ABSTRACT
Phosphorus (P) is the major essential macronutrient of plants. But its availability in Indian soil is relatively low due to high rate of P fixation. The use of phosphate solubilizing microorganism (PSM) to solubilize the fixed form of P is economically reasonable and ecologically safe as compared to chemical phosphatic fertilizers. Fungi have been reported to possess greater ability to solubilize insoluble phosphate than bacteria. However, phosphate solubilizing efficiency of PSM is found to be affected by different environmental factors. The main objective of this work was to optimize different process parameters for the solubilization of rock phosphate (RP) by the phosphate solubilizing fungus, Penicillium purpurogenum Stoll (NFCCI 3788) isolated from paddy field. The concentration of soluble P release by the isolate was tested in Pikovskaya’s broth supplemented with RP. Glucose (169 µg/ml) and sucrose (153 µg/ml) significantly promoted P solubilization as compared to other carbon sources. Ammonium sulphate was found to be optimum for maximum RP solubilization. Change in medium pH and mycelial dry weight were also recorded in all the tested groups. The fungus showed different levels of phosphate solubilization under different NaCl concentration tolerating maximum upto 6% sodium chloride concentration.
Key words: Penicillium purpurogenum Stoll, carbon source, nitrogen source, optimization, rock phosphate, Chhattisgarh.
Phosphorus (P), the second most essential element after nitrogen, is required by the plants for their growth and development. Apart from its abundance in soil in both organic and inorganic forms, the availability of soluble P in the soil is limited, explaining the need for the application of chemical phosphatic fertilizers for adequate plant growth. But, use of chemical fertilizers on a regular basis has become costly affair and environmentally undesirable. Moreover, large fraction of applied P as chemical fertilizers becomes immobile through precipitation reaction with highly reactive Al3+ and Fe3+ ions in acidic soil and Ca2+ in calcareous or normal soil (Gyaneshwar et al., 2002). Natural phosphate rocks have been recognized as a valuable alternative for synthetic P fertilizers. Conventionally, RP is processed through chemical methods that increases fertilizer cost and makes the environment worse (Panda et al., 2008). The involvement of soil microorganisms in dissolution of fixed forms of P and solubilization of RP is well documented by many researchers (Goldstein, 1986; Kucey et al., 1989; Khan et al., 2007; Xiao et al., 2008). These micro-organisms are known as phosphate solubilizing microorganisms (PSM) and include bacteria, fungi and actinomycetes. Several strategies for the solubilization of P are adapted by these organisms, the potential mechanisms either by proton extrusion associated with ammonium assimilation (Roos and Luckener, 1984), or by organic acid production (Cunningham and Kuiack, 1992). The availability of P in soil is regulated by several environmental factors (Hameeda et al., 2008; Srividya et al., 2009), such as nutritional parameters, physiological functions and growth dynamics of the microorganisms (Chen et al., 2006). Therefore, PSMs can be very effective in solubilizing the insoluble phosphate with (Vassilev et al. 1996) or without (Illmer and Schinner 1995) organic acid production. Furthermore, in most studies, ammonium was found to be better nitrogen source than nitrate (Asea et al., 1988) while in some studies, nitrate was reported as better nitrogen source (Relwani et al., 2008). The solubilizing ability has also been influenced by the P source (Nahas, 1996). These findings suggest that phosphate solubilization was affected by various carbon and nitrogen sources. Moreover, the performance of PSM has also been found to be severely affected by stressors (Yadav et al., 2010) such as low and high temperature, pH, salinity, etc. These stressors affect the plant physiology and growth and the activity of plant beneficial microbes including PSM (Mussarat and Khan, 2014). Thus, it seems to be important to evaluate the effect of different factors on solubilization of P by the fungus. The main objective of this work was to examine the effect of carbon and nitrogen sources, RP concentration and salinity on fungal growth and solubilization of RP by Penicillium purpurogenum Stoll.
Isolation of fungi
Fungal strain was isolated by serial dilution agar plating method on potato dextrose agar (PDA from Hi Media, India) plates from rhizosphere soil (pH 5.61) of paddy plant from Raipur, Chhattisgarh, India. The PDA plates were incubated at 28±2°C for five days. Based on distinct morphological characteristics, fungal colony was selected and purified by repeated sub culturing. The pure culture was maintained in PDA slants by sub culturing once in a month.
Screening for phosphate solubilization
Qualitative P solubilization assay
The fungal isolate was subjected to qualitative assay for P solubilization using Pikovskaya’s Agar (PKA from Hi Media, India) medium. For this, spore suspension of 5-day old fungal isolate cultivated on PDA was prepared in normal saline. Optical density of the culture was adjusted to 0.3 using spectrophotometer (Elico SL27) at 520 nm wavelength. 10 µl suspension of the culture was inoculated on PKA plates in triplicates and incubated at 28±2°C for 5 days. A clear halozone around the colony indicated P solubilization and expressed as solubilization index (SI) which in turn was calculated using the following formula (Premono et al., 1996):
Quantitative phosphate solubilization assay
The fungal isolate was further subjected to second step of selection based on the quantitative P solubilization in Pikovskaya’s broth (Hi Media, India) amended with 0.5% of RP/TCP as insoluble P sources. The initial pH of the broth was adjusted to 7.00±0.03 with 1 N HCl and 1 N NaOH using pH meter (Elico LI610). Flasks containing fungal culture were incubated at 28±2°C upto 5th, 7th and 9th days along with uninoculated control. Cultures were harvested after incubation periods by filtering with Whatman no. 42 filter paper in order to record the change in pH and concentration of released phosphorus in the filtrate. Activated charcoal was added in filtrate in order to avoid color produced by the fungal culture. The final pH of the culture filtrate was measured with a pH meter. Soluble phosphate concentration in filtrate was quantified by the vanado-molybdate method (APHA, 1999). It was expressed as µg/ml phosphorus released in culture medium.
Identification of fugal isolate
The fungus was inoculated aseptically on PDA plates and incubated at 28±2°C for five days and assessed for its morphological characteristics. Further, it was characterized through National Fungal Culture Collection (NFCCI), Agharkar Research Institute, Pune, Maharashtra, India.
Effect of different nutritional parameters and salinity on P solubilization
To evaluate the most significant medium constituents affecting phosphate solubilization, three factors viz. carbon, nitrogen and RP concentration were initially chosen to find out the possible best sources. The optimization was performed using one factor at a time (OFAT) to determine optimum conditions. The fungal isolate was inoculated in 250 ml flasks containing 100 ml of Pikovskaya’s broth amended with 0.5 g of RP. To study the effect of carbon sources on the P solubilization, six different carbon sources, that is, glucose, sucrose, fructose, maltose, mannitol, xylose and starch along with control (without carbon source) were used. Each carbon source was added at a concentration of 10.0 g L-1. For selection of most appropriate nitrogen source, six different sources, that is, ammonium sulphate (AS), ammonium chloride (AC), sodium nitrate (SN), potassium nitrate (KN), glycine (GL) and urea (UR) along with control (without nitrogen source) were used. Each nitrogen source was added at a concentration of 0.5 g L-1. The efficacy of RP concentration on P solubilization was studied by varying RP concentrations from 1 to 6%. Flasks containing 0.5% of RP was used as control. The effect of sodium chloride concentration on the P solubilization was performed in seven different concentrations of sodium chloride ranging from 0.2 to 6%. Flasks without sodium chloride were used as control. Initial pH of the medium was adjusted to 7.00±0.03. Flasks were inoculated with 5% v/v spore suspension (2 x 106spores/ml) and incubated at 28±2°C for five days. Final pH of the filtrate and soluble phosphate concentration were estimated as mentioned above. The fungal biomass (mycelial dry weight) was determined by weight loss after drying at 105°C in a hot air oven (Tempo SM 1063) for 48 h (Aneja, 2003).
Analysis
Data (three replicates) were subjected to ANOVA and Duncan multiple range test (0.05 level of probability) using SPSS version 16 software.
Fungal strain
The fungus was identified as Penicillium purpurogenum Stoll (NFCCI 3788) by NFCCI, Pune, Maharashtra, India.
Solubilization assay
A clear halozone around the colony having SI 2.29±0.03 was observed. Also, a significant quantity of soluble P was detected in medium supplemented with RP/TCP. Phosphate solubilization was accompanied by a decline in pH of the broth. The fungal isolate solubilized 188.33, 212.5 and 206.67 µg/ml of P from TCP with decrease in pH from 7.0 to 3.02, 3.21 and 3.39 and 84.17, 106.67 and 100.83 µg/ml of P from RP with decrease in pH 3.51, 4.29 and 5.49 after 5, 7 and 9 days of incubation, respectively (Figure 1). However, solubilization was found to be higher in case of TCP as compared to RP.
Effect of carbon source
The results of optimization using different carbon sources are summarized in Figures 2 and 3. The maximum P solubilization was detected with glucose (169±10.83 µg/ml) followed by sucrose, maltose, xylose, mannitol and fructose, whereas the minimum level of solubilization was recorded with starch (22.5±1.44 µg/ml). Values were significant at the level of p<0.001. A significant (p<0.001) drop in pH from initial value of 7.0 was recorded in all treatments and the value being lowest with mannitol (3.02±0.03). Though high solubilization was observed with glucose, more growth was found with mannitol (0.19±0.01g) and lowest growth was recorded with starch (0.04±0.003 g). In the control (without any added carbon source), solubilization of phosphate was not observed while some growth and pH drop was recorded due to the presence of yeast extract in the culture broth.
Effect of nitrogen source
Conversely, a higher concentration of soluble P was found in the case of the control (189±7.26 µg/ml). This may be due to the presence of yeast extract in the medium which is a source of nitrogen. In the tested groups, maximum solubilization was observed in the presence of ammonium sulphate (105±2.88 µg/ml), whereas lowest level of solubilization was observed with sodium nitrate (10.0±1.44 µg/ml). The values were significant at the level of p<0.001. pH of the culture medium significantly (p< 0.001) declined from initial pH of 7.0. The lowest value of pH was recorded in the case of the control (3.07±0.03) followed by ammonium chloride (4.23±0.06) (Figure 4). Although, higher soluble P concentration was recorded in the case of the control, significantly (p< 0.001) more growth was observed in the presence of potassium nitrate (0.37±0.003g) followed by urea (0.35±0.005 g), whereas lowest value was observed for the control (0.13±0.006g) and sodium nitrate (0.14±0.01g) (Figure 5).
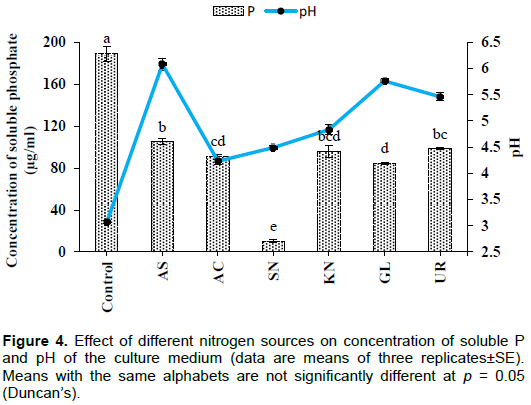
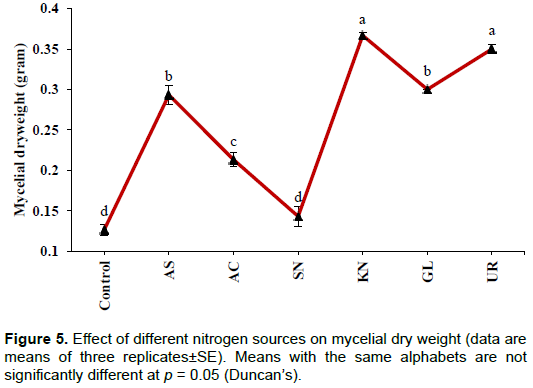
Effect of RP concentration
A continuous decrease in the level of soluble P was observed with successive increase in RP concentration which is significant (p< 0.001) when the amount of RP increased from 1 to 2%. However, the optimum solubilization was observed in the case of control (0.5% RP) and the value was 108±2.20µg/ml. Significant (p< 0.01) drop in pH was observed at 5% (4.15±0.13) and 6% (4.17±0.06) (Figure 6). Significant (p< 0.001) increase in mycelial dry weight was observed with increasing concentration of RP (Figure 7).
Effect of salinity
The concentration of soluble P was found to be significantly (p< 0.001) higher in the case of control (129±5.06 µg/ml) and a subsequent decrease in the concentration of soluble P was observed with the increasing concentration of sodium chloride. Significant (p< 0.001) reduction in medium pH of test groups was recorded as compared to the control. The lowest value of pH was observed at 6% sodium chloride (2.80±0.03) followed by 5% (3.04±0.07) and 3% (3.22±0.04) (Figure 8). Initially an increase in fungal biomass was observed upto 1% sodium chloride concentration and then decreased with the increasing level of sodium chloride (Figure 9) which is significant at the level of p<0.001.
In agreement with several published reports, this study confirms phosphate solubilizing efficiency of a soil fungus. A number of different filamentous fungi have been reported as potential P solubilizers by many researchers and among these fungi, Aspergillus and Penicillium are predominant (Mahamuni et al., 2012; Posada et al., 2013). Penicillium pupurogenum has been reported as phosphate solubilizer by earlier researchers (Scervino et al., 2010; Yadav and Tarafdar, 2011).
The appearance of clear halozone around the colony indicated phosphate solubilization by the fungus which is in accordance with the findings of Gupta et al. (2007). The SI value for the fungus was found to be 2.29. Similarly, Seshadri et al. (2004) reported the SI values for ten different strains of Aspergillus niger that ranged from 1.2-3.4 on the fifth day of incubation. In another study, the SI for P. purpurogenum was found to be 1.3 after four days of incubation (Yasser et al., 2014). The RP was poorly solubilized as compared to TCP. The most likely explanation is that the RP is more complex in structure than TCP. In addition to containing P-Ca, the RP contain other elements such as Mn, Zn, Fe and Cu which certainly affect organic acid production even in low concentration (Gadd, 1999; Mendes et al., 2014). Our data showed reduction in pH of the medium. Several earlier studies have reported that P solubilization is associated with the production of organic acids, which chelate cations through their carboxylic acid group and convert it into soluble form (Kpomblekou and Tabatabai, 1994; Sane and Mehta, 2015). However, with further incubation, phosphate solubilization increased with increase in pH. These results support earlier reports that phosphate solubilization is not necessarily associated with organic acid production (Abd Alla, 1994; Nautiyal et al., 2000). Cause of increase of pH of the medium after a certain period can be explained as the catabolic activity of fungi on organic acid as reported by Nielsen et al. (1994).
Different fungi use different carbon sources, and based on the carbon source, fungi use alternative metabolic pathways to produce organic acids. The process of P solubilization is greatly influenced by the organic acids produced in the medium because it provides a source of protons for solubilization (Gadd, 1999). The organic acids are the product of the microbial metabolism; mostly by oxidative respiration or by fermentation of organic carbon sources (For example, glucose) (Atlas and Bartha, 1997). Such organic acids can either directly dissolve the mineral P as a result of anion exchange or by chelating cations associated with P (Omar, 1998). It has been also reported that the nature of acid produced is more important than the quantity of the acid (Srividya et al., 2009). Glucose and xylose was found to be the best source of energy for the fungi (Rose, 1957). Penicillium citrinum Thom. showed maximum significant P solubilization in the presence of glucose followed by glycerol, maltose and sucrose. Drop in pH was also recorded for all the tested carbon sources. Similar results were reported by many researchers for various other fungi (Yadav et al., 2011; Bhattacharya et al., 2015). The maximum wet fungal biomass of A. niger was observed in the presence of mannitol (Seshadri et al., 2004).
For many fungi, NH4+ driven proton release seems to be the sole mechanism to promote P solubilization (Illmer and Schinner, 1992). Further, Reyes et al. (1999) reported the involvement of the H+ pump mechanism in the solubilization of small amounts of P in Penicillium rugulosum. Ammonium sulphate was found to be the best source of nitrogen for the solubilization of P. Similar results have been highlighted by earlier researchers (Pradhan and Shukla, 2005; Srividya et al., 2009). Increase in the level of soluble P was recorded in medium supplemented with ammonical nitrogen as nitrogen source by Penicillium bilaji (Asea et al., 1988). However, any significant relationship between the level of soluble P and pH drop could not be found. This indicates that acid production is not the only reason for P solubilization. Yeast extract was reported as the best nitrogen source utilized by P. chrysogenum for maximum phosphate solubilization (El-Badry et al., 2015). Though, the higher P solubilization was observed in the case of control, lowest fungal mycelial dry weight was recorded in control as compared to other nitrogen sources. As the amount of fungal biomass continued to increase slowly, the determined phosphate in the solution probably corresponded to the amount which was not consumed by the mycelium (Vassilev et al., 1996). Thus, the nature of carbon and nitrogen source used by the microbes directly influence the organic acid production and thereby the solubilization activity.
The highest level of P solubilization was found at 0.5% RP concentration, the value decreases with increase in RP concentration. A. niger (PSF4) showed highest soluble P content at 1% RP concentration. Also, the greater conversion of RP into soluble P was observed in the presence of lower concentration of RP. A sharp decline in the soluble P content was recorded with increasing concentration of RP (Panda et al., 2008). This may be due to the inhibitory effect of metals like Al, Ca and Fe present in RP. These metals inhibit the growth and activity of fungi or cause change in pH of the medium, which in turn affect P solubilization (Gaur and Sacher, 1980). Xiao et al. (2008) also reported a decrease in soluble P content when concentration of RP increased from 2.5 to 4.0 gL-1 by Penicillium expansum, Mucor ramosissimus and Candida krissi. However, increase in mycelial dry weight was observed with increase in RP concentration. Under limited P availability (when the very high P fixing soils were present), Mortierella sp. tend to accumulate P in its cells instead of releasing it into the growth medium (Osorio et al., 2015).
A simultaneous decrease in the solubilization of P and mycelial dry weight were recorded with increasing oncentration of sodium chloride. Similar finding was reported by many workers (Rosado et al., 1998; Nautiyal, 1999; Kang et al., 2002; Saber et al., 2009). Xiao et al. (2011) tested the P-solubilizing efficiency of A. niger, A. japonicus and Penicillium simplicissium in the presence of 0% to 3.5% NaCl concentration. They observed an increase in the level of soluble P and the growth of fungal spores when the NaCl concentration was increased from 0 to 1.0%. But, a gradual decrease in both the parameters was observed above 1.0%. The reduction in P activity under high salt environment can be explained by the adverse effect of salts in growth and cell proliferation resulting in a loss of solubilization efficiency and sequestration or neutralization of protons or acids by chloride ions resulting in a reduction of solubilization activity. Also, the decrease in microbial population with increasing concentration of NaCl can be attributed to decrease in cytoplasmic water activity of the microbes caused by the exposure of organisms to the conditions of hyper osmolarity (Mussarat and Khan, 2014).
These findings clearly indicated that for higher P solubilization, optimization of process parameter is required. Phosphate solubilization and growth were found to be highly influenced by both carbon and nitrogen sources. Further, solubilization of P was influenced by varying the initial concentration of RP. In addition, a significant P solubilization was also observed in different saline concentration. One factor at a time (OFAT) offers the possible finding of the parameters for optimization process, though it is not of first priority in the examination of the interaction between the variables. The fungal strain Penicillium purpurogenum Stoll could also be helpful in maintaining the available P in saline soil and its application as biofertilizer could be of great advantage in RP amended soil.
The authors have not declared any conflict of interests.
REFERENCES
|
Abd Alla MH, (1994). Phosphatases and the utilization of organic phosphorus by Rhizobium leguminosarum biovar viceae. Lett. Appl. Microbiol. 18:294-296.
Crossref
|
|
|
|
Aneja KR (2003). Experiment in microbiology, plant pathology and biotechnology. 4th ed. New Age International Publisher (P) Limited, New Delhi, India. pp. 213-214.
|
|
|
|
APHA (1999). Standard methods for the examination of water and wastewater. 21th ed. American Public Health Association, Washington, DC, USA.
|
|
|
|
Asea PEA, Kucey RMN, Stewart JWB (1988). Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol. Biochem. 20:459-464.
Crossref
|
|
|
|
Atlas R, Bartha R (1997). Microbial Ecology. Addison Wesley Longman, New York.
|
|
|
|
Bhattacharya S, Das A, Bhardwaj S, Rajan SS (2015). Phosphate solubilizing potential of Aspergillus niger MPF-8 isolated from Muthupettai mangrove. J. Sci. Ind. Res. 74:499-503.
|
|
|
|
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006). Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate abilities. Appl. Soil. Ecol. 34:33-41.
Crossref
|
|
|
|
Cunningham JE, Kuiack C (1992). Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 52:1451-1458.
|
|
|
|
El-Badry MA, Elbarbary TA, Ibrahim IA, Abdel-Fatah YM (2016). Evaluation and optimization of Abu Tartur Egyptian phosphate ore dissolution. Int. J. Innov. Sci. Eng. Technol. 3:386-412.
|
|
|
|
Gadd GM (1999). Fungal production of citric and oxalic acid: important in metal speciation, physiology and biogeochemical processes. Adv. Microbial. Physiol. 41:47-92.
Crossref
|
|
|
|
Gaur AC, Sacher S (1980). Effect of rock phosphate and glucose concentration on phosphate solubilization by Aspergillus awamori. Curr. Sci. 49:553-554.
|
|
|
|
Goldstein AH (1986). Bacterial solubilization of mineral phosphates: historical perspectives and future prospects. Am. J. Altern. Agric. 1:51-57.
Crossref
|
|
|
|
Gupta N, Sabat J Parida R, Kerkatta D (2007). Solubilization of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Bot. Croat. 66:197-204.
|
|
|
|
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002). Role of soil microorganisms in improving phosphrus nutrition of plants. Plant Soil 245:83-93.
Crossref
|
|
|
|
Hameeda B, Harini G, Rupela OP, Wani SP, Reddy G (2008). Growth promotion of maize by phosphate solubilizing bacteria isolated from compost and microfauna. Microbiol. Res. 163:234-242.
Crossref
|
|
|
|
Illmer P, Schinner F (1992). Solubilization of inorganic phosphates by microorganisms isolated from forest soil. Soil Biol. Biochem. 24:389-395.
Crossref
|
|
|
|
Illmer P, Schinner F (1995). Solubilization of inorganic calcium phosphates-solubilization mechanisms. Soil Biol. Biochem. 27:257-263.
Crossref
|
|
|
|
Kang SC, Ha CG, Lee TG, Maheshwari DK (2002). Soluibilization of insoluble inorganic phosphate by a soil-inhabiting fungus Fomitopsis sp. PS 102. Curr. Sci. 82:439-442.
|
|
|
|
Khan MS, Zaidi A, Wani PA (2007). Role of phosphate solubilizing microorganisms in sustainable agriculture- a review. Agron. Sustain. Dev. 27:29-43.
Crossref
|
|
|
|
Kpomblekou K, Tabatabai MA (1994). Effect of organic acids on release of phosphate rocks. Soil Sci. 158:442-432.
Crossref
|
|
|
|
Kucey RMN, Janzen HH, Legget ME (1989). Microbial mediated increases in plant available phosphorus. Adv. Agron. 42:199-288.
Crossref
|
|
|
|
Mahamuni SV, Wani PV, Patil AS (2012). Isolation of phosphate solubilizing fungi from rhizosphere of sugarcane and sugar TCP and RP solubilization. Asian J. Biochem. Pharm. Res. 1:237-244.
|
|
|
|
Mendes GO, Freitas ALM, Pereira OL, Silva IR, Vassilev NB, Costa MD (2014). Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann. Microbiol. 64(1):239-249.
Crossref
|
|
|
|
Mussarat J, Khan MS (2014). Factors affecting phosphate solubilizing activity of microbes: current status. In. Khan MS, Zaidi A, Mussarat J (eds) Phosphate solubilizing microorganisms: principles and application of microphos technology. Springer, Switzerland. pp. 63-85.
Crossref
|
|
|
|
Nahas E (1996). Factors determining rock phosphate solubilization by microorganisms isolated from soil. World J. Microbiol. Biotechnol. 12:567-572.
Crossref
|
|
|
|
Nautiyal CS (1999). An efficient microbiological grown medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 170:265-270.
Crossref
|
|
|
|
Nautiyal CS, Bhaduria S, Kumar P, Lal H, Mandal R, Verma D (2000). Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol. Lett. 182:291-296.
Crossref
|
|
|
|
Nielsen J, Johansen CL, Villadsen J (1994). Culture fluorescence measurements during batch and fed-batch cultivation with Penicillium chrysogenum. J. Biotechnol. 38:52-62.
Crossref
|
|
|
|
Omar SA (1998). The role of rock phosphate solubilizing fungi and vesicular arbuscular mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J. Microbiol. Biotecnol. 14:211-219.
Crossref
|
|
|
|
Osorio NW, Habte M, Leon JD (2015). Effectiveness of a rock phosphate solubilizing fungus to increase soil solution phosphate impaired by the soil phosphate sorption capacity. Rev. Fac. Nal. Agr. 68:7627-7636.
Crossref
|
|
|
|
Panda R, Panda SP, Kar RN, Panda CR (2008). Influence of environmental factors and salinity on phosphate solubilization by Aspergillus niger PSF4 from marine sediment. e-planet 9:1-7.
|
|
|
|
Posada RH, Heredia-Abarca G, Sieverding E, De Prager MS (2013). Solubilization of iron and calcium phosphates by soil fungi isolated from coffee plantations. Arch. Agron. Soil Sci. 59:185-196.
Crossref
|
|
|
|
Pradhan N, Shukla LB (2005). Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr. J. Biotechnol. 5:850-854.
|
|
|
|
Premono ME, Moawad AM, Vlek PLG (1996). Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indonesian. J. Crop Sci. 11:13-23.
|
|
|
|
Relwani L, Krishna P, Reddy MS (2008). Effect of carbon and nitrogen sources on phosphate solubilization by a wild-type strain and UV-induced mutants of Aspergillus tubingensis. Curr. Microbiol. 57:401-406.
Crossref
|
|
|
|
Reyes I, Bernier L, Simard RR, Antoun H (1999). Effect of nitrogen source on the solubilization of different inorganic phosphates by an isolate of Penicillium rugulosum and two UV-induced mutants. FEMS Microbiol. Ecol. 28:281-290.
Crossref
|
|
|
|
Roos W, Luckener K (1994). Relationship between proton extrusion and fluxes of ammonium ions and organic acids in Penicillium cyclopium. J. Gen. Microbiol. 130:1007-1014.
|
|
|
|
Rosado AS, de Azevedo FS, da Cruz DW, van Elsasand JD, Seldin L (1998). Phenotypic and genetic diversity of Paenibacillus azotofixans strains isolated from the rhizoplane or rhizosphere soil of different grasses. J. Appl. Microbiol. 84:216-226.
Crossref
|
|
|
|
Rose RE (1957). Techniques for determining the effect of microorganisms on insoluble inorganic phosphates. NZ J. Sci. Technol. 38:773-780.
|
|
|
|
Saber WIA, Ghanem KM, El Hersh MS (2009). Rock phosphate solubilization by two isolates of Aspergillus niger and Penicillium sp. and their promotion to mungbean plants. Res. J. Microbiol. 4:235-250.
Crossref
|
|
|
|
Sane SA, Mehta SK (2015). Isolation and evaluation of rock phosphate solubilizing fungi as potential biofertilizer. J. Biofertil. Biopestici. 6:156.
Crossref
|
|
|
|
Scervino JM, Mesa MP, Monica ID, Recchi M, Moreno NS, Godeas A (2010). Soil fungi isolates produced different organic acid patterns involved in phosphate salts solubilization. Biol. Fertil. Soils 46:755-763.
Crossref
|
|
|
|
Seshadri S, Ignacimuthu S, Lakshminarasimhan C (2004). Effect of nitrogen and carbon sources on the inorganic phosphate solubilization by different Aspergillus niger strains. Chem. Eng. Commun. 191:1043-1052.
Crossref
|
|
|
|
Srividya S, Soumya S, Pooja K (2009). Influence of environmental factors and salinity on phosphate solubilization by a newly isolated Aspergillus niger F7 from agricultural soil. Afr. J. Biotechnol. 8:1864-1870.
|
|
|
|
Vassilev N, Fenice M, Federici F (1996). Rock phosphate solubilization with gluconic acid produced by immobilized Penicillium variable P16. Biotechnol. Tech. 10:585-588.
Crossref
|
|
|
|
Xiao C, Chi R, Li X, Xia M, Xia Z (2011). Biosolubilization of rock phosphate by three stress tolerant fungal strains. Appl. Biochem. Biotechnol. 165:719-727.
Crossref
|
|
|
|
Xiao CQ, Chib RA, Huang XH Zhang WX, Qiu GZ, Wang DZ (2008). Optimization of rock phosphate solubilization by phosphate-solubilizing fungi isolated from phosphate mines. Ecol. Eng. 33:187-193.
Crossref
|
|
|
|
Yadav BK, Tarafdar JC (2011). Penicillium purpurogenum a unique P mobilize in arid agro-ecosystems. Arid Land Res. Manage. 25:87-99.
Crossref
|
|
|
|
Yadav J, Verma JP, Tiwari KN (2011). Solubilization of tricalcium phosphate by fungus Aspergillus niger at different carbon source and salinity. Trends Appl. Sci. Res. 6:606-613.
Crossref
|
|
|
|
Yadav J, Verma JP, Yadav SK, Tiwari KN (2010). Effect of salt concentration and pH on soil inhabiting fungus Penicillium citrinum Thom. for solubilization of tricalcium phosphate. Microbiol. J. 72:625-630.
|
|
|
|
Yasser MM, Mousa AS, Massoud ON, Nasr SH (2014). Solubilization of inorganic phosphate by phosphate solubilizing fungi isolated from Egyptian soils. J. Biol. Earth Sci. 4:B83-B90.
|