Full Length Research Paper
ABSTRACT
The use of extracts from plants and/or allelopathic compounds from natural products can minimize the use of pesticides in agriculture. This is a chemical study of Machaerium eriocarpum branch extract and the evaluation of the allelopathic potential of Machaerium hirtum (Vell.) Stellfed and M. eriocarpum Benth (Fabaceae) leaves and branches extracts. The triterpene lupeol was isolated from the hydroethanolic extract of M. eriocarpum branches. The quantification of this compound in all extracts was performed by HPLC-APCI-MS/MS and the highest amount of lupeol was observed in the leaves extract of M. eriocarpum. Allelopathy tests were carried out against seeds of Sorghum bicolor L. (sorghum), evaluating the final percentage of germination, germination speed index (GSI), root growth and number of lateral roots. The leaves extracts of both species were effective in the inhibition of the root growth as well as of the lateral roots. The branches extract of M. hirtum showed inhibitory effect in the root growth and lateral roots only in 50 and 100 mg.L-1 doses. The lupeol showed mainly a strong inhibition of seed germination, root growth and lateral root appearance at 100 μM. This study showed the potentiality of Machaerium extracts and lupeol as bioherbicides.
Key words: Allelopathy, lupeol, Machaerium, Fabaceae, Sorghum bicolor, phytoxicity
INTRODUCTION
The continuous practice of agricultural synthetic herbicides can increase human health risks through the non-biodegradable compounds accumulation in the soil and the development of microorganisms resistance (Luz et al., 2010). Finding alternative strategies for weed management is now being focused on to reduce the dependence of farm chemicals by a sustainable agriculture based on plant derived materials, developing environmentally friendly alternatives approach for the weed control (Kuk et al., 2001). Substances produced by plants can offer new and excellent opportunities to diversify the control of invasive plants in agriculture, decreasing or eradicating environmental contamination, preserving natural resources and ensuring the availability of quality agricultural products. Theoretically, natural products and plant extracts with allelopathic activity with phytotoxic effect can be used directly in the formulation of bioherbicides or be modified in order to increase their biological activity (Yamagushi et al., 2011). According to Todero (2017), until now there are thirteen bioherbicides originating from fungi, bacteria and plant, available on the international market. However, bioherbicides show a shorter half-life when compared with synthetic herbicides (Duke et al., 2000; Bailey, 2014; Todero, 2017). Despite its natural origin, it is important to take into account the toxic potential for living beings when produced on a large scale, as well as the environmental impact of this production due to the demand for food for a growing population.
According to Abd-ElGawad et al. (2020) the essential oil of Bassia muricata reduces the germination and seedling development of the weed Chenopodium murale. The aqueous extract of Sphenoclea zeylanica Gaertn. had an inhibitory effect on rice germination and seedling growth at concentrations higher than 50 g.L-1, while completely inhibited rice seed germination at the concentration of 100 g.L-1 (Krumsri et al., 2020). The flavonol quercetin and its derivatives from Fagopyrum esculentum root showed on haustorium induction in Phelipanche ramose at concentration range 0.1 to 0.05 mM (Fernández-Aparicio et al., 2021). The methyl oleate, methyl stearate, methyl palmitate and methyl linoleate inhibited the seed germination of Avena fatua, Chenopodium album, Euphorbia helioscopia, Rumex dentatus and Phalaris minor in a dose-dependent manner (Anwar et al., 2021). The main compound of Glycyrrhiza uralensis Fisch, liquiritin displays inhibitory effects on the growth of lettuce (Yan et al., 2020).
Chou and Waller (1980), caffeine, theobromine, theophylline, paraxanthine, scopoletin, chlorogenic, vanillic, ferulic and p-hydroxybenzoic acids, which are present in the Coffea arabica extract showed allelopathic action on lettuce growth at the concentration of 100 ppm. Jose et al. (2016) described the allelopathic effect of Merostachys riedeliana extract, fractions and phenolic acids on Mimosa bimucronata seeds. The constituents present in the extract of Cinnamomum septentrionale leaves presented potent phytotoxic action on corn growth (Yang et al., 2017); while the naphtoquinones presented potent allelopathic effects on the roots of Echium plantagineum (Zhu et al., 2016). According to De Oliveira et al. (2005) the leaf extract of Machaerium scleroxylon showed 100% of inhibitory effect in the length of the seedlings of Lactuca sativa.
Machaerium is one of the largest tropical arboreal genera of Papilionoideae, with approximately 160 known taxa and popularly known as "jacarandás" (Plant, 2020; Bastos, 1987; Polido and Sartori, 2011). According to the literature, this genus is recognized by the occurrence of different class of natural products such as triterpenes, proanthocyanidins, cinnamylphenols, alkaloids, benzoquinones, steroids and the incisive presence of flavonoids (Ignoato et al., 2013; Kurosawa et al., 1978a, b; Ollis et al., 1978, 1968; Alves et al., 1966; Bento et al., 2018; Gregson et al., 1968; elSohly et al., 1999; Seo et al., 2001; Adrian et al., 2007; Santos et al., 2009; Oliveira et al., 1968; Braga et al., 1968). Based on previous studies involving the allelopathic activity of isovitexin and Machaerium eriocarpum leaf extract, associated with the absence of phytochemical studies of branch extract of this species, in this work the triterpene lupeol was isolated and its quantification was performed by HPLC-APCI-MS/MS and their allelopathic action.
MATERIALS AND METHODS
Plant material
The plant material M. eriocarpum Benth was collected in Porto Murtinho-MS, by A. L. B. Sartori, E. S. S. Lima and F. J. Kochanovski. An exsiccate of the species is deposited in the Herbarium of the Federal University of Mato Grosso do Sul, Campo Grande campus (CGMS411323). The branches and leaves of Machaerium hirtum (Vell.) Stellfeld were collected by Almeida, L. F. R. in Botucatu, São Paulo. The exsiccate is deposited in the Herbarium of the Department of Botany (BOTU 027643), Institute of Bioscience, Sao Paulo State University, Botucatu campus. The data collected was recorded in the SisGen platform (National System of Management of Genetic Heritage and Associated Traditional Knowledge) as genetic patrimony under the registration number A32A22B and A1BB684.
All material was dried at 40°C and ground using a knife mill with an average particle size of 1 to 3 mm.
Extract fractionation and lupeol isolation
Separately, the plant materials of M. eriocarpum and M. hirtum were submitted to the percolation process in 70% ethanol. The material was weighed and swollen in 2 L of 70% (v/v) ethanol. The solvent was evaporated under reduced pressure at a temperature of 40°C and then stored in a sealed amber bottle (Table 1).
The branches extract of M. eriocarpum (1.54 g) was solubilized in 12 mL of methanol/H2O (8:2, v/v) and centrifuged at 10,000 rpm during 10 min. The supernatant was fractionated using a Sephadex LH-20 column (57 cm × 3.0 cm d.i) and eluted with methanol with a flow rate of 4 mL min-1. The 230 subfractions were analyzed by TLC using CHCl3/methanol/H2O 80:18:2 (v/v), which in turn, produced 11 groups of fractions (G1-G11). The G4 fraction group (50 mg) was applied on preparative TLC which was divided in two sides. On the left side, G4 was applied as a line (15 cm) and on the right side as a point using a capillary glass tube. After elution of the chromatographic plate using CHCl3/methanol 98:2 (v/v) as mobile phase, the left side was covered with a glass plate and the right side sprayed with sulfuric anisaldehyde. After sprayed and heated with a thermic pistol the plate showed a pink spot on the right side. Then, a region on the left side with the same retention factor as the pink spot was removed using a spatula and extracted with methanol (subfraction 2). A TLC was made to verify the purity of the subfraction 2 (15 mg).
Clean-up of fractions
The clean-up was performed with all leaves and branches extracts. Solid phase extraction (SPE) C18 cartridges (50 mg) were used. Each cartridge was previously activated in pure methanol and then set to 5.0 mL methanol/H2O (8:2, v/v). The sample was filtered through the cartridge and eluted with 5.0 mL of the same methanol/H2O mixture. The obtained fraction was dried at room temperature and the resulting solid was resuspended in methanol/H2O (8:2, v/v), then filtered through a PTFE filter (0.22 μm) and stored in a glass vial.
Quantification of lupeol contained in the extracts of the branches and leaves
Analysis of lupeol was performed in a HPLC system Thermo Scientific® (Thermo Fischer Scientific Inc., Waltham, MA, USA) consisted of an Accela 600 pump, an autosampler, a PDA detector and a mass spectrometer LTQ XL equipped with an Atmospheric-pressure chemical ionization (APCI) source and a linear ion-trap analyzer. All ionization and chromatographic parameters showed here were specifically optimized for the quantification of lupeol. Source parameters were operated in positive mode and adjusted as follows: capillary temperature at 320°C, vaporizer temperature at 450°C, sheath gas flow 30 (arb), auxiliary gas flow 15 (arb), sweep gas flow 10 (arb), tube lens of 55 V, capillary voltage of 33.0 V and source voltage of 6.00 kV. Lupeol detection was made by single reaction monitoring (SRM), which detected transitions 409>257, 409>203 and 409>191 for confirmation. The parent ion for monitored lupeol was the [M-H2O+H]+ adduct. Such water loss (due to the high temperature required in the APCI source) and product ions have been reported before in the literature (Mo et al., 2013). The product ions utilized were selected after direct injection of lupeol standard, followed by fragmentation in collision-induced dissociation (CID) mode against helium for ion activation at collision energy of 20%, as well as activation time of 30 min/s, in the same source conditions as described earlier. Chromatographic method consisted of A (0.1% formic acid in H2O) and B (0.1% of formic acid in MeOH), in a gradient elution system (92-100%) of B in 6 min followed by a hold of 100% B for 3 min, where the first 0.8 min were discarded, flow of 0.4 mL min-1, injection volume 10 µL, column Waters® Acquity BEH C18 1.7 µm, 2.1 × 50 mm. For the direct infusion the software LTQ Tune Plus, version 2.7.0.1093 was utilized and for LC-MS analysis the Thermo Xcalibur 2.2 SP 1.48 was used.
Quantification was performed using the external standard method and the calibration curve was made with lupeol concentrations of 0.0781, 0.156, 0.313, 0.625, 1.25, 2.50, 5.00 and 10.0 ppm, in triplicate and linear regression. Extracts were injected at a concentration of 100 ppm each, in triplicate. Thus, for the quantification of this compound in the extracts data was obtained by taking into consideration the calibration curve 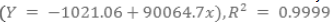 . The detection and quantification limits were determined, respectively as 0.11 μg mL-1 and 0.037 mg mL-1, and then calculated according to the literature (Ribani et al., 2004).
. The detection and quantification limits were determined, respectively as 0.11 μg mL-1 and 0.037 mg mL-1, and then calculated according to the literature (Ribani et al., 2004).
NMR spectroscopy analysis
The data of 1H NMR (500 MHz) and 13C NMR (125 MHz) were obtained on a Bruker Avance III 600 spectrometer using CDCl3 as solvent.
Allelopathy assays
The seeds of Sorghum bicolor L. were commercially acquired and the effects of the extracts and germinating seeds were tested adopting a full randomized design with three repetitions for each treatment and control groups.
The allelopathic potential of leaf and branch extracts of M. eriocarpum and M. hirtum as well as lupeol compound were observed on sorghum (S. bicolor L.) seed germination and seedlings growth. The seeds were previously treated against contamination in 2% sodium hypochlorite solution for 2 min and then washed in distilled water. Stock solutions were prepared for each extract tested, and then diluted for application in assays at the following concentrations: 30, 50, 80, 100 and 1000 g L-1. The lupeol was diluted in the concentration of 100 μM. The treatments were carried out in 9 cm Petri dishes, with 10 sorghum seeds and 10 mL of solution. A control (conventional sorghum without M. eriocarpum or M. hirtum extract) was performed using only deionized water. The treatments were carried out in triplicates. The seeds were kept in a germination chamber programmed with photoperiod of 12/12 h light/dark at 25°C. The number of germinated seeds was counted after 24, 48, 72, 96 and 120 h after application of the treatments. After 120 h the following parameters were analyzed: final germination percentage, germination speed index (GSI), root growth and number of lateral roots. The GSI was obtained with the equation (Al-Mudaris, 1998):
where G1, G2, G3, ..., Gn = number of seedlings computed in the first, second, third, and last count.
N1, N2, N3, ..., Nn = number of days from seeding to first, second, third, and last count.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) with significant differences in p < 0.001.
RESULTS AND DISCUSSION
In order to obtain the extracts, the leaves and branches of M. eriocarpum and M. hirtum were submitted to the extraction process by percolation. The yield of each extract obtained in this process is shown in Table 1. Highest percentage of yield was found in M. hirtum leaf extract (17% w/w), while M. eriocarpum leaf extract was slightly lower (11.6% w/w) (Table 1).
The fractionation of the hydroethanolic extract of M. eriocarpum branches by gel permeation chromatography followed by a preparative thin layer chromatography led to isolation of the lupeol. In the 1H NMR spectrum (300 MHz, CDCl3) two broad singlets, were observed at d 4.65 and 4.55 ppm, referring to the two hydrogens of the terminal double bond (H-29). The double doublet at d 3.18 (J = 5.8 and 8.6 Hz) refers to H-3. In the region between d 0.8 and 1.8 of the spectrum, the presence of the signals related to the methyl and methylene hydrogens were detected. The structural identification was established by 1H NMR and mass spectrometry data analysis compared with the literature (Jácome et al., 2004; Branco and Pizzoletti, 2002; Magalhães et al., 2011).
The lupeol was used as TLC standard to evaluate the presence of this metabolite in the other extracts studied. Using the solvent mixture of chloroform/methanol/water (80:18:2, v/v) as mobile phase, the lupeol presented the RF value of 0.8 and pink coloration after using anisaldehyde as spray reagent. After comparison, the lupeol was detected in all evaluated Machaerium extracts (Wagner and Bladt, 1996).
This triterpene is commonly found in many plants and it was previously identified in the extract of M. hirtum (Ignoato et al., 2013). The literature reports numerous biological activities associated with lupeol, such as glucose absorption, insulin secretion, vascular diabetic dysfunction, nephropathy, retinopathy, as well as anti-inflammatory, antimalarial and antiarthritic (Chaturvedi et al., 2008; Hamid et al., 2015). These data showed the potentiality of this natural product.
Resistance or tolerance to secondary metabolites is a species-specific characteristic, with the most sensitive species being S. bicolor L. (sorghum), Lactuca sativa L. (lettuce), Lycopersicon esculentum Miller (tomato) and Cucumis sativus L. (cucumber), which are considered to be indicative plants of allelopathic activity. According to Wang et al. (2014), the ursolic acid identified in the Alstonia scholaris leaf, impact the growth of neighboring weeds by inhibiting seed germination and radicle growth of Bidens pilosa. Experimental trials have shown phytotoxic effects of lupeol and lupenone applied on the weeds of Mimosa pudica and Senna obtusifolia (Luz et al., 2010). The lupeol completely inhibited the etiolated wheat coleoptile elongation at 10-3 M and the bioactivity was retained upon dilution (Nebo et al., 2015).
The qualitative analyses by HPLC-APCI-MS/MS showed the presence of this compound in all extracts studied. In order to quantify this metabolite in all extracts, the quantitative analysis was performed on lupeol, under analytical conditions using HPLC-APCI-MS/MS (Table 2). The calibration curve was obtained using standard solutions of lupeol in eight different concentrations (0.0781, 0.156, 0.313, 0.625, 1.25, 2.50, 5.00 and 10.0 ppm).
The results showed a higher content of lupeol in M. eriocarpum species and the highest value was observed in the branches extract with 2.31 ppm while in leaves, was 1.51 ppm. Comparatively, a higher lupeol content was detected in the branch extracts, when compared with the leaves in both species studied (Table 2). The obtained results indicate that HPLC-APCI-MS/MS is a rapid and valid method for lupeol quantification.
All extracts of the two Machaerium spp. were submitted to the allelopathy tests with Sorghum bicolor L. seeds for the evaluation of the final percentage of germination, germination speed index (GSI), root growth and number of lateral roots.
All extracts did not show statistical differences compared to the control in the S. bicolor germination seeds (Table 2). The Machaerium branches extract show a significant phytotoxic effect in the three doses (30, 50 and 100 mg L-1) tested during the evaluation of the germination speed index (Table 2). The reduction of the germination speed investigated by IVG indicated the allelochemical activity affected by the mechanisms of stretching and cell division. Even with the reduced germination speed, the final count of germinated seeds does not differ from the control. This process can occur by the activation of cellular detoxification mechanisms that prevents the substances from inhibiting germination through enzymes of oxidative metabolism (Hoagland and Williams, 2004; Martinez et al., 2018).
According to the data obtained in Table 2, the leaves and branches extract of M. hirtum had a inhibitory effect on the growth of the root, when compared with the control. M. hirtum extracts showing inhibitory effects for this parameter only at doses of 50 and 100 mg L-1. An increase in the inhibitory effect on the growth of the root is observed with an increase in the dose of M. eriocarpum leaf extract.
Machaerium leaf extracts show lower values at root growth parameters than branch extracts. The root growth showed more sensibility to the effect of allelochemicals when compared with the germination process. According to Suzuki et al. (2009), the root growth reduction can be one of the first evident effects of allelochemical exhibition probably by the premature lignification of cell walls. The inhibition of root system leads to a reduction in the competitive pressure of the plant favoring neighboring species (Belter and Cahill, 2015; Jacob et al., 2017). All extracts were significantly relevant in the inhibition of lateral root growth at 50 and 100 mg L-1 (Table 2). Only, M. eriocarpum extracts showed inhibitory effect of this parameter in the three doses tested; thus, as the observation of an increase of the inhibitory effect with the dose increment. The inhibitory effect of two M. hirtum extracts in this parameter was restricted to 50 and 100 mg L-1 doses.
The lupeol was evaluated by these parameters only at 100 µM. According to Table 2, this compound showed the GSI value of 55 ± 29 mm, 3.9 ± 0.5 mm for the growth root and 0 ± 0 mm value for lateral roots. The response was effective for the four parameters evaluated against sorghum seed. It is important to emphasize the inhibition of seed germination, root growth and lateral root appearance. The lupeol content in M. eriocarpum extracts (0.23 to 2.31 ppm, Table 2) may have an influence on phytotoxic activities of extracts. At low concentrations it can promote cell growth by increasing the efficiency of enzymes and proteins; but at high concentrations, it can hyperpolarize membranes, altering the functioning of ATP pumps, promoting toxicity to the cells and causing growth reduction (Arora et al., 2015; de Martino et al., 2012). Inhibition of root growth and lateral root development may be directly related to the presence of lupeol, which in turn, may modulate auxin transport by altering membrane polarization patterns, and promoting the cells homeostasis of the root tissue. The stress caused by the presence of allelochemicals can divert the auxin pathway to cell division and become a secondary marker for the expression of stress tolerance genes, reducing root development (Berleth et al., 2007).
CONCLUSION
All extracts had an allelopathic effect on sorghum seeds, mainly in relation to root growth and lateral root growth parameters. The Machaerium leaves extract presents inhibitory effect to root growth and lateral roots parameters. The M. hirtum branches extract shows inhibitory activity at root growth at 50 and 100 mg L-1 doses. Apparently, there is no difference in the inhibitory results obtained for lateral roots. A potent allelopathic effect was observed for lupeol, through the process of seed germination and, therefore, of root growth and appearance of lateral roots. The allelopathic action observed in extracts of the two species studied is directly associated with the content of lupeol associated to the synergistic effect of other natural products present in the extract. Further studies were necessary for the possible application of M. hirtum leaves as bioherbicide, showing the potential sustainable use of Brazilian biodiversity.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abd-ElGawad A, El Gendy AE, El-Amier Y, Gaara A, Omer E, Al-Rowaily S, Assaeed A, Al-Rashed S, Elshamy A (2020). Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi journal of biological sciences 27(7):1900-1906. ISSN 1319-562X, |
|
|
Adrian-Romero M, Blunden G, Patel AV, Armstrong N, Meléndez P, Cuervo AC (2007). Betaines and N-methylprolines from Venezuelan plants. Natural Product Communications, 2(8), 1934578X0700200815. |
|
|
Al-Mudaris MA (1998).Notes on various parameters recording the speed of seed germination. Der Tropenlandwirt-Journal of Agriculture in the Tropics and Subtropics 99(2):147-154. |
|
|
Alves HM, Arndt VH, Ollis WD, Eyton WB, Gottlieb OR, Magalhães MT (1966). Triterpenoids isolated from Machaerium incorruptibile. Phytochemistry 5(6):1327-1330. |
|
|
Arora K, Batish DR, Singh HP, Kohli RK (2015). Allelopathic potential of the essential oil of wild marigold (Tagetes minuta L.) against some invasive weeds. Journal of Environmental and Agricultural Sciences 3:56-60. |
|
|
Bailey KL (2014) The bioherbicide approach to weed control using plant pathogens. In:Abrol, D.P., Ed., Integrated Pest Management: Current Concepts and Ecological Perspectives, Elsevier pp. 245-266. |
|
|
Bastos MNC (1987). Contribuição ao estudo sistemático de algumas espécies do gênero Machaerium Persoon (Leguminoase Papilionoideade) ocorrentes na Amazônia brasileira. Boletim do Museu Paraense Emílio Goeldi 3(2):183-278. |
|
|
Braga A, Gottlieb OR, Eyton WB (1968). Constituents of Machaerium villosum. Anais da Academia Brasileira de Ciências 40 (2):147-150. |
|
|
Belter PR, Cahill JF (2015) Disentangling root system responses to neighbours: Identification of novel root behavioural strategies. AoB Plants 7:1-12. |
|
|
Bento CC, Tangerina MMP, Zanatta AC, Sartori ALB, Franco DM, Hiruma-Lima CA (2018). Allelopathic activity of Machaerium eriocarpum Benth. Natural Product Research 32:1-5. |
|
|
Berleth T, Scarpella E, Prusinkiewicz P (2007). Towards the systems biology of auxin-transport mediated patterning. Trends in Plant Science 12 (4):151-159. |
|
|
Branco A, Pizzolatti MG (2002). CGAR e CGAR-EM na análise dos constituintes químicos isolados do extrato hexanico de Sebastiania argutidens (Euphorbiaceae). Química Nova 25(1):15-19. |
|
|
Chaturvedi PK, Bhui K, Shukla Y (2008). Lupeol: Connotations for chemoprevention. Cancer Letters 263(1):1-13. |
|
|
Chou CH, Waller GR (1980). Possible allelopathic constituents of Coffea arabica. Jourmal of Chemical Ecology 6(3):643-654. |
|
|
De Martino L, Mencherini T, Mancini E, Aquino RP, De Almeida LF, De Feo V (2012). In vitro phytotoxicity and antioxidant activity of selected flavonoids. International Journal of Molecular Sciences 13(5):5406-5419. |
|
|
De Oliveira MN, Mercadante-Simões MO, Ribeiro LM, Lopes PSN, Gusmão E, Dias BAS (2005). Efeitos alelopáticos de seis espécies arbóreas da família Fabaceae. Unimontes científica Montes Claros 7(2):121-128. |
|
|
Duke SO, Dayan FE, Romagni JG, Rimando AM (2000). Natural products as sources of herbicides: current status and future trends. Weed Research (Oxford) 40(1):99-111. |
|
|
ElSohly HN, Joshi AS, Nimrod AC (1999). Antigiardial isoflavones from Machaerium aristulatum. Planta Medica 65(5):490. |
|
|
Fernández-Aparicio M, Masi M, Cimmino A, Vilariño S, Evidente A (2021) Allelopathic Effect of Quercetin, a Flavonoid from Fagopyrum esculentum Roots in the Radicle Growth of Phelipanche ramosa: Quercetin Natural and Semisynthetic Analogues Were Used for a Structure-Activity Relationship Investigation. Plants 10:543. |
|
|
Gregson M, Kurosawa K, Ollis WD, Redman BT, Roberts RJ, Sutherland IO,Dietrichs HH (1968). The natural occurrence of cis-and trans-cinnamylphenols. Journal Chemical Communication 22:1390-1392. |
|
|
Hamid K, Alqahtani A, Kim MS, Cho JL, Cui PH, Li CG, Groundwater PW, Li GQ (2015) Tetracyclic triterpenoids in herbal medicines and their activities in diabetes and its complications. Current Topics in Medicinal Chemistry 15(23):2406-2430. |
|
|
Hoagland RE, Williams RD (2004) Allelopathy: chemistry and mode of action of allelochemicals, in: Macias AF, Galindo, JCG, Molinillo JMG, Cutler HG (Ed.). CRC Press, Boca Raton 372 p. |
|
|
Ignoato MC, Fabrão RM, Schuquel ITA, Botelho MFP, Bannwart G, Pomini AM, Arruda LLM, Bersani-Amado CA, Santin SMO (2013). Chemical constituents of Machaerium hirtum Vell. (Fabaceae) leaves and branches and its anti-inflammatory activity evaluation. Natural Product Research 27(17):1556-1561. |
|
|
Jacob CE, Tozzi E, Willenborg CJ (2017). Neighbour presence, not identity, influences root and shoot allocation in pea. PLoS One 12 (3): 1-12. |
|
|
Jácome, RLRP, de Oliveira AB, Raslan DS, Wagner H (2004). Estudo químico e perfil cromatográfico das cascas de Aspidosperma parvifolium A. DC. ("paupereira"). Química Nova 27(6):897-900. |
|
|
Jose CM, Torres LMB, Torres MAMG, Shirasuna RT, Farias DA, dos Santos NA, Grombone-Guaratini MT (2016). Phytotoxic effects of phenolic acids from Merostachys riedeliana, a native and overabundant Brazilian bamboo. Chemoecology 26(6):235-246. |
|
|
Kuk Y-I, Burgos NR, Talbert RE (2001). Evaluation of rice by-products for weed control. Weed Science 49(1):141-149. |
|
|
Kurosawa K, Ollis WD, Sutherland IO, Gottlieb OR, De Oliveira AB (1978). Mucronustyrene, mucronulastyrene, and villostyrene, cinnamylphenols from Machaerium mucronulatumand and M. Villosum. Phytochemistry 17(8):1389-1394. |
|
|
Kurosawa K, Ollis WD, Sutherland IO, Gottlieb OR, de Oliveira AB (1978). Mucronulatol, mucroquinone and mucronucarpan, isoflavonoids from Machaerium mucronulatum and M. villosum. Phytochemistry 17(8):1405-1411. |
|
|
Kurosawa K, Ollis WD, Redman BT, Sutherland IO,Gottlieb OR (1978). Vestitol and vesticarpan, isoflavonoids from Machaerium vestitum. Phytochemistry 17(8):1413-1415. |
|
|
Luz SM, Souza-Filho APS, Guilohn GMSP, Vilhena KSS (2010). Atividade alelopática de substâncias químicas isoladas da Acacia mangium e suas variações em função do PH. Planta Daninha 28(3):479-487. |
|
|
Magalhães CG, Ferrari FC, Guimarâes DA, Silva GD, Duarte LP, Figueiredo RC (2011). Maytenus salicifolia: triterpenes isolated from stems and antioxidant property of extracts from aerial parts. Revista Brasileira de Farmacognosia 21(3):415-419. |
|
|
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM (2018). Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 23(3):1-20. |
|
|
Mo S, Dong L, Hurst WJ, van Breemen RB (2013). Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. Lipids 48(9):949-956. |
|
|
Nebo L, Varela RM, Molinillo JMG, Severino VGP, Sarria ALF, Cazal CM, Fernandes MFG, Fernandes JB, Macías FA (2015). Phytotoxicity of triterpenes and limonoids from the Rutaceae and Meliaceae. 5α,6β,8α,12α-Tetrahydro-28-norisotoonafolin--a Potent Phytotoxin from Toona ciliata. Natural Product Communications 10(1):17-20. |
|
|
Oliveira AB, Gottlieb OR, Ollis WD (1968). Chemistry of brazilian Leguminosae. XVII. Constituents of Machaerium villosum. Anais da Academia Brasileira de Ciências 40(:147-150. |
|
|
Ollis WD, Redman BT, Roberts RJ, Sutherland IO, Gottlieb OR (1968). New neoflavanoids from Machaerium kuhlmannii and Machaerium nictitans and the recognition of a new neoflavanoid type, the neoflavenes. Chemical Communications (London) (22):1392-1393. |
|
|
Ollis WD, Sutherland IO, Alves HM, Gottlieb OR (1978). Duartin, an isoflavan from Machaerium opacum. Phytochemistry 17(8):1401-1403. |
|
|
Plant List - A Working List of all Plant Species (2020). Version 1.1 |
|
|
Polido CA, Sartori ÂLB (2011). Machaerium (Leguminosae, Papilionoideae, Dalbergieae) nos estados de Mato Grosso e Mato Grosso do Sul, Brasil. Rodriguésia 62(1):107-122. |
|
|
Ramida Krumsri,Kato-Noguchi H, Poonpaiboonpipat T (2020). Allelopathic effect of Sphenoclea zeylanica Gaertn. on rice (Oryza sativa L.) germination and seedling growth. Australian Journal of Crop Science 14(9):1450-1455. ISSN:1835-2707 |
|
|
Ribani M, Grespan Bottoli CB, Collins CH, Fontes-Jardim ICS, Costa-Melo LF (2004). Validação em métodos cromatográficos e eletroforéticos. Química Nova 27(5):771-780. |
|
|
Santos ABD, Silva DHS, Bolzani VDS, Santos LÁ, Schmidt TM, Baffa O (2009). Antioxidant properties of plant extracts: an EPR and DFT comparative study of the reaction with DPPH, TEMPOL and spin trap DMPO. Journal of the Brazilian Chemical Society 20(8):1483-1492. |
|
|
Seo EK, Kim NC, Mi Q, Chai H, Wall ME, Wani MC, Navarro HA, Burgess JP, Graham JG, Cabieses F, Tan GT (2001). Macharistol, a new cytotoxic cinnamylphenol from the stems of Machaerium aristulatum. Journal of Natural Products 64(11):1483-1485. |
|
|
Suzuki LS, Zonetti PC, Ferrarese MLL, Ferrarese-Filho O (2008). Effects of ferulic acid on growth and lignification of conventional and glyphosate-resistant soybean. Allelopathy Journal 21(1):155-164. |
|
|
Tauseef A, Huma Q, Mater H, Mahnashi, FK, Nusrat P, Dawood A, Umara A, Salma B, Muhammad A, Saleh Ahmed A, Hussain AA (2021). Bioherbicidal ability and weed management of allelopathic methyl esters from Lantana camara, Saudi Journal of Biological Sciences 2021. |
|
|
TODERO I (2017). Desenvolvimento de um bioherbicida a partir de metabólitos de Phoma sp. para o manejo de plantas daninhas. Tese de mestrado em engenharia agrícola, Santa Maria, RS pp. 12-18. |
|
|
Wagner H, Bladt S (1996). Plant Drug Analysis: a thin layer chromatography atlas. Berlin, Springer 2:1-2. |
|
|
Wang C-M, Li T-C, Weng J-H, Jhan Y-L, Cho C-H (2014). The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. Journal of Chemical Ecology 40(1):90-98. |
|
|
Yamagushi MQ, Gusman GS, Vestena S (2011). Efeito alelopático de extratos aquosos de Eucalyptus globulus Labill. e de Casearia sylvestris Sw. sobre espécies cultivadas. Semina Ciências Agrarias 32(4):1361-1374. |
|
|
Yang S, Hu H, Hu T, Wang Q, Ye M, Luo J, Peng Y, Zhang R (2017). Chemical constituents of Cinnamomum septentrionale leaf litter and its allelopathic activity on the growth of maize (Zea mays). Natural Product Research 31(11):1314-1317. |
|
|
Zhu X, Skoneczny D, Weidenhamer JD, Mwendwa JM, Weston PA, Gurr GM, Callaway RM, Weston LA (2016) Identification and localization of bioactive naphthoquinones in the roots and rhizosphere of Paterson's curse (Echium plantagineum), a noxious invader. Journal of Experimental Botany 67(12):3777-3788. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0