ABSTRACT
Fermentation of worts with high concentrations of fermentable sugars has become common practice in large clear beer breweries that use high gravity worts containing 16-18% (w/v) dissolved solids to obtain high alcohol contents. Normal and high gravity worts, ranging from 12 to 20% (w/v) initial dissolved solid contents were used in industrial opaque beer brewing and the biochemical characteristics of the brews were determined. Characteristics of the beers varied according to wort gravity. Decreases in dissolved solids and maltose contents were recorded during fermentation for all the brews. Higher residual dissolved solids, ranging from 6.5 to 11% (w/v), were recorded for the high gravity brews as compared to the normal brews, which had 3.5% (w/v) residual dissolved solids. An increase in ethanol levels was recorded during alcoholic fermentation for all the brews and the maximum ethanol levels, recorded after 72 h of fermentation for all the brews, were 3.5 and 6.0% (v/v) for the normal and high gravity beers, respectively. The optimal wort dissolved solids content recorded with regard to ethanol productivity was 18% (w/v). Increases in lactic acid and acetic acid levels were recorded for the brews, reaching maximum levels of 2.25 and 0.48 g/L, respectively, after 96 h of fermentation. No propionic acid was detected in any of the brews. Considering the quality of the beer, there was no settling observed for all the beers at 48 h of aging. Viscosity values, ranging from 100 to 190 mPa.s were higher than the expected levels for the industrial opaque beer (60-80 mPa.s) and values recorded for head were within the expected range for the industrial opaque beer (3 to 4).
Key words: High gravity, opaque beer, dissolved solids, ethanol.
Beer is a product of alcoholic fermentation of worts obtained from malted cereals, unmalted cereals and sugar sources (adjuncts) (Mathias et al., 2014). The production of opaque beer on an industrial scale in Southern African countries such as South Africa and Zimbabwe is well described and documented (Steinkraus, 1996; Togo et al., 2002). Chibuku is a popular industrialised opaque beer in Zimbabwe (Kutyauripo et al., 2009). The process of brewing of Zimbabwean industrial opaque beer begins with the cooking of the unmalted cereal adjunct, together with commercial lactic acid for souring. Mashing is carried out by incubating the cooked adjunct with malt, then straining is carried out. The mixture is then heated for pasteurisation, cooled and more malt is added for the second conversion to produce the final brewing wort. The mixture is left to cool, after which active dried yeast is added to commence the alcoholic fermentation. The beer is left to cool to between 26 and 28°C and at this stage it is ready for consumption. The industrial process makes use of Saccharomyces cerevisiae yeast for alcoholic fermentation (Kutyauripo et al., 2009).
Wort composition is extremely important in determining microbial activity and final beer quality (Mathias et al., 2014). Traditional brewing of clear beer results in the production of beers of 4-5% (v/v) ethanol from worts of 11 to 12% (w/v) (Casey et al., 1984; Puligundla et al., 2011). High gravity (HG) fermentation technology, which involves the fermentation of media containing normally 16 to 18% (w/v) initial dissolved solids, has been adopted in many clear beer breweries nationwide (Younis and Stewart, 1999; Patkova et al., 2000; Dragone et al., 2007; Mathias et al., 2014). Ethanol levels in the range of 9 to 11% (v/v) can be achieved when HG technology is applied to the brewing of clear beer. The high gravity beers are then diluted to equivalent ethanol levels to the normal beers (Patkova et al., 2000). The advantages of high gravity brewing are well-documented for clear beer production and include considerable saving of process water, efficient utilisation of fermenter space and production of more alcohol per given plant capacity and labour costs (Erten et al., 2007; Dragone et al., 2007; Puligundla et al., 2011). In the brewing of clear beer, high gravity fermentation technology has proved to be a technique that allows for high utilisation of raw materials and equipment while maintaining the quality of the beer. Disadvantages of HG technology reported for clear beer brewing include longer fermentation times to allow efficient fermentable substrate use, decreased foam stability and possible different flavour characteristics from normal gravity fermentations (Fernandez et al., 1985; Younis and Stewart, 1999; Erten et al., 2007; Dragone et al., 2007). While HG technology has many advantages, most research has mainly been directed to its application in clear beer. Our previous work reported the application of HG technology to opaque beer following the traditional procedure used in rural homes (Bvochora and Zvauya, 2001). Results of our previous studies showed that the application of HG fermentation technology to the brewing of traditional opaque beer resulted in the production of 4.74% (v/v) ethanol, higher than the 2.66% (v/v) recorded under normal fermentation conditions (Bvochora and Zvauya, 2001). To the best of our knowledge, there are no published reports on the application of HG technology to the brewing of industrial opaque beer. Potentially, the technique can be applied to industrial opaque beer brewing and improve the productivity of fermentation. The aim of this study therefore was to determine the biochemical characteristics of industrial opaque beer brewed using high gravity fermentation technology.
The work was carried out at an opaque beer brewery in Harare, Zimbabwe. The yeast used in this study was S. cerevisiae, strain Y2/49, obtained from an opaque beer brewery in Harare, Zimbabwe. The sucrose used in the study was household sucrose obtained from a sugar refinery in Harare, Zimbabwe. The wort used in the study was obtained from an industrial fermentation process at an opaque beer factory.
Preparation of high gravity wort
Samples of wort (4 L) were collected from a 75 000 L brewing tank at 50°C, just before the addition of yeast. The wort samples were left to cool to 35°C. The wort samples were inoculated with dry S. cerevisiae yeast (1.2 g), and varying amounts of sucrose were added, with stirring, to obtain a range of initial dissolved solid contents of 12 (w/v) to 20% (w/v). An Agato N1 Brix sugar refractometer was used to determine the initial dissolved solids contents.
Fermentation and opaque beer sample collection
Fermentations were carried out in 5 L plastic buckets covered with aluminium foil at room temperature (approximately 27°C). After thorough mixing, samples (250 ml) were collected at intervals, filtered through Whatman 1 filter paper and kept at -20°C until required for analysis. All fermentations were carried out in duplicate.
Analysis of opaque beer samples
pH was measured immediately after sample collection, at room temperature (approximately 27°C), using a Mettler Delta pH meter. Dissolved solids contents were measured on the opaque beer supernatant using an Agato N1 Brix sugar refractometer. Opaque beer supernatant was used for analysis to determine sugar, ethanol and organic acid levels with fermentation time by HPLC analysis. HPLC analysis was carried out on a Shimadzu HPLC using an Aminex HPX 87H column, with column temperature 65°C, flow rate 0.6 ml/min and a sample injection volume of 5 µl. H2SO4 (4 mM) was used as the mobile phase. A refractive index detector was used at a wavelength of 215 nm. Samples were filtered through 0.45 µm syringe filters before HPLC analysis. A standard mixture was prepared from 0.1% v/v concentrations of formic acid, lactic acid, propionic acid and butyric acid and was run together with the beer samples to determine organic acid concentrations in the beers.
Beer quality determinants such as viscosity, head and settling were determined. A sample of opaque beer (250 ml) was placed in a plastic container and the viscosity of the beer was measured using a Brookfield Dial Viscometer at room temperature (approximately 27°C), according to the instructions of the manufacturer. The head of the beer was measured by placing 200 ml of opaque beer in a 500 ml glass measuring cylinder and recording the volume occupied by the head, which is the foam of the beer. Head was expressed as a percentage of the beer volume. To determine the settling of the solids in the beer, an opaque beer sample (200 ml) was placed in a 500 ml glass measuring cylinder and left to stand for 30 min. The amount of liquid above the settled solids of the opaque beer was noted and expressed as a percentage of the beer volume. The higher the amount of liquid recorded, the higher the settling value of the beer.
The results presented in this section refer to biochemical changes occurring during the alcoholic fermentation stage of industrial opaque beer brewing. All results are means of duplicate determinations. Where no error bars are visible, standard deviation = 0.
Changes in pH
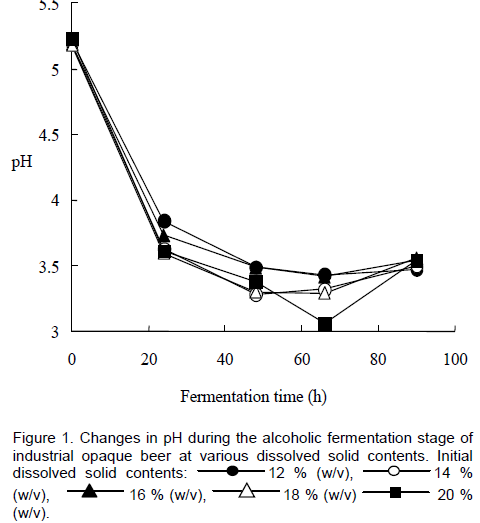
A general decrease in pH was recorded with fermentation time for the brews at all the initial dissolved solid contents used (Figure 1). pH values dropped from an initial maximum of 5.25 to a final minimum of 3.50. There was apparently no relationship between the rate of pH decrease and the initial dissolved solids contents of the wort for the range studied. Thomas and co-workers (2002) reported poor growth of S. cerevisiae as a result of a very rapid decrease of pH to values below 2.5 and that the inhibitory effect of low pH on yeast growth was compounded by the presence of organic acids such as acetic and lactic acid in the medium. Low pH values, below 4.5, may have had inhibitory effects on yeast growth as no buffering system was present in the beers. Raising the external pH places less stress on yeast cells (Thomas et al., 2002).
Changes in dissolved solid contents
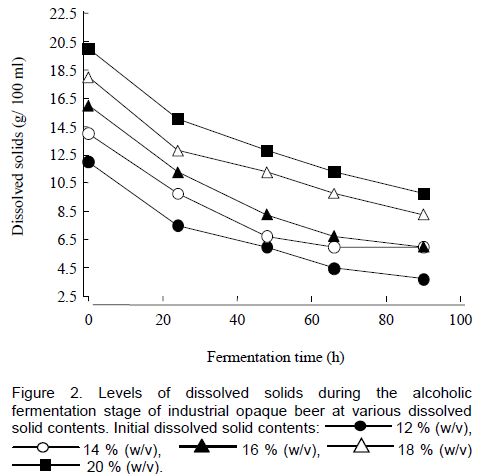
A decrease in dissolved solid contents was recorded during fermentation for all the brews due to the use of sugars during fermentation (Figure 2). For all the fermentations, there were residual dissolved solids when fermentation was terminated. Fermentable sugars remained in the wort as a result of incomplete fermentation. Dragone and co-workers (2007) reported similar results when determining the effect of wort original gravity on fermentation performance. Residual dissolved solid contents were higher for the HG brews as compared to the normal brew where an initial dissolved solids content of 12% (w/v) was used. The residual dissolved solids content in the normal Zimbabwean industrial opaque beer is about 3.6% (w/v) (Bvochora and Zvauya, 2001), which is lower than levels obtained in the HG beers, ranging from 6.5 to 11% (w/v). There is therefore, a need to optimize fermentation conditions to allow for maximal use of dissolved solids during fermentation to result in a product of acceptable quality as high levels of residual dissolved solids may result in an unacceptably sweet beer.
Changes in ethanol
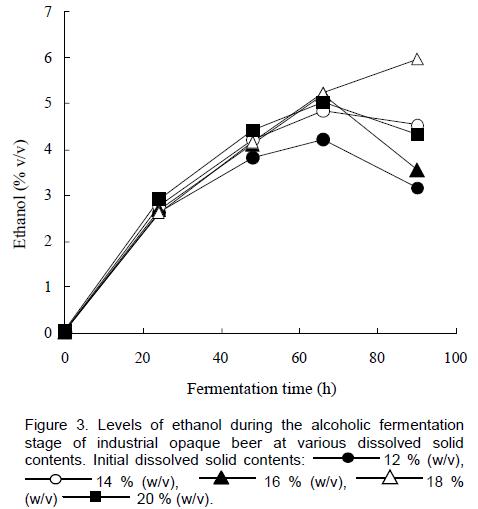
An increase in ethanol levels was recorded during alcoholic fermentation for all the brews and the maximum ethanol level recorded was 6% (v/v) after 96 h of fermentation when an initial dissolved solids content of 18% w/v was used (Figure 3). Under normal fermentation conditions (12% w/v dissolved solids), the maximum ethanol level obtained was 3.5% (v/v) after 72 h. A maximum ethanol level of 4.74% (w/v) was obtained in our previous studies where we applied HG technology to the brewing of traditional opaque beer, at an initial dissolved solids content of 16.89% (w/v) (Bvochora and Zvauya, 2001). Patkova and co-workers (2000) reported ethanol levels of about 9% (v/v) at an initial dissolved solids content of 20°Plato, after about 8 days of fermentation when HG technology was applied to clear beer brewing. There may be a need to increase yeast pitching levels proportionally to the increase in wort gravity and to optimize temperature conditions, as well as increase nutritional conditions if higher levels of ethanol are to be attained. Increased levels of new yeast cell mass synthesis have been reported when a nitrogen source is added to very high gravity worts (Casey et al., 1984; Betite et al., 2012; Mathias et al., 2014).
Changes in organic acid contents
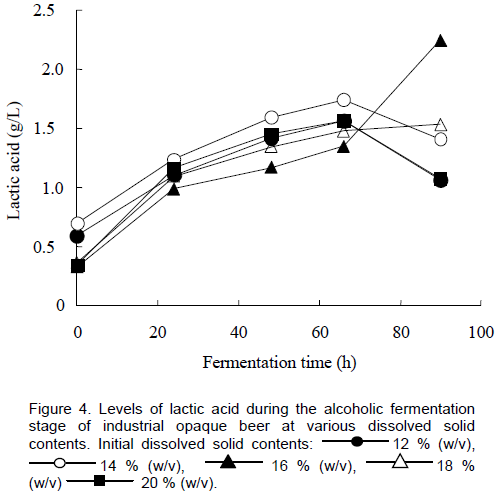
An increase in lactic acid was recorded during the alcoholic fermentation for all the brews as proliferation of lactic acid bacteria occurred (Figure 4). Despite the addition of commercially prepared lactic acid during wort preparation, spontaneous lactic acid fermentation has been demon-strated to occur during the later stages of Chibuku brewing (Togo et al., 2002). There were no apparent differences in the rate of increase of the lactic acid for the brews at varying initial dissolved solid contents. An increase in acetic acid was also recorded, reaching a maximum level of 0.48 g/L after 96 h of fermentation at an initial dissolved solids content of 18% (w/v) (Figure 5). There was no acetic acid detected in the first 20 h for the brews with initial dissolved solid contents of 18 and 20 % (w/v). Thomas and co-workers (2002) reported that acetic acid (167 mM) and lactic acid (548 mM) completely inhibited growth of S. cerevisiae both in minimal medium and nutritionally supplemented media. However, the yeast grew when the pH of the medium containing acetic acid or lactic acid was adjusted to 4.5. Based on the report by Thomas et al. (2002), acetic and lactic acid levels may have been inhibitory to yeast growth in the beers in this study. Lactic acid levels above 0.5% (v/v) have been reported to be undesirable in Chibuku (Kutyauripo et al., 2009). No propionic acid was detected by HPLC analysis. Propionic acid imparts off flavours in beers. Organic acid contents are useful as an indicator of spoilage and acceptability of opaque beer (Kutyauripo et al., 2009).
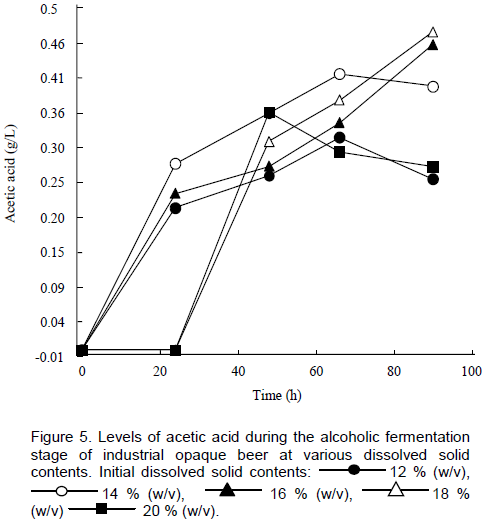
S cerevisiae yeasts, under aerobic conditions, can use ethanol and short-chain organic acids, such as acetic acid and lactic acid, as carbon sources, which may explain the slight decreases in ethanol and lactic acids in some beers after 72 h fermentation (Thomas et al., 2002).
Changes in maltose
A general decrease in maltose was recorded during the alcoholic fermentation for all the brews due to the use of the sugars by fermenting microbes (Figure 6). Constant levels of maltose were recorded during the first 24 and 48 h of fermentation for brews with initial dissolved solids contents of 18 (w/v) and 20% (w/v), respectively. This may have been due to either the high levels of sugars initially present inhibiting yeast fermentation or the need to induce maltase enzyme under the conditions. Maltose levels were negligible for brews with initial dissolved solid levels of 12and 14 % (w/v) at 96 h of fermentation.
Determination of beer quality parameters
Considering the quality of the beer, there was no settling observed for all the beers. Settling is an undesirable characteristic in opaque beer brewing. Viscosity values were higher than the expected levels for the industrial beer and values recorded for head were within the expected range (Table 1). While our values for head were within the expected range, other workers have reported that HG fermentation results in a reduction in the formation and stability of head in beer due to greater losses of hydrophobic proteins in the beers (Cooper et al., 1998; Patkova et al., 2000). Different brews of opaque beer will vary in product quality depending on the ratio and quality of raw materials used and initial wort gravity. The need arises to employ a taste panel to determine the quality and acceptability of the beer. To reduce the adverse effects of HG fermentation on the behaviour of yeast, it may be necessary to modify the medium by adding various nutrients such as amino acids, unsaturated fatty acids and sterols (Fernandez et al., 1985).
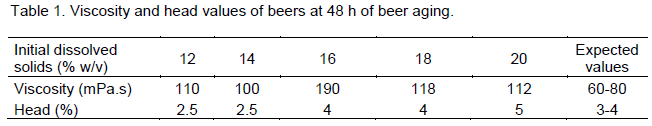
The study showed that higher ethanol levels can be achieved when HG technology is applied to the brewing of industrial opaque beer. However, further research would be necessary to determine suitable substrates for attaining the desired gravities and suitable diluents to produce beers with acceptable qualities and similar ethanol levels with the normal opaque beers. The study forms a basis for further investigation, which includes scaling up the process, carrying out a sensory evaluation of the beers and aiming to produce beers with acceptable properties.
The authors did not declare any conflict of interest.
Financial support for this study was provided by the Swedish Agency for Research Co-operation with Developing countries (SAREC) and the University of Zimbabwe Research Board. We thank Chibuku Breweries, Harare, for providing laboratory facilities. The technical support of Raymond Murimba and Sharai Mashanda is gratefully acknowledged.
REFERENCES
|
Betite VC, Junior MM, de Oliveira JE, Ernades JR (2012). Very high gravity sucrose fermentation by Brazilian industrial yeast strains: effect of nitrogen supplementation. J. Inst. Brew. 118:174-178.
Crossref
|
|
|
|
Bvochora JM, Zvauya R (2001). Biochemical changes occurring during the application of high gravity fermentation technology to the brewing of Zimbabwean traditional opaque beer. Process Biochem. 37: 365-370.
Crossref
|
|
|
|
|
Casey G, Magnus CA, Ingledew WM (1984). High Gravity Brewing: Effects of nutrition on yeast composition, fermentative ability, and alcohol production. Appl. Environ. Microbiol. 48(3): 639-646.
|
|
|
|
|
Cooper DJ, Stewart GG, Bryce JH (1998). Some reasons why high gravity brewing has a negative effect on head retention. J. Inst. Brew. 104: 83-87.
Crossref
|
|
|
|
|
Dragone G, Mussatto SI, Almeida e Siva JB (2007). High gravity brewing by continuous process using immobilised yeast: Effect of wort original gravity on fermentation performance. J. Inst. Brew. 113 (4):391-398.
Crossref
|
|
|
|
|
Erten H, Tanguler H, Cakiroz H (2007). The effect of pitching rate on fermentation and flavour compounds in high gravity brewing. J. Inst. Brew. 113(1):75-79.
Crossref
|
|
|
|
|
Fernandez S, Machuca N, Gonzalez MG, Sierra JA (1985). Accelerated fermentation of high gravity worts and its effect on yeast performance. J. Am. Soc. Brew. Chem. 43:109-113.
|
|
|
|
|
Kutyauripo J, Parawira W, Tinofa S, Kudita I, Ndengu C (2009). Investigation of the shelf-life extension of sorghum beer (Chibuku) by removing the second conversion of malt. Int. J. Food Microbiol. 129:271-276.
Crossref
|
|
|
|
|
Mathias TR, Moretzsohn de Mello PP, Servulo EF (2014). Nitrogen compounds in brewing wort and beer: A review. J. Brew. Distilling 5(2): 10-17.
|
|
|
|
|
Patkova J, Smogrovicova D, Domey Z, Bafrncova P (2000). Very high gravity fermentation by immobilised yeast. Biotechnol. Lett. 22:1173-1177.
Crossref
|
|
|
|
|
Puligundla P, Smogrovicova D, Obulam VSR, Ko S (2011). Very high gravity (VHG) ethanolic brewing and fermentation: a research update. J. Ind. Microbiol. Biotechnol. 38:1133-1144.
Crossref
|
|
|
|
|
Steinkraus KH (1996). Handbook of Indigenous Fermented Foods. 2nd edition, Marcel Dekker, New York.
|
|
|
|
|
Thomas KC, Hynes SH, Ingledew WM (2002). Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl. Environ. Microbiol. 68(4):1616-1623.
Crossref
|
|
|
|
|
Togo CA, Feresu SB, Mutukumira AN (2002). Identification of lactic acid bacteria isolated from opaque beer (Chibuku) for potential use as a starter culture. J. Food Technol. Afr. 7(3):93-97.
Crossref
|
|
|
|
|
Younis OS, Stewart GG (1999). Effect of malt wort, very high gravity malt wort, and very high gravity adjunct on volatile production in Saccharomyces cerevisiae. J. Am. Soc. Brew. Chem. 57:39-45.
|
|