ABSTRACT
Momordica charantia and Morinda lucida are Benin’s pharmacopeia plants that are used traditionally for the treatment of infectious diseases. This study aims to investigate phytochemical profile and antimicrobial activity of both plants. The dried leaf powder is used for extraction with different solvents by ultrasonication (35 Hz) at room temperature for 2 h. TLC and the method based on coloring and precipitation differential reactions were used for preliminary screening. HPTLC analysis was performed on silica gel 60 F254, 20.0 X 10.0 cm HPTLC plates, with Toluen: Ethyl acetate: Formic Acid: Methanol (3:4:0.8:0.7 v/v) as a mobile phase. The antibacterial and antifungal activities were assessed in vitro by the method of macrodillution and solid medium agar diffusion. TLC analysis showed many spots which suggest that both of the plants extracts contain various secondary metabolites. HPTLC revealed the presence of Quercetine, cafeic acid and vanilic acid in the plants’ extract. M. charantia extracts have shown the largest inhibition diameters (25.00±0.00 mm) and inhibit more strains than M. lucida extracts. From all the tested strains, only P. aeruginosa was the most sensitive to M. charantia extracts with 50% bactericidal effect.
Key words: Phytochemical screening, antimicrobial activity, Momordica charantia and Morinda lucida, Benin.
Plants have been for centuries the source of molecules and food for humans and wildlife. One of the surveys conducted by the World Health Organization (WHO) reports that more than 80% of the world’s population is still depending on the traditional medicines for various diseases (Atef et al., 2019). In the same line, most of the West African population lives in the rural areas and depends on natural resources for their own subsistence and for their cash income (Achigan-Dako et al., 2011). Mbuni et al. (2020) also reported that rural dwellers prefer traditional medicines because of their closiness to the traditional healers and the fact that the healers understand their culture and environment as well as their patients. Indeed, these plants are used to treat all kinds of chronic diseases (Petrovska, 2012) among which are infectious diseases.
Today, there are more than 250 types of infections and food poisoning caused by bacteria and fungi (Hernández-Cortez et al., 2017). Of these infections and intoxications, the most frequently isolated pathogenic bacteria are Staphylococci, Pseudomonas, Streptococcus and Escherichia coli (Elisha et al., 2017; Hernández-Cortez et al., 2017). Otherwise, Pallavali et al. (2017) and Bassetti et al. (2018) reported that Gram-positive cocci such as Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus spp. and Gram-negative bacilli such as E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Proteus species are the most common pathogenic bacteria isolated from wound infections and are also an important cause of wound infections in diabetic individuals and infected wound following surgeries. These bacteria are also mostly responsible for toxins production. Apart from bacterial toxins, mycotoxins are fungal secondary metabolites that can cause the serious infection (Benedict et al., 2016). Indeed, fungal diseases are severe and have very high morbidity as well as up to 60% mortality for patients diagnosed with invasive fungal infection (Staniszewska, 2020). It is the case of Candida albicans with many virulence factors implicated in the invasive diseases, that have become common of human infections worldwide (Nouraei et al., 2020; Köhler et al., 2020).
Indeed, the treatment of infections due to bacteria and phytopathogens requires the use of several methods; the best known are the treatments with synthetic products, which is not without consequences on the environment and human health. Nowadays, there is a phenomenon of resistance of bacteria and fungi to most conventional antibiotics. Antibiotic resistance among bacterial or fungal strains strains is a serious situation. It may be so rapid that the effectiveness of common antibiotics may be lost within a span of 5 years due to genetic changes (Chandra et al., 2017). It therefore seems important to explore other alternative for fighting infectious diseases. An alternative is the use of medicinal plants. Many studies show that medicinal plants contain many biologically active secondary metabolites such as tannins (Chokki et al., 2020), terpenoid (Frezza et al., 2019), alkaloids (Vanderplanck and Glauser, 2018), glycosides (Pertuit et al., 2018), flavonoids, phenols (Frezza et al.,2019; Chokki et al., 2020) and other compounds which display various pharmacological activities: antioxidant, anti-inflammatory, anti-allergic, anti-cancer, analgesic, anti-diabetic, antibacterial, antifungal, antiviral activities (Ksouri et al., 2007; Forni et al., 2019; Senhaji et al., 2020). In the socio-economic and health context of developing countries, including Benin, the study of plants can lead to obtaining adequate and low-cost therapeutic responses, with proven scientific efficacy and optimal cultural acceptability.
Momordica charantia and Morinda lucida are frequently used in Benin traditional pharmacopiea against various diseases. Morinda lucida Benth., belonging to the family Rubiaceae is a tropical rainforest tree. It is known as xwesué (in Benin) and is one of the most used plants in the preparation of traditional medicines against fever (Lawal et al., 2012). The leaves are used as “oral teas”, which are usually taken orally for the traditional treatment of malaria, and as a general febrifuge, analgesic, laxative and anti-infections (Adeyemi et al., 2014). M. charantia (bitter melon) is a tropical and subtropical vine of the family cucurbitaceous widely grown in India, South Asia, China, Africa (Kubola and Siriamornpun, 2008) and particularly in Benin. Leaf aqueous macerate is used without combination with other plants in the treatment of microbial and viral infections (measles virus). Considering the vast potentiality of plants as sources for antimicrobial drugs, the present research aims to carry out preliminary phytochemical screening and evaluates antimicrobial activity of M. charantia and M. lucida leaf extracts.
Plant material
M. lucida leaf samples were collected from Agata (06°30'28''N, 002°38'44''E), which is located in the Department of Oueme, Benin, while those of M. charantia were collected from Dangbo (06°35'19''N, 002°33'15''E) located in the same department. A voucher specimens No. AAC8100/HNB and No. AAC8101/HNB respectively for M. lucida and M. charantia were deposited at the Benin national herbarium, University of Abomey-Calavi, Cotonou, Benin. All samples were collected in the morning at 7 am. They were air-dried (23°±2°C) for 15 days before powdered using grinder Retsch type SM 2000/1430/Upm/Smf, Haan Germany.
Preliminary screening
The preliminary phytochemical profiling of leaf powders of M. charantia and M. lucida to determine the major constituents (nitrogenous, polyphenolic, terpenic compound and glycosides) was done using a qualitative analysis based on coloring reactions and/or precipitation described by Senhaji et al. (2020).
Preparation of plant extracts
The extracts were prepared with 9 polar solvents [water, water-ethanol 30:70 (v / v) methanol, methanol-HCl 1%, ethanol, acetone, ethyl acetate, dichloromethane] and 2 non-polar solvents (chloroform and petroleum ether). For the polar solvents, 1 g of powder in 100 mL of solvent was subjected to ultrasonication (35 Hz) at room temperature for 2 h. The same operation was carried out with non-polar solvents under reflux system. A total of 24 extracts were thus obtained, 12 per plant. In addition, the residues obtained after the ethyl acetate and petroleum ether extractions were extracted again using methanol and methanol/1% HCl. These extracts are coded Methanol-EA and Methanol/HCl-PE, respectively. Each mixture was filtered through Whatman N° 1 paper (125 mm ø, Cat No. 1001 125) and concentrated under reduced pressure using a rotary evaporator before being oven dried at 40°C. The aqueous extract was lyophilized to dryness. The extraction yields were determined by the ratio between the mass of powder and extract obtained.
Thin layer chromatography (TLC) analysis
TLC of the two plants extracts was carried out using pre-coated silica gel and alumina plate (TLC-grade ; Merck 20 20 cm, 0.2 mm thikness). Each extract was dissolved in the extraction solvent at a concentration of 1 mg/ml and about 2µl of this solution was applied 1 cm from the base of the TLC with cappillar tube. Development of the chromatograms was done in a closed tank in which the atmosphere was saturated with the eluent vapor to separate various constituents of the extract by lining the tank with filter paper wetted with the eluent and dried at the end. Solvent systems used as eluent were (1) toluen-ethyl acetate 9:1, (2) ethyl acetate-formic acid-water 8:1:1, (3) toluen-acetic acid-formic acid 5:4:1, (4) toluen-acetic acid 4:6, (5) toluen-ethyl acetate-formic acid-methanol 3:4:0.8:0.7. TLC spot was visualized under UV light fluorescent at 254 and 366 nm. The best solvent system was used for HPTLC analysis.
High performance thin-layer chromatography (HPTLC)
Chromatography was performed on silica gel 60 F 254, 20.0 X 10.0 cm HPTLC plates manufacturer Merck, with Toluen-Ethyl acetate-Formic Acid-Methanol (3:4:0.8:0.7 v/v) as a mobile phase. The standard (rutin, quercetin, galic acid, tanic acid, cafeic acid, vanilic acid and clorogenic acid) solutions (2.0 µL of 1 mg/mL) were applied to the plates as 7.0 mm bands; samples were applied with CAMAG-Linomat V automated spray on band applicator equipped with a 100 µL syringe and operated with the following settings : band length of 3.0 mm, application rate of 10 s/ µL, migration distance of 80 mm.
Assessment of antimicrobial activity
Organisms and growth conditions
10 reference strains used in this study included Gram+ bacteria (Staphylococcus aureus ATCC 29213, S. epidermidis T22695, M. luteus ATCC 10240, S. oralis, Enterococcus faecalis ATCC 29212), Gram- bacteria (E. coli ATCC 25922, Proteus mirabilis A24974, Proteus vulgaris A25015, Pseudomonas aeruginosa ATCC 27853) and yeast (Candida albicans MHMR). Overnight (18h) cultures were prepared by inoculating 1 mL Muller Hinton broth with 1-2 young colonies of each organism obtained from 24 h-old Muller Hinton Agar cultures. Broths were incubated overnight at 37°C with shaking. Inocula were prepared by diluting overnight cultures in saline to approximately 108 cfu mL−1 for bacteria and 107 cfu mL−1 for C. albicans. These suspensions were further diluted with sterile saline as required.
Antibiogramme
The disc diffusion method described by Trinh et al. (2020) with slight modifications was used to evaluate the effects of the extracts on the strains. Briefly, two to three sterile paper discs (6 mm in diameter) were lodged, under aseptic conditions, on Mueller Hinton agar Petri dish previously flooded with the appropriate inoculum. The discs were aseptically impregnated with 25 µL of plant extract solution (30 mg/mL) and kept for 15 min at room temperature before incubation at 37°C for 24 h. After the incubation period, the dishes were examined for inhibitory zones. Each sample was performed in triplicate.
Determination of Minimal Inhibitory Concentrations (MIC)
The more effective plant extracts, which exhibited antibacterial activity at 30 mg.mL-1, were used to determine their MIC using the macrodilution method described by Dah-Nouvlessounon et al. (2015). Different concentrations of the plant extracts (30, 15, 7.5, 3.75, 1.875, 0.937, 0.468, 0.234, 0.117 and 0.058 mg.mL-1) sterilized through Millipore ï¬lter were prepared separately in screw tubes. To 1 mL of the above concentrations, 1 mL of the bacterial inoculum (106 CFU/mL) was added to obtain 2 mL as a final volume. Culture medium without samples and others without microorganisms were used in the tests as control. Tubes were incubated at 37°C for 18–24 h and growth was evaluated using turbidity measurements. The MIC is the lowest concentration of the compound at which the tested microorganism does not demonstrate visible growth (turbidity).
Dertermination of Minimal Bactericidal/Fungicidal Concentrations (MBC/MFC)
Streaks were taken from the MIC to the highest concentration of plant extracts exhibiting invisible growth, subcultured onto a fresh MH agar medium and incubated at 37°C for 18-24 h. The concentration that yielded no bacterial growth on solid medium was considered the MBC or MFC (Farshori et al., 2013).
Statistical anlysis
Antibiogramme experiment was done in double and data obtained were reported as a mean ± standard deviation (SD). The data were analyzed using Graph Pad Prism 7 software. Differences of p <0.05 were considered significant.
Preliminary phytechemical screening
The preliminary phytochemical analysis performed on the two plants revealed the presence of several secondary metabolites (Table 1). It was noted an uneven distribution of these metabolites from one plant to another. Indeed, 78.57% of the studied secondary metabolites were present in M. charantia leaf powder against 71.42% in M. lucida leaf powder.
Thin layer chromatography (TLC) analysis
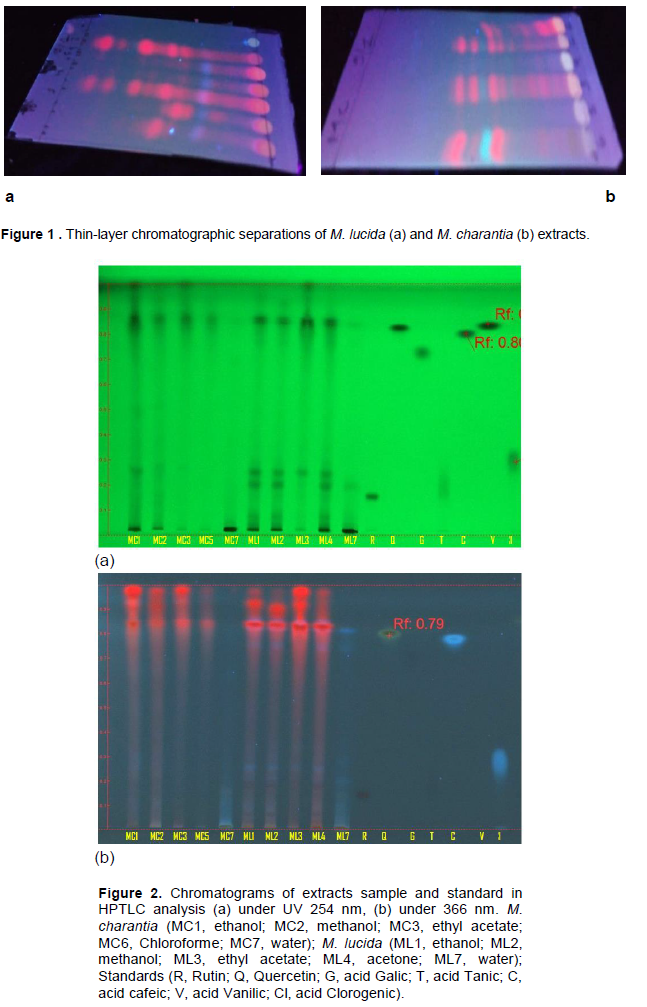
TLC profiling showed the good separation of the metabolites with system solvent : toluen-ethyl acetate-Formic Acid-Methanol (3:4:0.8:0.7 v/v) used as eluent. As shown in Figure 1, the TLC profiling showed the presence of various components in both plant extracts. After the observations made on the plants powder, the TLC shows the efficiency of the extraction method used to extract the maximum compounds contained in the plants.
HPTLC analysis
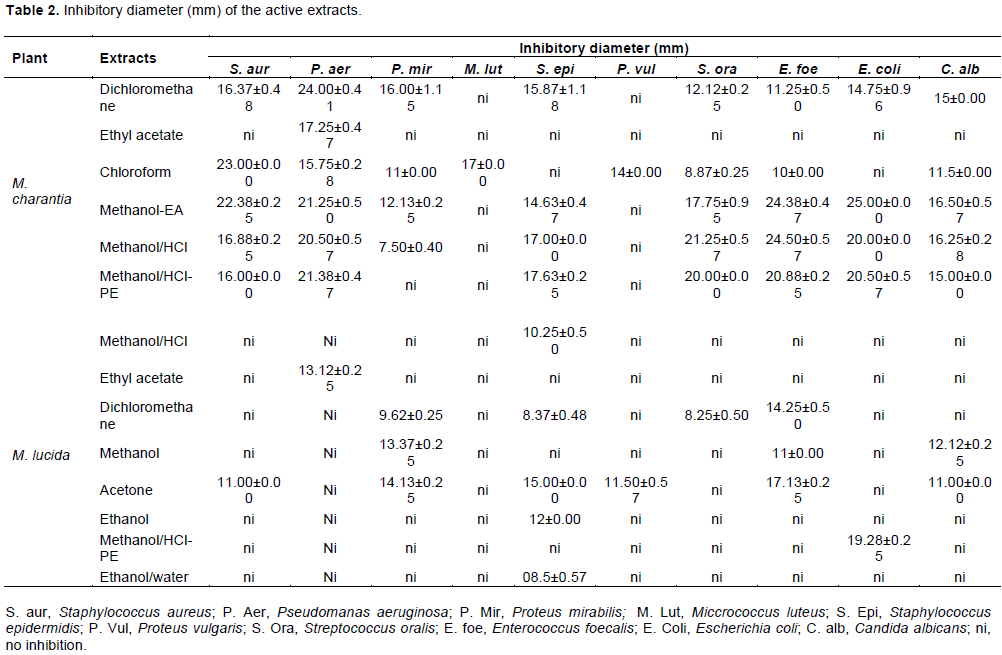
Figure 2 shows the HPTLC profil of phenolics compunds standards and tests extracts. The florescence bands of most of the phenolics compunds are not visible at 366 nm wavelength but they are visible at 254 nm. Netherveless, the chromatograms showed that the compound available are satandard; Quercetine Rf = 0.79 was present in all of the test plant extracts. In addition, cafeic acid (Rf = 0.80) and vanilic acid were found in most of the test extracts.
Antimicrobial activity
Sensibility test
The results of the antibiogram are presented in Table 2. The analyses have determined that 50% of M. charantia extracts inhibit the proliferation of at least one of the tested microorganisms against 66.66% of M. lucida extracts. In addition, among the strains sensitive to extracts from both plants, M. luteus showed more resistance to all active extracts followed by P. vulgaris. Inhibition diameters vary depending on strains and extract types. M. charantia extracts have shown the largest inhibition diameters and inhibit more strains than M. lucida extracts (Table 2).
For M. charantia, the smallest diameter (8.87±0.25 mm) was obtained with chloroform extract on S. oralis, while the largest diameter of inhibition (25.00 ± 0.00 mm) was obtained with methanol-EA extract on E. coli strain. The analysis of variance ANOVA shows a highly significant variation (p <0.0001) by considering the response of the strains in terms of inhibition diameter and also a significant variation (p <0.05) compared to the power of the extracts in terms of the number of inhibited strains. Regarding M. lucida, the smallest inhibition diameter (8.25 ± 0.50 mm) was obtained with the dichloromethane extract on S. oralis strain, while the largest diameter (19.28 ± 0.25 mm) was obtained with the Methanol/HCl-PE extract on E. coli strain.
Minimal inhibitory concentration (MIC)
The MIC of the active extracts of the two plants are determined and presented in Table 3. These concentrations vary according to the extract types.
With M. charantia extracts, the lowest inhibitory concentration (0.117 mg.mL-1) was obtained with the chloroform extract on the S. aureus strain while the highest concentration (7.5 mg/mL-1) was obtained with the dichloromethane extract with P. mirabilis and S. oralis. For each extract type, a significant difference (p < 0.05) was observed between the tested strains. For each strain, however, there is a variation in the threshold of significance. Indeed, for the strains S. epidermidis and E. coli, no difference was observed (p ˃ 0.05).
With M. lucida, the lowest concentration (0.234 mg.mL-1) was obtained with the methanol, acetone and methanol/HCl-PE extracts respectively against P. mirabilis, E. foecalis and E. coli strains. On the other hand, the lowest inhibition (7.5 mg.mL-1) was obtained with the dichloromethane and ethanol/water extracts on S. epidermidis.
Minimal bactericidal concentration (MBC)
Minimal bactericidal concentrations (MBC) of the active extracts of both plants are presented in Table 4. Like MICs, MBCs vary depending on the types of extract. With M. charantia, the lowest concentration (0.234 mg.mL-1) was obtained with Methanol-EA extract against E. coli while the highest concentration obtained is greater than 30 mg.mL-1. This concentration was obtained with the dichloromethane extract on the P. mirabilis strain. The lowest inhibitory concentration (0.468 mg.mL-1) of M. lucida extracts was obtained with the methanol extract. The ratio of the two MIC and MBC parameters showed bactericidal and bacteriostatic effects (Table 4).
Indeed, for M. charantia, the dichloromethane extract has a bactericidal effect on 71.42% of the bacterial strains that are sensitive to it, followed by the chloroform extract which has a bactericidal effect on 42.85% of the sensitive bacteria. In contrast to the ethyl acetate extract which has a bacteriostatic effect, all the other extracts of M. charantia showed a bactericidal effect on at least one sensitive bacterial strain. Of all the tested strains, only P. aeruginosa was the most sensitive to M. charantia extracts with 50% bactericidal effect. On the other hand, P. mirabilis strain was the most resistant to all extracts of M. charantia.
Contrary to the observations made with M. charantia, P. mirabilis is the strain which presented more sensitivity to M. lucida extracts with two bactericidal effects obtained with dichloromethane and methanol extracts. In addition to the ethanolic extract that had a bactericidal effect on the S. epidermidis strain, all other M. lucida extracts had bacteriostatic effects on all susceptible strains. The tested strains showed more resistance to M. lucida extracts than those of M. charantia.
The extracts used in this study were prepared using ultrasonication. The choice of this method is based on the fact that the mechanical effects of ultrasound induce a disruption of the cell walls. This leads to greater intraparticle penetration of the solvent into the cells, thus facilitating the rapid release of their contents and the acceleration of the kinetics extraction (Landoulsi, 2016). The efficiency of cell disruption and mass transfer are the main factors responsible for the good performance of ultrasound extraction (Romdhane, 1993). Ultrasound has the advantage of considerably reducing the extraction time and increasing the extraction yield (Bourgou et al., 2016). The yields obtained during the extraction varied from one plant to another and according to the solvents. Since for the same solvent yields vary according to the plant, while the same amount of plant powder was extracted with the same amount of solvent under the same conditions, the explanation of the difference would be related to the chemical composition of the plants that would not be the same. Phytochemical screening showed that both plants are source of secondary metabolites. The preliminary screening reveals, in nitrogen compunds group, the presence of alkaloïds. This observation was similar to those made by Adomi and Umukoro (2010) in Nigeria. Tanin and flavonoid found in both plants were reported to have antibacterial, anti fungal, antiviral and antioxydant activity (Leelaprakash et al., 2011; Manandhar et al., 2019). Ndam et al. (2014) in Cameroon and Kazeem et al. (2013) in Nigeria have the same observations. Dandawate et al. (2016), De Oliveira et al. (2018) and Khatun et al. (2020) reported that phytochemical screening of M. charantia revealed the presence of secondary metabolite such as alkaloids, flavonoids, , tannins, saponins and terpenoids.
In drug analysis, analytical methods used are diversified and are still being improved to find better solutions in pharmaceutical analysis. In this study, we use also HPTLC technique. HPTLC is an analytical technique based on TLC, but with enhancements intended to increase the resolution of the compounds to be separated and to allow quantitative analysis of the compounds. The usage of HPTLC is well appreciated and accepted all over the world. HPTLC is an ideal screening tool, that not only confirms but also establishes its identity (Wang et al., 2010). A simple and reproducible method using HPTLC was successfully performed for the qualitative and quantitative analysis of medicinal plant (Yadav et al., 2011; Puranik et al., 2010). Therefore, HPTLC analysis showed in our extracts, the presence of some phenolics compunds such as: Quercetine Rf = 0.79 present in all of test plant extracts, cafeic acid (Rf = 0.80) and vanilic acid were found in most of the test extracts. For M. charantia, the same observation was made by Shodehinde et al. (2016) for quercetin and acid cafeic. In the same way, Thiruvengadam et al. (2014) found vanilic acid in M. charantia leaf extracts. Phytochemical studies by several authors have shown the presence of several secondary metabolites in the organs of M. lucida (Owolabi et al., 2014; Adebayo et al., 2020). The diversity of these secondary metabolites at the level of each plant gives it a wide range of biological activities. Indeed, some authors have shown the traditional use of these plants in the treatment of several diseases such as microbial and viral infections, diabetes, malaria, cancer (Ezuruike and Prieto, 2014; Kumar et al., 2010). Therefore, antimicrobial activity of the extracts of these two plants was evaluated in vitro in our study.
The antimicrobial activity has shown that the susceptibility of the microorganisms to tested extracts varies according to the plants and the types of extracts. The variation observed at the plant level is due to the chemical composition of each plant which can be influenced by several factors such as: the soil and pedological conditions that highlight the plant's nutrition (Durand, 2007; Stewart et al., 2001) on which the formation and expression of secondary metabolites depends (Fritz et al., 2006). Moreover, these observations can be explained on the one hand by the conditions and harvesting period of organs. Indeed, some authors (Slimestad and Verheul, 2005; Toor et al., 2006) showed the unequal distribution of secondary metabolites in plant organs between time intervals. These variations are due, among other things, to light and temperature conditions (Riga et al., 2008). Moreover, regarding the extracts, the affinity that the extraction solvents exhibit according to their polarity with the phytomolecule (Bourgou et al., 2016) would be the basis of the intrinsic difference (for the same plant) observed. The extracts inhibited the proliferation of microorganisms tested with inhibition diameters ranging from 7.50±0.40 to 25.00±0.00 mm. The ratio of Minimal Bactericidal Concentrations (MBC) and Inhibition (MIC) according to a previous study (Berche et al., 1991) showed that some extracts have bactericidal effects which shows a good antibacterial activity. The antimicrobial activity observed with these plants is attributed to their chemical composition. In addition, other authors like Naqvi et al. (2020) showed promising antibacterial activities, >18.5 ± 0.21 mm zone-of-inhibition against S. aureus, and 18.4 ± 0.17 mm zone-of-inhibition against Escherichia coli with methanol extract of M. charantia. Ale (2020) reported that the aqueous extracts of different parts of M. Lucida were found to be effective against S. aureus, B. subtilis, E. coli, and P. aeruginosa. In addition, Olawuwo et al. (2020) investigate the in vitro antifungal activity of acetone and aqueous extracts of M. lucida (Rubiaceae) against ATCC strains of Aspergillus fumigatus , A. flavus, C. albicans and Cryptococcus neoformans as well as clinical isolates of A. fumigatus and C. albicans and showed that the minimum inhibitory concentration (MIC) of both extracts against tested organisms ranged from 0.11 to 2.50 mg/ml and 0.03 to 2.50 mg/ml after 48 and 72 h respectively. In the present study, MIC obtained with M. lucida extracts range from 0.468 to 3.75 mg/ml respectively for methanol and acetone extracts. This similitude between the results shows that M. lucida extract has antifungal activity.
From the results obtained, TLC analysis showed many spots that suggest that both plants’ extracts contain many secondary metabolites. HPTLC revealed the presence of Quercetine, cafeic acid and vanilic acid in the plant extract. The presence of those compounds confers on these plants the antimicrobial activity. M. charantia extracts inhibited the proliferation of the test strains than those of M. lucida extracts. The Dichloromethane extract of M. charantia displays more bactericidal effect of references strains than those of M. lucida. The purified extracts of M. charantia and M. lucida can be useful both in food conservation and in human medicine.
The authors have not declared any conflict of interests.
The authors are indebted to Francophony Universitary Agency (AUF) and Romania Government through Eugen Ionescu Mobility Programme and also the University of “Dunarea de Jos” of Galati, Romania.
REFERENCES
|
Achigan-Dako E, N'Danikou S, Assogba-Komlan F, Ambrose-Oji B, Ahanchede A, Pasquini M (2011). Diversity, geographical, and consumption patterns of traditional vegetables in sociolinguistic communities in Benin: Implications for domestication and utilization. Economic Botany 65:129-145.
Crossref
|
|
|
|
Adebayo NS, Abubakar AA, Emmanue AS, Oluwabunmi SB, Ifeoluwa JD, Oladele B (2020). Phyto Chemical Screening and Antiplasmodial Potential of Morinda Lucida (Brimstone Leave) In Infected Mice. The Journal of Middle East and North Africa Sciences 6(06):24-31.
|
|
|
|
|
Adeyemi TOA, Ogboru RO, Idowu OD, Owoeye EA, Isese MO (2014). Phytochemical screening and health potentials of Morinda lucida Benth. International Journal of Innovation and Scientific Research 11(2):515-519.
|
|
|
|
|
Adomi PO, Umukoro GE (2010). Antibacterial activity of aqueous and ethanol crude extracts of the root barks of Alstonia boonei and preliminary phytochemical test of Morinda lucida. Journal of Medicinal Plants Research 4(8):644-648.
|
|
|
|
|
Ale E (2020). Assessment of antioxidant properties of N-Hexane extract of Morinda lucida as a link to its pharmacological actions. Pharmacy and Pharmacology International Journal 8(3):174-178.
|
|
|
|
|
Atef NM, Shanab SM, Negm SI, Abbas YA (2019). Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bulletin of the National Research Centre 43(144):1-11.
Crossref
|
|
|
|
|
Bassetti M, Vena A, Croxatto A, Righi E, Guery B (2018). How to manage Pseudomonas aeruginosa infections. Drugs Context 7:212-227.
Crossref
|
|
|
|
|
Benedict K, Chiller TM, Mody RK (2016). Invasive Fungal Infections Acquired from Contaminated Food or Nutritional Supplements: A Review of the Literature. Foodborne Pathogens and Disease 13(7):343-349.
Crossref
|
|
|
|
|
Berche P, Gaillard JL, Simonet M (1991). Bacteriologie : Les bactéries des infections humaines Paris: Flammarion Médecine-Sciences Publications pp 500-660.
|
|
|
|
|
Bourgou S, Serairi Beji R, Medini F, Ksouri R (2016). Effet du solvant et de la méthode d'extraction sur la teneur en composés phénoliques et les potentialités antioxydantes d'Euphorbia helioscopia. Journal of New Science 28(12):1649-1655.
|
|
|
|
|
Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR (2017). Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials-A Review. Plants (Basel, Switzerland) 6(2):1-16.
Crossref
|
|
|
|
|
Chokki M, Cudălbeanu M, Zongo C, Dah-Nouvlessounon D, Ghinea IO, Furdui B, Raclea R, Savadogo A, Baba-Moussa L, Avamescu SM, Dinica RM, Baba-Moussa F (2020). Exploring Antioxidant and Enzymes (A-Amylase and B-Glucosidase) Inhibitory Activity of Morinda lucida and Momordica charantia Leaves from Benin. Foods 9(434):1-26.
Crossref
|
|
|
|
|
Dah-Nouvlessounon D, Adoukonou-Sagbadja H, Diarrassouba N, Sina H, Adjanohoun A, Inoussa M, Akakpo D, Gbenou JD, Kothoni SO, Dicko MH, Baba-Moussa L (2015). Phytochemical analysis and biological activities of Cola nitida bark. Biochemistry Research International 2015:1-12.
Crossref
|
|
|
|
|
Dandawate PR, Subramaniam D, Padhye SB, Anant S (2016). Bitter melon: a panacea for inflammation and cancer. China Journal of Natural Medicine 14:81-100.
Crossref
|
|
|
|
|
de Oliveira MS, da Costa WA, Bezerra FWS, Araújo ME, Ferreira GC, de Carvalho RNJ (2018). Phytochemical profile and biological activities of Momordica charantia L. (Cucurbitaceae): A review. African Journal of Biotechnology 17(26):829-846.
Crossref
|
|
|
|
|
Durand JL (2007). Les effets du déficit hydrique sur la plante: Aspects physiologiques. Fourrages 190:181-195.
|
|
|
|
|
Elisha IL, Botha FS, McGaw LJ, Eloff JN (2017). The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complementary and Alternative Medicine 17(133):1-10.
Crossref
|
|
|
|
|
Ezuruike UF, Prieto JM (2014). The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. Journal of Ethnopharmacology 155(2):857-924.
Crossref
|
|
|
|
|
Farshori NN, Al-Oqail MM, Al-Sheddi ES, Siddiqui MA, Rauf A (2013). Antimicrobial potentiality of Polyalthia longifolia seed oil against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. African Journal of Microbiology Research 7(19):1977-1982.
Crossref
|
|
|
|
|
Forni C, Facchiano F, Bartoli M, Pieretti S, Facchiano A, D'Arcangelo D, Norelli S, Valle G, Nisini, Beninati S, Tabolacci C, Jadeja RN (2019). Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Research International 2019:1-16.
Crossref
|
|
|
|
|
Frezza C, De Vita D, Spinaci G, Sarandrea M, Venditti A, Bianco A (2019). Secondary metabolites of tilia tomentosa moench inflorescences collected in central Italy: Chemotaxonomy relevance and phytochemical rationale of traditional use. Natural Product Research 12:1-8.
Crossref
|
|
|
|
|
Fritz C, Palacios-Rojas N, Feil R, Stitt M (2006). Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. The Plant Journal 46(4):533-48.
Crossref
|
|
|
|
|
Hernández-Cortez C, Palma-Martínez I, Gonzalez-Avila LU, Guerrero-Mandujano A, Solís RC, Castro-Escarpulli G (2017). Food Poisoning Caused by Bacteria (Food Toxins). Chapter 3 : Poisoning - From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis. 40p.
Crossref
|
|
|
|
|
Kazeem MI, Adamson JO, Ogunwande IA (2013). Modes of Inhibition of -Amylase and -Glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Research International 2013:527-570.
Crossref
|
|
|
|
|
Khatun F, Akhlas MB, Parvin T, Akter A, Torequl IM, Rouf R (2020). Phytochemical and Pharmacological Investigations of Crude Aqueous Extract of Momordica charantia. Toxicology and Applied Pharmacology Insights 3(1):20-26.
Crossref
|
|
|
|
|
Köhler JR, Acosta-Zaldívar M, Qi W (2020). Phosphate in Virulence of Candida albicans and Candida glabrata. Fungi 6(40):1-16.
Crossref
|
|
|
|
|
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007). Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiology and Biochemistry 45(34):244-249.
Crossref
|
|
|
|
|
Kubola J, Siriamornpun S (2008). Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chemistry 110(4):881-890.
Crossref
|
|
|
|
|
Kumar DS, Sharathnath KV, Yogeswaran P, Harani A, Sudhakar K, Sudha P, Banji DA (2010). Medicinal potency of Momordica charantia. International Journal of Pharmaceutical Sciences Review and Research 1(2):95-100.
|
|
|
|
|
Landoulsi A (2016). Etude chimiotaxonomique et activités biologiques des métabolites secondaires des plantes du genre Eryngium Thèse de Doctorat de pharmacie en sciences du médicament et des autres produits de santé Médecine humaine et pathologie Université du Droit et de la Santé, Lille II 216p.
|
|
|
|
|
Lawal HO, Etatuvie SO, Fawehinmi AB (2012). Ethnomedicinal and pharmacological properties of Morinda lucida. Journal of Natural products 5(12):93-99.
|
|
|
|
|
Leelaprakash G, Rose JC, Gowtham BM, Javvaji PK, Prasad SA (2011). In vitro antimicrobial and antioxidant activity of Momordica charantia leaves. Pharmacophore 2(4):244-252.
|
|
|
|
|
Manandhar S, Luitel S, Dahal RK (2019). In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. Journal of Tropical Medicine 2019:1-5.
Crossref
|
|
|
|
|
Mbuni YM., Wang S, Mwangi BN, Mbari NJ, Musili PM, Walter NO, Hu G, Zhou Y, Wang Q (2020). Medicinal Plants and Their Traditional Uses in Local Communities around Cherangani Hills, Western Kenya. Plants 9(331):1-16.
Crossref
|
|
|
|
|
Naqvi SAR, Ali S, Sherazi TA, Haq AU, Saeed M, Sulman M, Rizwan M, Alkahtani S, Abdel-Daim MM (2020). Antioxidant, Antibacterial, and Anticancer Activities of Bitter Gourd Fruit Extracts at Three Different Cultivation Stages. Journal of Chemistry 2020:1-10.
Crossref
|
|
|
|
|
Ndam LM, Mih AM, Fongod AGN, Tening AS, Tonjock RK, Enang JE, Fujii Y (2014). Phytochemical screening of the bioactive compounds in twenty (20) Cameroonian medicinal plants. International Journal of Current Microbiology and Applied Science 3(12):768-778.
|
|
|
|
|
Nouraei H, Sheykhi S, ZareShahrabadi Z, Khodadadi H, Zomorodian K, Pakshir K (2020). Comparative Analysis of Virulence Factors of Homozygous and Heterozygous Strains of Candida albicans Vaginal Isolates. International Journal of Microbiology 2020:1-5.
Crossref
|
|
|
|
|
Olawuwo SO, Aro AO, Kabongo-Kayoka PN, Eloff JN, McGaw LJ (2020). Antifungal and cytotoxic activities of acetone and aqueous extracts of Morinda lucida. FASEB Journal of Pharmacology 34(1).
Crossref
|
|
|
|
|
Owolabi MS, Padilla-Camberos E, Ogundajo AL, Ogunwande IA, Flamini G, Yusuff OK, Allen K, Flores-Fernandez KI, Flores-Fernandez JM (2014). Insecticidal Activity and Chemical Composition of the Morinda lucida Essential Oil against Pulse Beetle Callosobruchus maculatus. Scientific World Journal 2014:1-7.
Crossref
|
|
|
|
|
Pallavali RR, Degati VL, Lomada D, Reddy MC, Durbaka VRP (2017). Isolation and in vitro evaluation of bacteriophages against MDR bacterial isolates from septic wound infections. PLoS ONE 12(7):179-245.
Crossref
|
|
|
|
|
Pertuit D, Mitaine-Offer AC, Miyamoto T, Tanaka C, Delaude C, Lacaille-Dubois MA (2018). Terpenoid glycosides from the root's barks of eriocoelum microspermum radlk. ex engl. Phytochemistry 152:182-190.
Crossref
|
|
|
|
|
Petrovska BB (2012). Historical review of medicinal plants' usage. Pharmacognosy Review 6:1-5.
Crossref
|
|
|
|
|
Puranik M, Bhawsar DV, Rathi P, Yeole PG (2010). Simultaneous determination of ofloxacin and ornidazole in solid dosage form by RP-HPLC and HPTLC techniques. Indian Journal of Pharmaceutical Science 72(4):513-517.
Crossref
|
|
|
|
|
Riga P, Anza M, Garbisu C (2008). Tomato quality is more dependent on temperature than on photosunthetically active radiation. Journal of the Science of Food and Agriculture 88(1):158-166.
Crossref
|
|
|
|
|
Romdhane M (1993). Extraction solide-liquide sous ultrasons: mise en oeuvre d'un capteur de puissance locale ultrasonore Thèse de doctorat en Génie des procédés Université de Toulouse INPT 12 septembre 1993, 228p.
|
|
|
|
|
Senhaji S, Lamchouri F, Toufik H (2020). Phytochemical Content, Antibacterial and Antioxidant Potential of Endemic Plant Anabasis aretioïdes Coss. & Moq. (Chenopodiaceae). BioMed Research International 2020:1-16.
Crossref
|
|
|
|
|
Shodehinde SA, Adefegha SA, Oboh G, Oyeleye SI, Olasehinde TA, Nwanna EE, Adedayo BC, Boligon AA (2016). Phenolic Composition and Evaluation of Methanol and Aqueous Extracts of Bitter Gourd (Momordica charantia L) Leaves on Angiotensin-I-Converting Enzyme and Some Pro-oxidant-Induced Lipid Peroxidation in vitro. Journal of Evidence Based Complementary and Alternative Medicine 21(4):1-10.
Crossref
|
|
|
|
|
Staniszewska M (2020). Virulence Factors in Candida species. Current Protein Peptide Science 21(3):313-323.
Crossref
|
|
|
|
|
Stewart AJ, Chapman W, Jenkins GI, Graham I, Martin T, Crozier A (2001). The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell & Environment 24(11):1189-1197.
Crossref
|
|
|
|
|
Toor RK, Savage GP, Lister CE (2006). Seasonal variations in the antioxidant composition of greenhouse grown tomatoes. Journal of Food Composition and Analysis 19(1):1-10.
Crossref
|
|
|
|
|
Thiruvengadam M, Praveen N, Maria JKM, Yang YS, Kim SH, Chung IM (2014). Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell Tissue Organ Culture 118(3):545-557.
Crossref
|
|
|
|
|
Trinh PC, Thao LTT, Ha HTV, Nguyen T (2020). DPPH-Scavenging and Antimicrobial Activities of Asteraceae Medicinal Plants on Uropathogenic Bacteria. Evidence-Based Complementary and Alternative Medicine 2020:1-9.
Crossref
|
|
|
|
|
Vanderplanck M, Glauser G (2018). Integration of non-targeted metabolomics and automated determination of elemental compositions for comprehensive alkaloid profiling in plants. Phytochemistry 154:1-9.
Crossref
|
|
|
|
|
Wang J, Tang F, Yue Y, Guo X, Yao X (2010). Development and validation of an HPTLC method for simultaneous quantitation of isoorientin isovitexin orientin and vitexin in bamboo-leaf flavonoids. Journal of AOAC International 93(5):1376-1383.
Crossref
|
|
|
|
|
Yadav D, Tiwari N, Gupta MM (2011). Simultaneous quantification of diterpenoids in Premna integrifolia using a validated HPTLC method. Journal of Seperation Science 34:286-291.
Crossref
|
|