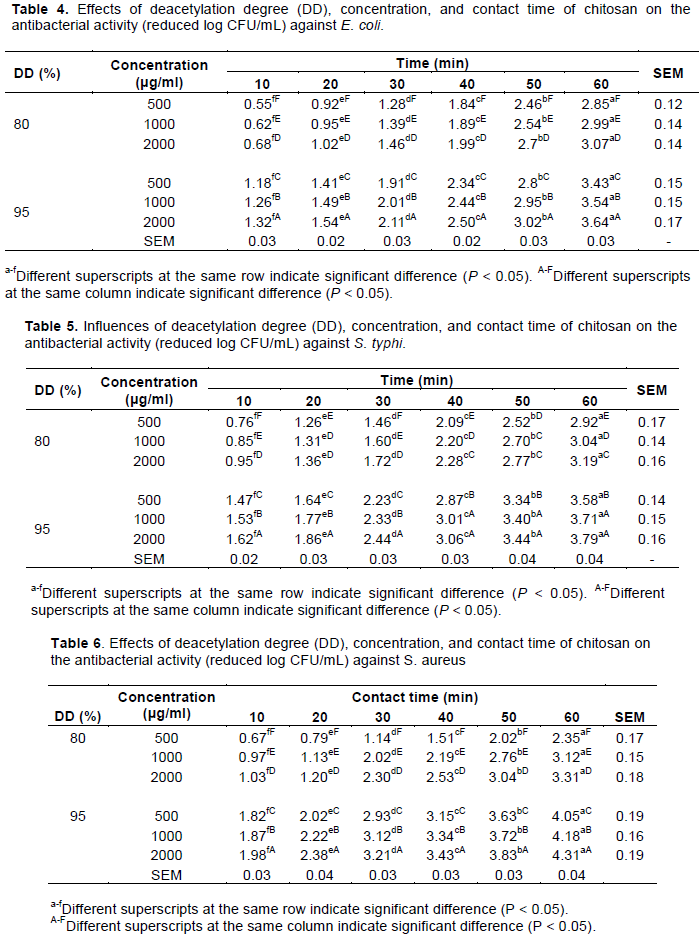
Table 4 illustrates the influence of deacetylation degree (DD), concentration and contact time of chitosan on antibacterial activity against E. coli. The results showed that the inhibition effects of chitosan against E. coli increased significantly as chitosan concentration increased (P < 0.05) at any contact time and with the same DD. For example, the bacterial count reduction increased significantly from 2.85 to 3.07 log CFU/mL when the chitosan concentration (80% DD) increased from 500 to 2000 μg/mL with contact for 60 min. These results agreed with the study conducted by Zheng and Zhu (2003) who reported that chitosan (305 kDa molecular weight) had a 0% inhibition rate at a concentration of 0.25%, whereas it had a 40% inhibition rate against E. coli when the chitosan concentration increased to 0.5%. This inhibition rate further increased to 100% when the chitosan concentration increased to 1.0%. Dorsa et al. (1997) also explained that higher NH3+ concentration, which was due to a higher chitosan concentration in the medium, contributed to increased chitosan antibacterial activity. Liu et al. (2004) reported that chitosan at the higher concentration of 0.5% caused more cell membrane damage to E. coli than chitosan at the lower level concentration of 0.25%.
In this study, the reduction in E. coli bacterial counts also significantly increased (P < 0.05) as the contact time increased at the same DD and concentration of chitosan. For example, the bacterial count reduction increased significantly from 1.32 to 3.64 log CFU/mL when contact time increased from 10 to 60 min for 2000 μg/mL (95% DD) chitosan solution. Liu et al. (2004) found that the permeability of the outer and inner membranes of E. coli increased with increased chitosan contact time. A significant reduction in the numbers of Vibrio parahaemolyticus, which was artificially inoculated in shrimp, was observed when the chitosan exposure time increased (Chaiyakosa et al., 2007). Similarly, the growth of E. coli was inhibited when the chitosan exposure time increased (Liu et al., 2004). A study performed by Chung et al. (2003) also illustrates that the antibacterial activity of chitosan inhibits E. coli and S. aureus increased with the contact time. Moreover, chitosan with low molecular weight possesses a grander flexibility to bind more than one cell. This situation causes the bridge between polymer chains of chitosan and bacteria cells rapidly formed and inhibits bacteria (Wu et al., 2006). Helander et al. (2001) reported that chitosan displays stronger antimicrobial activty in acid condition. The activity decreases with the increasing pH.
In this experiment, it was found that contact time (that is, 10-60 min) had a greater influence on E. coli inhibition than the concentration (that is, 500-2000 μg/mL) of chitosan. For example, count reduction increased by approximately 2.35 log CFU/mL (that is, from 0.62 to 2.97 log CFU/mL) when the chitosan contact time increased from 10 to 60 min at all chitosan concentrations (80% DD) from 500 to 2000 μg/mL. However, the count reduction only increased by approximately 0.22 log CFU/mL (that is, from 2.85 to 3.07 log CFU/mL) when the contact time was 60 min and when the concentration increased from 500 to 2000 μg/mL. Liu et al. (2004) stated that the permeability of the outer and inner membranes of E. coli increased with increased chitosan contact time. Another study by Chung et al. (2003) illustrates that an increase of the contact time increases the antibacterial activity of chitosan on E. coli and S. aureus.
Moreover, with regard to DD bacterial count, reduction with 95% DD was higher than for 80% DD when chitosan concentrations and contact time were maintained at the same conditions. For example, chitosan with 95% DD resulted in a significantly higher count reduction for E. coli (1.18 to 1.32 log CFU/mL) than for 80% DD (that is, 0.55 to 0.68 log CFU/mL) when contact time was 10 min at concentrations varying from 500 to 2000 μg/mL. This higher inhibition efficiency due to higher deacetylation degrees of chitosan solutions was also observed for different contact times in this study, which agrees with Liu et al. (2001) who reported that the antibacterial activities of chitosan against E. coli increased when the DD increasing from 74 to 96%. Similar increases in antibacterial activities with increased DD were also reported by Hongpattarakere and Riyaphan (2008).
The antibacterial effects of chitosan with different DD concentrations and contact time for S. Typhi and S. aureus are shown in Tables 5 and 6. The inhibition effects of chitosan against S. Typhi and S. aureus increased significantly as the concentrations and contact time increased (P < 0.05) and these results were similar to E. coli in the previous experiment. However, antibacterial activity of the same DD concentrations and contact time was higher for S. aureus and S. Typhi than for E. coli. For example, a 1000 μg/mL chitosan solution with 95% DD and a contact time of 60 min utilized against E. coli, S. Typhi and S. aureus reduced bacterial counts by 3.54, 3.71 and 4.18 log CFU/mL, respectively. In summary, the data in this study demonstrate that better antibacterial activity was achieved against S. aureus, regardless of DD concentration and contact time. Zheng and Zhu (2003) showed that chitosan (305 kDa molecular weight) had a 99% inhibition rate against S. aureus at a concentration of 0.25% and a 100% inhibition rate when the concentration increased to 0.5%. In this study, antibacterial efficiency was more profound with increases in chitosan contact time compared with increased concentrations of chitosan. Moreover, for the same concentrations and contact times, chitosan with higher DD resulted in higher antibacterial efficiency against S. typhi and S. aureus.
Antibacterial efficiency of sanitizers with chitosan and organic acids at pH 3
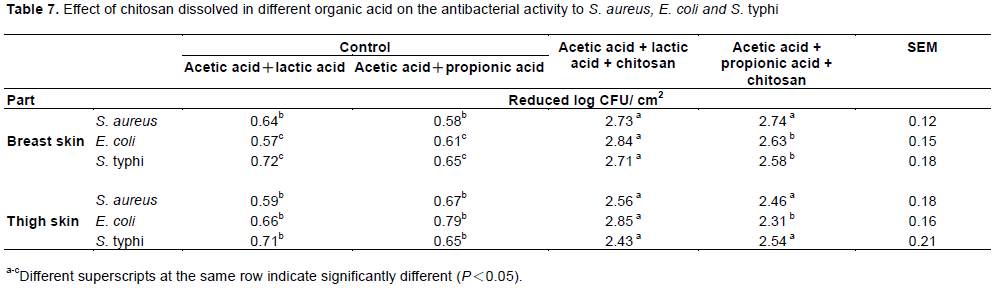
Four sanitizers, including: acetic acid+lactic acid (2:1), acetic acid+propionic acid (2:1), acetic acid + lactic acid (2:1) + chitosan 1000 μg/mL and acetic acid + propionic acid (2:1) + chitosan 1000 μg/mL was separately prepared. Broiler carcasses were individually inoculated with selected bacteria (S. aureus, E. coli and S. Typhi) and then, the 4 sanitizers were applied by spraying on the broiler carcass surfaces (breast and thigh). The bacterial inhibition for S. aureus, E. coli and S. Typhi when the sanitizers were sprayed individually are shown in Table 7. The results showed that sanitizers formulated with 1000 μg/mL chitosan and organic acids (acetic acid + lactic acid or acetic acid + propionic acid) significantly inhibited the growth of S. aureus, E. coli and S. Typhi on breast and thigh surfaces of broiler carcasses when compared with sanitizers formulated only with organic acids. However, the sanitizer with the best inhibition efficiency for S. aureus, E. coli and S. Typhi was formulated with 1000 μg/mL chitosan and organic acid (acetic acid + lactic acid). The reduced counts for S. aureus, E. coli and S. Typhi were 2.73, 2.84 and 2.71 log CFU/cm2, respectively, on the breast surface and 2.56, 2.85 and 2.43 log CFU/cm2, respectively, on the thigh surface. It was determined that the bacterial inhibition efficiency was the same for all parts of the broiler carcasses examined in this study. Many reviews have also indicated that chitosan with acids has better antibacterial activity in foods. For example, chitosan (0.6%) mixed with a low concentration of sulfide (170 ppm) significantly inhibited growth of lactic acid bacteria and yeast, as determined by total plate count (Roller et al., 2002). Coma et al. (2003) reported that the addition of chitosan to cheese did not significantly affect the product’s components. Kanatt et al. (2008) reported that chitosan added to ground lamb and salami sausage can significantly increase shelf-life when stored at 0-3°C. Fruits with high commercial value can be corrupted when fruit frostbite, water loss and microbial contamination occur due to storage at low temperatures.
Some reports have shown that juiced fruit, mango, strawberry, orange and longan whose surfaces were covered with chitosan had significantly increased storage time and reduced drip loss (Chien et al., 2007; Jiang and Li, 2001; Pilar et al., 2008). Moreover, the chitosan layer can effectively inhibit bacterial contamination of the fruit. In this study, a sanitizer solution formulated with chitosan and organic acid at pH 3 effectively controlled and reduced the bacterial counts for S. aureus, E. coli and S. Typhi on the surface of broiler carcasses.
All food chemicals were considered to improve the microbial quality of food according to cost, safety and antibacterial ability. Although phosphoric acid was cheaper, all the organic acids in this study showed better bacterial inhibition capabilities than the inorganic acid, regardless of whether the acids were single or complex. The most effective acids for solutions formulated with chitosan were found in the acid complexes (2:1), such as acetic acid + lactic acid and acetic acid + propionic acid and these acid complexes were utilized to treat the breast and thigh surfaces by spraying and to determine the greatest sanitizer formulation. The solution consisting of 1000 μg/mL chitosan and an acid complex with acetic acid + lactic acid with ratio at 2:1 and pH 3 was the paramount optimal according to the antibacterial results shown in Table 7. Organic acid and chitosan are not only very safe and have good sterilization ability, but the pH of the solution (pH 3) was also shown to have similar antibacterial abilities when compared with 2% organic acids in this study. Therefore, this new formulation of organic acid and chitosan can be recommended as a sanitizer for use in the poultry slaughtering system.
The authors have not declared any conflict of interests.
REFERENCES
|
Acuff GR, Vanderzant C, Savell JW, Jones DK, Griffin DB, Ehlers JG (1987). Effect of acid decontamination of beef subprimal cuts on the microbiological and sensory characteristics of steaks. Meat Science 19(3):217-226.
Crossref
|
|
|
|
Awwad NS, El-Nadi YA, Hamed MM (2013). Successive processes for purification and extraction of phosphoric acid produced by wet process. Chemical Engineering and Processing Process Intensification 74:69-74.
Crossref
|
|
|
|
|
Ba HV, Seo HW, Seong PN, Kim YS, Park BY, Moon SS, Kang SJ, Choi YM, Kim JH (2018). The effects of pre-and post-slaughter spray application with organic acids on microbial population reductions on beef carcasses. Meat Science 137:16-23.
Crossref
|
|
|
|
|
Bracket RE, Hao YY, Doyle MP (1994). Ineffectiveness of hot acid sprays to decontaminate E. coli O157:H7 on beef. Journal of Food Protection 57(3):198-203.
Crossref
|
|
|
|
|
Brenneman KE, Willingham C, Kong W, Curtiss R, Roland KL (2013) low-pH rescue of acid-sensitive Salmonella enterica serovar typhi strain by a rhamnose-regulated arginine decarboxylase system. Journal of Bacteriology 195(13):3062-3072.
Crossref
|
|
|
|
|
Buchanan RL, Golden MH (1994). Interaction of citric acid concentration and pH on the kinetics of Listeria monocytogenes inactivation. Journal of Food Protection 57(7):567-570.
Crossref
|
|
|
|
|
Carpenter CE, Broadbent JR (2009). External concentration of organic acid anions and pH: key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. Journal of Food Science 74(1):R12-15.
Crossref
|
|
|
|
|
Chaiyakosa S, Charernjiratragul W, Umsakul K, Vuddhakul V (2007). Comparing the efficiency of chitosan with chlorine for reducing Vibrio parahaemolyticus in shrimp. Food Control 18(9):1031-1035.
Crossref
|
|
|
|
|
Chauret CP (2014). Sanitization. Encyclopedia of Food Microbiology (2ed) pp. 360-364.
Crossref
|
|
|
|
|
Chien PJ, Sheu F, Lin HR (2007). Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chemistry 100(3):1160-1164.
Crossref
|
|
|
|
|
Chung YC, Wang HL, Chen YM, Li SL (2003). Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresource Technology 88(3):179-184.
Crossref
|
|
|
|
|
Coma V, Deschamps A, Martial-Gros A (2003). Bioactive packaging materials from edible chitosan polymer antimicrobial activity assessment on dairy related contaminants. Journal of Food Science 68(9):2788-2792.
Crossref
|
|
|
|
|
Dan SM, Mihaiu M, Rotaru O, Dalea I (2007). Microbial changes on the surface of pork carcasses due lactic and acetic acids decontamination. USAMV-CN 64(1-2):403-408.
|
|
|
|
|
Dorsa WJ, Siragusa GR, Cutter CN, Berry ED, Koohmaraie M (1997). Efficacy of using a sponge sampling method to recover low levels of Escherichia coli O157:H7, Salmonella typhimurium, and aerobic bacteria from beef carcass surface tissue. Food Microbiology 14(1):63-69.
Crossref
|
|
|
|
|
Dubal ZB, Paturkar AM, Waskar VS, Zende RJ, Latha C, Rawool DB, Kadam MM (2004). Effect of grade organic acids on inoculated S. aureus, L. monocytogenes, E. coli and S. typhimurium in sheep/goat meat stored at refrigeration temperature. Meat Science 66(4):817-821.
Crossref
|
|
|
|
|
FDA (2003). Code of federal regulations title 21. Government Printing Office, USA.
|
|
|
|
|
Garbutt J (1997). Essentials of food microbiology. Hodder Headline Group, London UK pp. 135-174.
|
|
|
|
|
Gonzalez-Garcia RA, McCubbin T, Navone L, Stowers C, Nielsen LK, Marcellin E (2017). Microbial propionic acid production. Fermentation 3(2):21.
Crossref
|
|
|
|
|
Haque MN, Chowdhury R, Islam KMS, Akbar MA (2009). Propionic acid is an alternative to antibiotics in poultry diet. Bangladesh Journal of Animal Science 38(1-2):115-122.
Crossref
|
|
|
|
|
Harris D, Brashears MM, Garmyn AJ, Brooks JC, Miller MF (2012). Microbiological and organoleptic characteristics of beef trim and ground beef treated with acetic acid, lactic acid, acidified sodium chlorite, or sterile water in simulated commercial processing environment to reduce Escherichia coli O157:H7 and Salmonella. Meat Science 90(3):783-788.
Crossref
|
|
|
|
|
Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S (2001). Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. International Journal of Food Microbiology 71(2-3):235-244.
Crossref
|
|
|
|
|
Hong KN, Park NY, Lee SH, Meyers SP (2002). Antibacterial activity of chitosan and chitosan oligomers with different molecular weights. International Journal of Food Microbiology 74(1-2):65-72.
Crossref
|
|
|
|
|
Hongpattarakere T, Riyaphan O (2008). Effect of deacetylation conditions on antimicrobial activity of chitosans prepared from carapace of black tiger shrimp (Penaeus monodon). Songklanakarin Journal of Science and Technology 30:1-9.
|
|
|
|
|
Jiang Y, Li Y (2001). Effect of chitosan coating on postharvest life and quality of longan fruit. Food Chemistry 73(2):139-143.
Crossref
|
|
|
|
|
Kandil AT, Mira HI, Taha MH, Kamel MF (2017). Production of pure phosphoric acid from El-Sebaeya low-grade phosphate ore. Separation Science and Technology 52(4):679-690.
Crossref
|
|
|
|
|
Kahya N (2019). Water soluble chitosan derivatives and their biological activities: a review. Polymer Sciences 5(3):1-11.
|
|
|
|
|
Kanatt SR, Chander R, Arun S (2008). Chitosan glucose complex - A novel food preservative. Food Chemistry 106(2):521-528.
Crossref
|
|
|
|
|
Laury AM, Alvarado MV, Nace G, Alvarado CZ, Brook JC, Echeverry A, Brashears MM (2009). Validation of a lactic acid- and citric acid-based antimicrobial product for chicken carcass. Journal of Food Protection 72(10):2208-2211.
Crossref
|
|
|
|
|
Lipnizki F (2010). Basic Aspects and Applications of Membrane Processes in Agro-Food and Bulk Biotech Industries. Comprehensive Membrane Science and Engineering pp. 165-194.
Crossref
|
|
|
|
|
Liu H, Du YM, Wang XH, Sun LP (2004). Chitosan kills bacteria through cell membrane damage. International Journal of Food Microbiology 95(2):147-155.
Crossref
|
|
|
|
|
Liu X, Yun L, Dong Z, Zhi L, Kang D (2001). Antibacterial action of chitosan and carboxymethylated chitosan. Journal of Applied Polymer Science 79(7):1324-1335.
Crossref
|
|
|
|
|
Lucera A, Costa C, Conte A, Del Nobile MA (2012). Food applications of natural antimicrobial compounds. Frontiers in Microbiology 3(287):1-13.
Crossref
|
|
|
|
|
Mani-López E, García HS, López-Malo A (2012). Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Research International 45:713-721.
Crossref
|
|
|
|
|
Nguyen DH, Seok WJ, Kim IH (2020). Organic acids mixtures as a dietary additive for pigs-a review. Animals 10(952):1-12.
Crossref
|
|
|
|
|
Pilar HM, Eva A, Valeria DV, Dinoraz V, Rafael G (2008). Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria ananassa) quality during refrigerated storage. Food Chemistry 110:428-435.
Crossref
|
|
|
|
|
Raftari M, Jalilian FA, Abdulamir AS, Son R, Sekawi Z, Fatimah AB (2009). Effect of organic acids on Escherichia coli O157:H7 and Staphylococcus aureus contaminated meat. The Open Microbiology Journal 3:121-127.
Crossref
|
|
|
|
|
Roller S, Sagooa S, Boarda R, O'Mahonyb T, Capliceb E, Fitzgeraldb G, Fogdenc M, Owenc M, Fletcherc H (2002). Novel combinations of chitosan, carnocin and sulphite for the preservation of chilled pork sausages. Meat Science 62:165-177.
Crossref
|
|
|
|
|
Sagoo S, Board R, Roller S (2002). Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiology 19:175-182.
Crossref
|
|
|
|
|
Sallam KI, Abd-Elghany SM, Hussein MA, Imre K, Morar A, Morshdy AE, Sayed-Ahmed MZ (2020). Microbial decontamination of beef carcass surfaces by lactic acid, acetic acid, and trisodium phosphate sprays. BioMed Research International 2324358:1-11.
Crossref
|
|
|
|
|
SkÅ™ivanová E, Marounek M (2007). Influence of pH on antimicrobial activity of organic acids against rabbit enteropathogenic strain of Escherichia coli. Folia Microbiology 52(1):70-72.
Crossref
|
|
|
|
|
Smulders FJM, Greer GG (1998). Integrating microbial decontamination with organic acids in HACCP programs for muscle foods: prospects and controversies. International Journal of Food Microbiology 44(3):149-169.
Crossref
|
|
|
|
|
Sohaib M, Anjum FM, Arshad MS, Rahman UU (2016). Postharvest intervention technologies for safety enhancement of meat and meat based on products; a critical review. Journal of Food Science and Technology 53(1):19-30.
Crossref
|
|
|
|
|
Stivarius MR, Pohlman FW, McElyea KS, Waldroup AL (2002). Effects of hot water and lactic acid treatment of beef trimmings prior to grinding on microbial, instrumental color and sensory properties of ground beef during display. Meat Science 60(4):327-334.
Crossref
|
|
|
|
|
Sudarshan NR, Hoover DG, Knorr D (1992). Antibacterial action of chitosan. Food Biotechnology 6(3):257-272.
Crossref
|
|
|
|
|
Tepe B, Daferera D, Sokmen M, Polissiou M, Sokmen A (2004). In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii M. Zohary et PH Davis. Journal of agricultural and food chemistry 52(5):1132-1137.
Crossref
|
|
|
|
|
Wang GH (1992). Inhibition and inactivation of five species of foodborne pathogens by chitosan. Journal of Food Protection 55(11):916-919.
Crossref
|
|
|
|
|
Wang L, Liu F, Jiang Y, Chai Z, Li P, Cheng Y, Jing H, Leng X (2011). Synergistic antimicrobial activities of natural essential oils with chitosan films. Journal of agricultural and food chemistry, 59(23):12411-12419.
Crossref
|
|
|
|
|
Wee YJ, Kim JN, Ryu HW (2006). Biotechnological production of lactic acid and its recent applications. Food Technology and Biotechnology 44(2):1-11.
|
|
|
|
|
Wu XY, Zeng QX, Mo SF, Ruan Z (2006). Antibacterial activities of chitosan with different degree of deacetylation and molecular masses. Journal of South China University Technology 34:58-62.
|
|
|
|
|
Xiong X, Hirata M, Takanashi H, Lee MG, Hano T (1998). Analysis of acclimation behavior against nitrification inhibitors in activated sludge processes. Journal of Fermentation and Bioengineering 86(2):207-214.
Crossref
|
|
|
|
|
Yang ZP, Li YB, Slavik M (1998). Use of antimicrobial spray applied with an inside-outside birdwasher to reduce bacterial contamination on prechilled chicken carcasses. Journal of Food Protection 61(7):829-832.
Crossref
|
|
|
|
|
Yu G, Huang GH, Zhang XD, Li Y (2010). Inhibitory effects of organic acids on bacteria growth during food waste composting. Compost Science & Utilization 18(1):55-63.
Crossref
|
|
|
|
|
Zheng LY, Zhu JF (2003). Study on antimicrobial activity of chitosan with different molecular weights. Carbohydrate Polymers 54(4):527-530.
Crossref
|
|