ABSTRACT
The phytochemical screening of Citharexylum spinosum L. aerial parts resulted in the presence of flavonoids, tannins, carbohydrates and/or glycosides, triterpenes and/or sterols and saponins. The percentage of hydrocarbons and sterols in C. spinosum petroleum ether extract were 99.57 and 0.3%, respectively. In petroleum ether extract, saturated fatty acids (78.76%) and unsaturated fatty acids (9.14%) were found. Chromatographic fractionation of 80% aqueous, methanol and chloroform extracts of C. spinosum resulted in isolation of 10 compounds; β-Sitosterol, β-Sitosterol 3-O-β-D-glucopyranoside, Oleanolic acid, Gallic acid, Quercetin, 6-Methoxy acacetin 7-O-β-D-glucopyranoside, Naringenin, Quercetin 3-O-α-L-rhamnopyranoside (Quercetrin), 1, 2, 6-tri-O-galloyl-β-D-glucopyranoside and Rutin. The antipyretic activity of aqueous methanolic residue using Brewer's yeast-induced pyrexia in rats was significant at dose 300 mg/kg. All tested samples had no analgesic activity. The major isolated compounds were quercetin and quercetrin, their biological activities, antimicrobial and cytotoxic activities, were determined parallel to the extracts. It was found that the aqueous methanolic residue, chloroform extract, quercetin and quercetrin exerted significant antimicrobial activity. From 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay on A2780 human ovarian cell line, quercetrin showed moderate cytotoxic activity, whereas quercetin showed significant cytotoxic activity.
Key words: Citharexylum spinosum, lipoidal matter, phenolics, antipyretic, antimicrobial.
Family Verbenaceae includes about 100 genera and more than 3000 species. Among the largest genera of Verbenaceae is Citharexylum which comprises 115 species (Dahiya, 1979; Starr et al., 2006; Mohammed et al., 2014). Genus Citharexylum was reported to contain triterpenes, sterols, irridoids, lignan glycoside, phenolic and flavonoids. Different species of genus Citharexylum are famous to have antiulcer, antihypertensive, hepatoprotective effects, immunomodulatory, antimicrobial, anti-Schistosomal, antioxidant, nephroprotective, radical scavenging, cytotoxic activities and regulating immediate type of allergic reaction (Khalifa et al., 2002; Ganapaty et al., 2010; Khan and Siddique, 2012; Kadry et al., 2013; Allam, 2014; Mohammed et al., 2016). Among these species is Citharexylum spinosum L. which is a popular ornamental tree in many tropical and subtropical regions and are known as fiddlewood. It has been used in folk medicine as diuretic, antipyretic, antiarthritic and in liver disorders (Lawrence, 1951; Turner and Wasson, 1997; Wagner et al., 1999; Starr et al., 2006).
Plant material
Aerial parts (leaves and stems) of C. spinosum L. were collected from Zoo garden, Giza, Egypt in January, 2014. The plant was identified by Mrs. Terase Labib, senior specialist of plant taxonomy, floral and taxonomy department, El-Orman garden, Giza, Egypt. Voucher specimens are kept in the herbarium of Pharmacognosy Department, Faculty of pharmacy, Helwan University, Cairo, Egypt.
The human ovarian cell line, RPMI-1640 media was supplemented with 10% heat inactivated foetal bovine serum (FBS), L-glutamine and 5% penicillin + streptomycin, MTT: 3-(4,5-Dimethyl
thiazol-2-yl)-2,5-diphenyl
tetrazolium bromide, Paracetamol, Saline (0.9%NaCl) and 20% aqueous suspension of Brewer's yeast in normal saline. All chemicals were from Sigma/Aldrich, USA. Multidrug-resistant strains of
Staphylococcus aureus, Escherichia coli and
Pseudomonas aeuroginosa were selected among clinical isolates obtained from Outpatient Clinics of the Research Institute of Ophthalmology (RIO) while Imipenem and Ciprofloxacin discs were purchased from
Oxoid , England.
Adult albino mice weighing 25 to 30 g and rats weighing 120-130 g of either sex were used in the present study. All animals were kept in a controlled environment of air and temperature with access to water and diet
ad libitum.
Anesthetic procedures and animal handling were in compliance with the ethical guidelines of Medical Ethics Committee of the National Research Centre; Polyamide S6 (50-160 μm, Fluka chemie AG, Switzerland) for column chromatography, Microcrystalline cellulose (E. Merck, Darmstadt, Germany) for column chromatography, Sephadex LH-20 (25-100μm, Pharmacia, Uppsala, Sweden) for column chromatography, Silica gel 60 F254, precoated aluminium sheets (20 x 20, 0.2mm thickness), (E. Merck, Darmstadt, Germany) for thin layer chromatography, Silica gel G 60 for column chromatography (70-230 mesh, 60 Aº, E. Merck, Germany) and Whatman No.1 for paper chromatography (Whatman Ltd., Maidstone, Kent, England). Spraying reagents were done according to common methods (Smith, 1960; Stahl, 1969; Balbaa et al., 1981; Markham, 1982).
NMR spectrometers
1H and 13C NMR spectra (University of Louisiana at Monroe) were recorded at 400 and 100 MHz, respectively, in appropriate deuterated NMR solvent, on a JEOL Eclipse ECS-400 NMR spectrometer (Boston, MA, USA). For analysis and spectral processing, chemical shifts reported δ ppm values relative to TMS using DettaTM NMR Data Processing Software (JEOL Inc, MA, USA). HP 5890 series Gas Chromatograph System with an FID/MS detector, Faculty of Agriculture, Cairo University was used for lipoidal matters analysis. We used UV lamp (Marne La Vallee, VL-215 LC, France) for visualization of spots on paper and thin layer chromatograms to follow up the columns fractionation on columns at 254 and/or 365 nm. Hot plate (Harvard Apparatus, Kent, UK), sterile pipettes and 96 well cell culture microplate were used for pharmacological studies.
Preliminary phytochemical screening
Air dried powdered aerial parts (leaves and stems) of C. Spinosum L. was subjected to preliminary phytochemical screening for its constituents, according to methods mentioned in the references of Trease and Evans (1989), Evans (1996) and the British Pharmacopea (1993).
Preparation and fractionation of lipoidal matter of C .spinosum L. aerial parts
The air-dried powder of C. spinosum L. aerial parts (90 g) was extracted with petroleum ether (b.p. 60 to 80°C) and evaporated to give residue (3 g). This residue was kept for the preparation of unsaponifiable matters (USM) and total fatty acids (TFA) according to previous studies (El-Said and Amer, 1965; British Pharmacopea, 1993). TFA and USM of C. spinosum L. aerial parts were subjected to methylation followed by GC-MS analysis. Tentative identification was carried out by comparison of their Rt-values. The relative concentration of each constituent was calculated based on the peak area integration (Vogel, 1961).
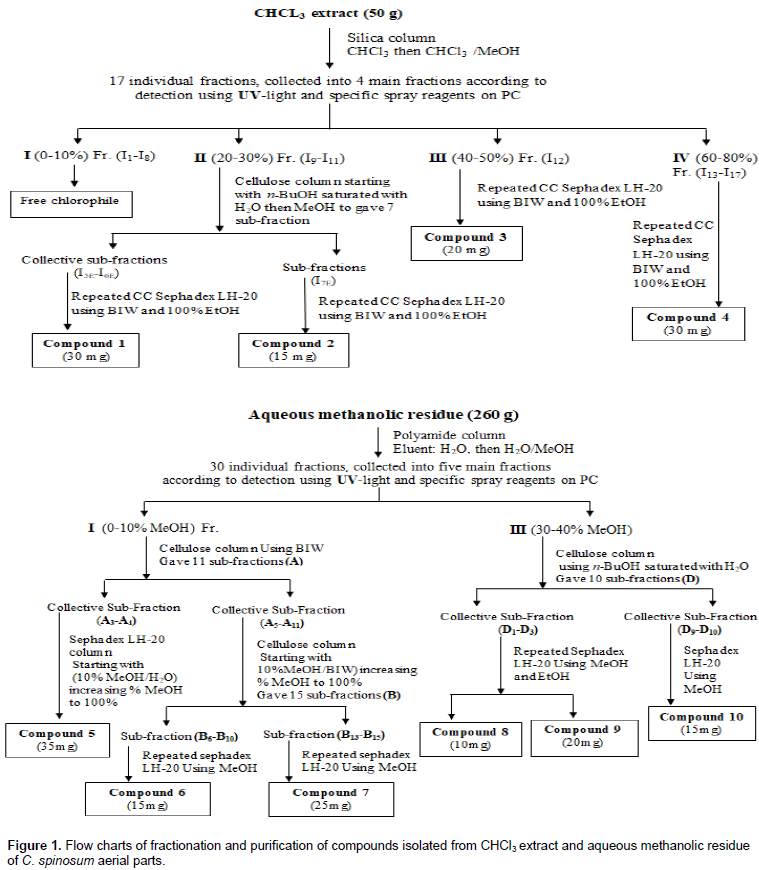
Extraction and purification of active constituents from C. spinosum L. aerial parts
The air-dried ground aerial parts (1350 g) of C. spinosum L. were subjected to exhaustive extraction with hot 80% aqueous methanol under reflux (50°C). The extract was dried under vacuum (50°C) to give dry total extract (360 g). This dry extract was defatted by petroleum ether which resulted in 20 g of dried petroleum ether residue, and 330 g of the remaining residue was successively extracted with chloroform, under reflux at 50οC to yield 50 g of chloroform extract, 2 g of ethyl acetate extract, 10 g of n-butanol extract and 260 g of remaining aqueous methanolic residue. The 2D-PC and TLC revealed that, ethyl acetate and n-butanol extracts had limited constituents, while concentrated in aqueous methanolic residue and chloroform extract. Fractionation, isolation and purification were performed as illustrated in Figure 1. Paper chromatography (PC) according to Mabry et al. (1970), column chromatography and thin layer chromatography (TLC) according to Stahl (1969), GC-MS conditions for unsaponifiable matters analysis and GC - MS conditions for fatty acid methyl esters analysis were performed according to Vogel (1961), mild and complete acid hydrolysis were done according to the methods described by Harborne (1984).
Cell culture and MTT cell proliferation assay
A human
ovarian cell line A2780 was incubated at 37°C in an atmosphere of 5% CO
2, 95% air and 100% relative humidity, to maintain continuous logarithmic growth. RPMI-1640 media was supplemented with 10% heat inactivated Foetal Bovine Serum (FBS), L-glutamine and 5% penicillin + streptomycin. Cells were checked for Mycoplasma, by measuring the bio-luminescence (Myco Alert sample detection kit; Lonza, Switzerland), using a multiplate reader (Synergy HT, BioTek, USA). The MTT
in vitro cell viability colorimetric assay was used for measuring cellular proliferation, inhibitory activity and cytotoxicity of the plant samples. The colour of MTT: 3-(4, 5-Dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide is yellow (tetrazole), which changed to purple (reduced to formazan). When mitochondrial dehydrogenase enzymes are active therefore, reduction indicates cell viability which can be measured as optical density (OD). Cells were incubated at 37°C overnight. Final concentrations of each sample (in DMSO was filtered with Nylon 0.22 µm × 25 mm) in wells were 1, 10, 25, 50 and 100 μg/ml in 200 μl of media (DMSO 0.1%). 20 μl medium was added to each control well, and incubated for 48 h.
Each concentration was tested in triplicates (n=3). MTT was added into each well. Plates were incubated for 3 h, supernatant was aspirated, and 100 μl of DMSO was added to each well. Plates were shaken for 5 min at 26°C using STUART scientific orbital shaker (Redhill, Surrey, UK) and absorbance was read on multi-plate reader (Synergy HT, BioTek, USA). The OD of the purple formazan A
570 is proportional to the number of viable cells. When the amount of formazan produced by treated cells is compared with the amount of formazan produced by untreated control cells, the strength of the drug in causing growth inhibition can be determined. Through plotting growth curves of absorbance against sample(s) concentration, thus formulation concentration causing 50% inhibition (IC
50) compared to control cell growth (100%) were determined (Hansen et al., 1989). GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA (
www.graphpad.com) was used for analysis.
Determination of LD50
The alcoholic sample was dissolved in distilled water then given orally to adult albino mice in graded doses up to 4 g/kg (the maximum given dose) and the control group received the same volume of the vehicle. The percentage mortality for samples as well as the general behavior of the animal was recorded 24 h later (Armitage, 1971).
Estimation of analgesic activity using hot Plate Test
Two doses of 100 and 300 mg/kg body weight for chloroform and methanolic extract each and 50 mg/kg paracetamol (as standard) was administered orally to adult albino mice weighing 25 to 30 g of either sex using 25-gauge needle (Farshchi et al., 2009). Tested animal was placed on a hot plate with fixed temperature 55±0.5°C (Harvard Apparatus Ltd., Kent, UK), till the appearance of withdrawal response in terms of hind paw licking, biting or jumped off. A cut-off time to remove mouse from the plate of 30 seconds was used to minimize the tissue damage (Pini et al., 1997; Lavich et al., 2005; National committee for clinical laboratory standard (NCCLS), 1997).
Estimation of antipyretic activity
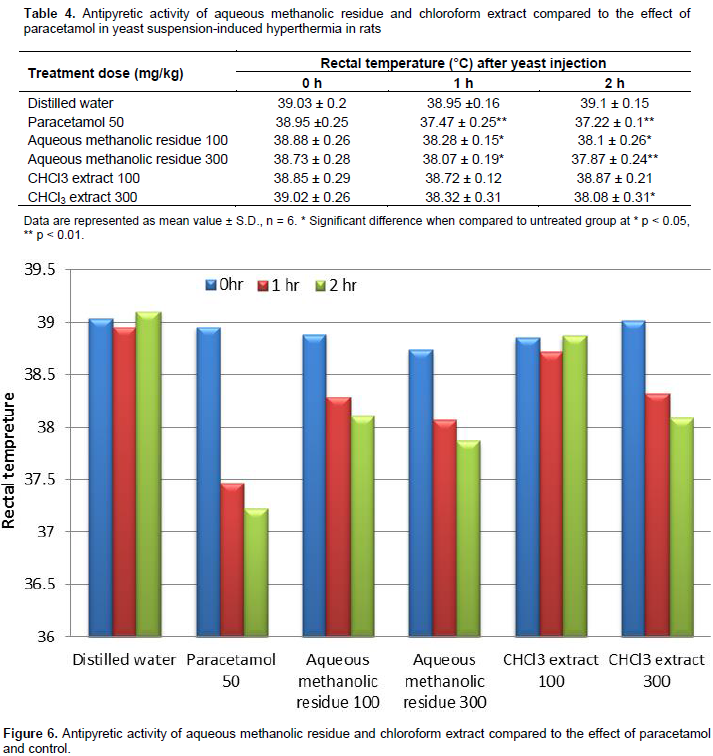
Aqueous methanolic residue and chloroform extract of C. spinosum L. aerial parts were used to evaluate their antipyretic activity using Brewer's yeast-induced pyrexia in rats as described by, Loux et al. (1972). Fever was induced by injecting 20 ml/kg of 20% aqueous suspension of Brewer's yeast in normal saline subcutaneously. Temperature across rectum (using thermal probe Eliab thermistor thermometer) was recorded after 18 h and served as base line of elevated body temperature. The extracts samples (100 and 300 mg/kg) was administered orally, using paracetamol (50 mg/kg, orally) as reference. Control group received distilled water. Rectal temperature was determined at 1 and 2 h after test samples/reference drug administration.
Preparation of the plant samples for antimicrobial evaluation
The antimicrobial activity of the aqueous methanolic residue, chloroform extract, compounds 5 and 8 obtained from C. spinosum L. aerial parts were evaluated using the agar well diffusion method as described by Rahbar and Diba (2010). All samples were dissolved in 0.5 ml methanol. A loopful of the tested organisms was inoculated into 5.0 ml of nutrient broth and incubated at 37°C for 24 h. 50 µl of 24 h culture organism was dispensed into 5 ml broth and incubated for 2 h to standardize the culture to 106 cfu/ml. Cotton swab was immersed into standardized culture to be spread onto the
surface of, the agar plate. Sterilized 6 mm cork borer was used to punch 5 wells for the extracts. From each of the 4 extract samples, 100 µl was dispensed into the corresponding 4 wells while the fifth was used for negative control (methanol). To allow diffusion of the tested extract samples, the plates were left at room temperature for at least 1 h. Two discs of antibiotic (imipenem and ciprofloxacin) were placed as positive control. These plates were incubated at 37°C for 18 to 24 h. Zones of inhibition surrounding the wells and discs were measured to evaluate their antimicrobial activity.
Preliminary phytochemical screening, hydrocarbon, sterol and fatty acid contents in C. spinosum
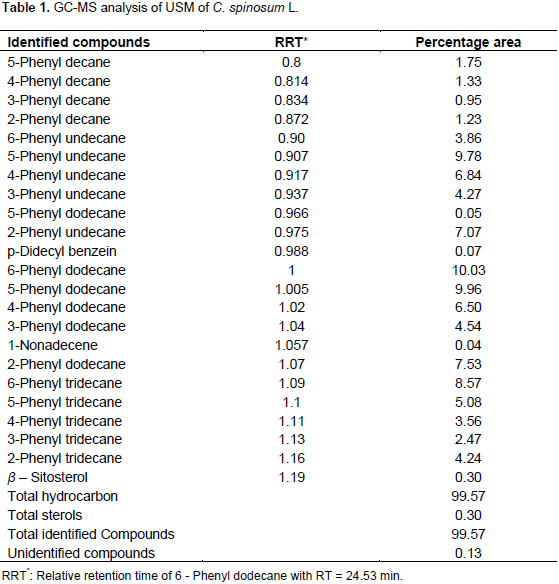
Phytochemical screening as preliminary tests of aerial parts of C. spinosum revealed the presence of carbohydrate and/or glycosides, tannins, flavonoids, irridoids, unsaturated sterols and/or triterpenes, saponins and the absence of anthraquinones, volatiles, coumarins, and alkaloids or compound containing nitrogenous bases. Identification of hydrocarbons and sterols content of USM fraction was carried out by GC-MS; the conditions were adopted as mentioned. Tentative identification of hydrocarbons and sterols was carried out by, comparison of their retention times. Quantitation was based on peak area integration.
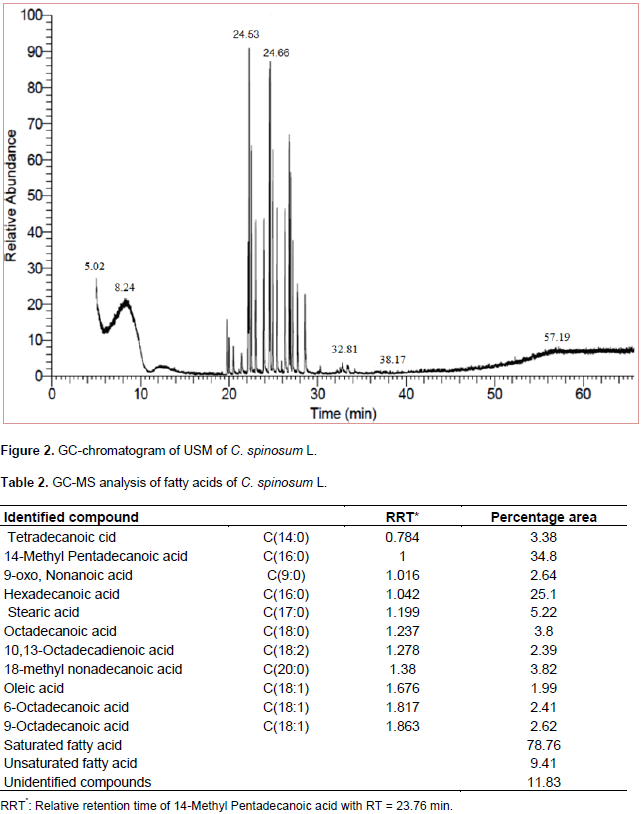
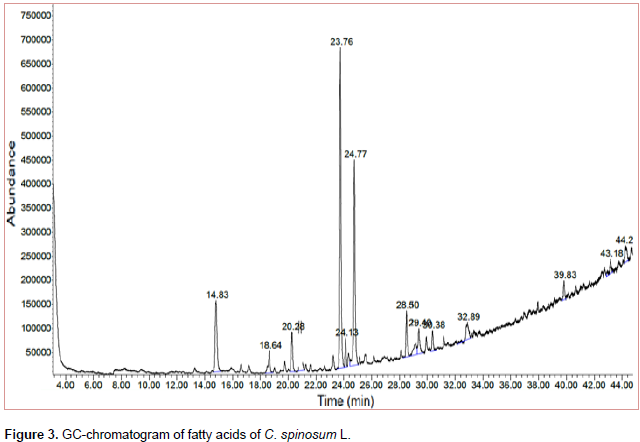
The results of USM analysis for C. spinosum L. are compiled in Table 1 and Figure 2. It was found that, hydrocarbons represented a higher percentage (99.57%) than that of sterols (0.30%). 6-Phenyldodecane (10.03%) and 5-Phenyldodecane (9.96%) represented the major hydrocarbons while β-Sitosterol (0.30%) represented the only sterol identified. It could be concluded that, the saturated fatty acids (78.76%) represented a higher percentage than that of unsaturated ones (9.41%). 14-methyl Pentadecanoic acid (34.8%) and Hexadecanoic acid (25.1 %) represented the major identified saturated fatty acids while 9-Octadecanoic acid (2.62 %) represented the major unsaturated fatty acid, Table 2 and Figure 3.
Characterization and identification of isolated compounds
Air dried powdered aerial parts of the plant under investigation (1350 g) was subjected to exhaustive extraction with 80% MeOH under reflux. After drying the extract under reduced pressure, the residue was defatted by petroleum ether and the remaining residue was fractionated by chloroform, ethyl acetate and n-butanol under reflux (50°C), respectively. The 2D-PC analysis proved that active constituents are concentrated in the chloroform extract and aqueous methanolic residue when compared to ethyl acetate and n-butanol extracts. Aqueous methanolic residue, and chloroform extract were subjected to fractionation according to the illustrated Figure 1. Identification of isolated compounds are based on chemical and physical methods including 1H/13C NMR and HMBC.
Based on these data and by comparison with reported literature data (Haddock et al., 1982; Barakat et al., 1987; Agrawal and Bansal, 1989; Mahmoud et al., 2001; Seebacher et al., 2003; Shalaby and Bahgat, 2003; Marzouk et al., 2004; Aboutabl et al., 2008; Rahmana et al., 2009; Ahmad et al., 2010; Kamal et al., 2012; Onoja and Ndukwe, 2013; Haggag et al., 2013; Allam, 2014; Khan and Hossain, 2015; Mohammed et al., 2016) and authentic samples, the compounds identified were ten; 1; β- Sitosterol, 3; Oleanolic acid and 4; Gallic acid were isolated once before from genus Citharexylum, while 2; β- Sitosterol 3-O- β -D-glucopyranoside 5; Quercetin , 6; 6-Methoxy acacetin 7-O-β-D-glucopyranoside, 7; Naringenin, 8; Quercetin 3-O-α-L-rhamnopyranoside (Quercetrin), 9; 1, 2, 6-tri-O-galloyl-β-D-glucopyranoside, 10; Rutin were isolated for the first time from genus Citharexylum (Figure 4). Two major compounds (5 and 8) subjected to biological activities, their spectral data are summarized as follow:
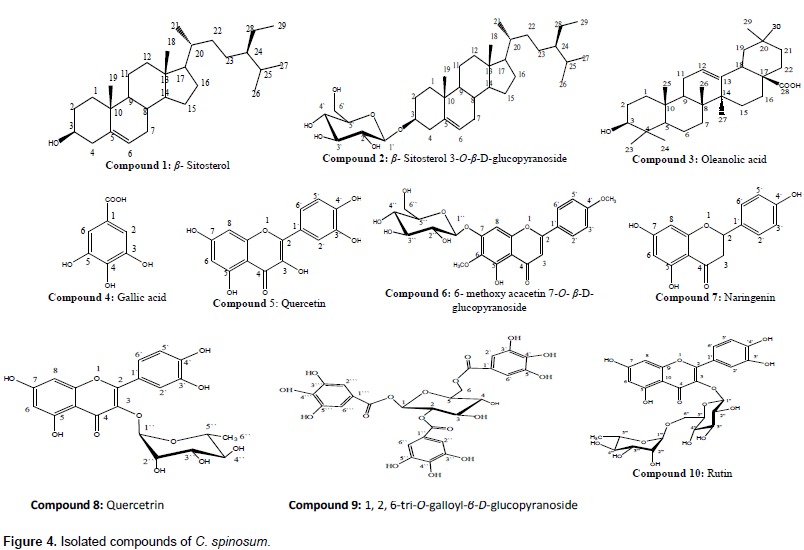
Compound 5
Is a yellow amorphous powder (20 mg), with chromatographic
properties: Rf values; 0.6 (S1), 0.4 (S2); brilliant yellow fluorescent spot by UV- light. It gave pale green color and orange fluorescence with FeCl3 and Naturstoff spray reagents, respectively. 1H-NMR (400 MHz, CD3OD): δ ppm 7.71 (1H, d, J=2.0 Hz, H-2`), 7.62 (1H, dd, J=8.3, 2.0 Hz, H-6`), 6.86 (1H, d, J=8.3 Hz, H-5`), 6.36 (1H, d, J=1.8Hz, H-8), 6.15 (1H, d, J=1.8Hz, H-6). 13C-NMR PENDANT (100 MHz, CD3OD): δ ppm 175.99 (C-4), 164.23 (C-7), 161.17 (C-5), 156.86 (C-9), 147.34 (C-2), 146.62 (C-4`), 144.88 (C-3`), 135.90 (C-3), 122.79 (C-1`), 120.29 (C-6`), 114.85 (C-2`), 114.48 (C-5`), 103.17 (C-10), 97.85 (C-6), 93.02 (C-8).
Compound 8
Is an orange amorphous powder (16 mg), with chromatographic properties: Rf values; 0.39(S1), 0.63 (S2) on PC; dark purple fluorescent spot under long UV-light which turned yellow fluorescence on exposure to ammonia vapors and gave a green color and orange fluorescence with FeCl3 and Naturstoff spray reagents, respectively. Complete acid hydrolysis resulted in Quercetin in organic layer and Rhamnose in aqueouslayer (CoPC). 1H-NMR spectrum (400MHz, CD3OD): δ ppm 7.30 (1H, d, J=2.2 Hz, H-2`), 7.27 (1H, dd, J=2.2,8.2 Hz, H-6`), 6.88 (1H, d, J=7.7 Hz, H-5`), 6.32 (1H, d, J=1.8 Hz, H-8), 6.15 (1H, d, J=1.8 Hz, H-6 ), 5.32 (1H, d, J=1.3 Hz, H- 1Ë‹`), 4.19 (1H, dd, J=1.3, 3.2 Hz, H-2``), 3.71 (1H, dd, J=3.2, 9.6 Hz, H-3``), 3.33 (1H, m, H-5``), 3.32 (1H, m, H-4``), 0.91(3H, d, J=5.94 Hz, H-6Ë‹Ë‹ ). 13C-NMR PENDANT (100 MHz, CD3OD): δ ppm 178.4 (C-4), 164.8 (C-7), 161.8 (C-5), 157.9 (C-2), 157.2 (C-9), 148.3 (C-4`), 145.1 (C-3`), 134.7 (C-3), 121.6 (C-1Ë‹), 121.5 (C-6Ë‹), 115.5 (C-2`), 115.0 (C-5`), 104.3 (C-10), 102.2 (C-1Ë‹`), 98.5 (C-6), 93.4 (C-8), 71.8 (C-4``), 70.6 (C-3``), 70.5 (C-2``), 70.5 (C-5``), 16.3 (CH3-6``).
Biological study
Cytotoxic activity
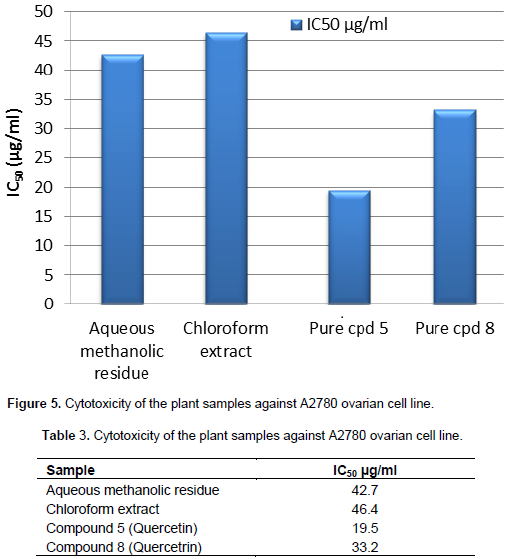
Cytotoxic activity of aqueous methanolic residue, chloroform extract, and pure compounds (5 and 8)obtained from the aerial parts of C. spinosum were examined against A2780, a human ovarian cell line. Activity was reported in terms of an IC50 (concentration in µg/ml necessary to produce 50% inhibition) (Figure 5) and (Table 3). The treatment of A2780 ovarian cell line with an aqueous methanolic residue, chloroform extract showed weak cytotoxic effect as their calculated IC50 which were 42.7 µg/ml and 46.4 µg/ml, respectively. While pure compound 8 (identified later as Quercetrin) showed moderate cytotoxic effect calculated (IC50) as 33.2 µg/ml, pure compound 5 (identified later as Quercetin) showed significant cytotoxic effect as IC50 19.5 µg/ml.
Determination of median lethal dose (LD50)
On low doses (less than 2 g/kg of total aqueous methanol extract of C. spinosum), it was observed that animals moved and fed normally. The behavior of mice has changed at a dose of 2 g/kg extract. Mice showed abnormal signs like fatigue, loss of appetite and mortality. The 50% of dead animals were estimated at 3 g/kg extract. In contrast, all animals died at a dose of 4 g/kg. LD50 value was calculated by-probit analysis which is 2.86 g/kg body weight.
Analgesic and antipyretic activities
Using hot plate test, the analgesic effect of plant samples was studied. All tested samples at both concentrations (100 mg/Kg) and (300 mg/Kg) showed non-significant analgesic activity as compared to paracetamol as standard and saline as control. As shown in (Table 4) and (Figure 6), aqueous methanolic residue at concentration 100 mg/kg and chloroform extract at 300 mg/kg showed moderate antipyretic activity while aqueous methanolic residue at 300 mg/kg showed significant antipyretic activity as compared to paracetamol as standard and distilled water as control.
Antimicrobial study
Aqueous methanolic residue of C. spinosum exerted marked antimicrobial activity against all tested multidrug-resistant Gram +ve and -ve bacteria. Chloroform extract exerted antimicrobial activity against the tested Gram +ve and -ve bacteria. It showed that, the activity on Gram +ve is higher than Gram -ve bacteria. Pure comound 5 exerted marked activities against the tested Gram +ve S. aureus and Gram -ve bacteria E. coli, but showed no antimicrobial activity against P. aeuroginosa. Compound 8 showed moderate antimicrobial activity against the tested Gram +ve S. aureus and Gram -ve bacteria E.coli but showed no antimicrobial activity against P. aeuroginosa. Fortunately, multidrug-resistant S. aureus and E. coli strains showed sensitivity to all tested samples. The results of agar well diffusion method are shown in (Figure 7 and Table 5).
A phytochemical screening of
C. spinosum aerial parts resulted in the presence of flavonoids, tannins, carbohydrates and/or glycosides, triterpenes and/or sterols and saponins. Also, it revealed the absence of alkaloids, volatiles, anthraquinones and coumarins. The percentages of hydrocarbons and sterols in
C. spinosum pet-ether extract were 99.57 and 0.3%, respectively. It was found that 6-phenyldodecane (10.03%) and 5-phenyldodecane (9.96%) represented the major hydrocarbons while
β- Sitosterol (0.30 %) represented only identified sterol. Concerning the composition of fatty acids content in pet-ether extract, it could be concluded that the percentage of saturated fatty acids (78.76%) represented higher percentage than that of unsaturated fatty acids (9.14%). 14-methyl Pentadecanoic acid (34.8%) and hexadecanoic acid (25.1%) represented the major identified saturated fatty acids while 9-octadecanoic acid (2.62%) represented the major unsaturated fatty acid.
These results are in accordance with previous studies of different species of genus Citharexylum (Khalifa et al., 2002; Ayers and Sneden, 2002; Shalaby and Bahgat, 2003; Balazs et al., 2006; Ganapaty et al., 2010; Allam, 2014; Mohammad et al., 2016). Furthermore, the 80% aq. methanolic residue and chloroform extract of C. spinosum were purified by employing diversity of chromatographic techniques to afford ten compounds, β- Sitosterol Gallic acid and Oleanolic acid were isolated once before from genus Citharexylum (Allam, 2014; Khan and Hossain, 2015; Mohammed et al., 2016; Allam, 2017), while; β- Sitosterol 3-O-β-D-glucopyranoside, 6-Methoxy acacetin 7-O-β-D-glucopyranoside, Naringenin, 1, 2, 6-tri-O-galloyl-β-D-glucopyranoside, Rutin were isolated for the first time from genus Citharexylum in addition to two major compounds (5 and 8).
According to chromatographic properties of compound 5 (Rf – value), fluorescent under UV-light and change in color with Fecl3 and Naturstoff reagents compound 5 was expected to be quercetin aglycone (Harborne, 1984). 1H-NMR spectrum showed two characteristic aromatic spin coupling system, the first ABX of three proton resonances at δ 7.71, 7.62, 6.86 were assignable to H-2`,6` and 5` of 3`,4` dihydroxy B-ring. The second coupling system was described as typical AM system of two meta-coupled doublets at δ 6.36 and 6.15 for H-8 and H-6 of 5, 7-dihydroxylated ring- A. The absence of any signals in the aliphatic region proved the aglycone structure. 13C-NMR spectrum exhibited fifteen 13C resonances of the Quercetin moiety with key carbon signals of quercetin nucleus at 175.99 (C-4), 146.62 (C-4`), 144.88 (C-3`), 120.29 (C-6`), 122.79 (C-1`), 114.85 (C-2`) and 114.48 (C-5`) (Agrawal and Bansal, 1989).
Based on the above discussed data and in comparison with previous reported data (Agrawal and Bansal, 1989) and authentic sample, compound 5 was identified as Quercetin which is isolated for the first time from genus Citharexylum. The chromatographic properties of compound 8 (Rf-values, fluorescence under UV-light and change in color with Fecl3 and natrustoff reagents) and products of acid hydrolysis, was expected to be quercetin rhamnoside (Harborne, 1984). 1H-NMR spectrum showed two characteristic aromatic spin coupling system, the first one ABX of three proton resonances δ7.30, 7.27, 6.88 were assignable to H-2Ë‹, 6Ë‹ and 5Ë‹ of 3Ë‹, 4` dihydroxylated B-ring. The second coupling system was described as typical AM system of two meta-coupled doublets at δ 6.32 and 6.15 for H-8 and H-6, respectively of 5, 7- dihydroxylated ring-A.
Concerning the sugar moiety and doublet signal at 5.32 ppm with J=1.3Hz (H-1Ë‹Ë‹), doublet of doublet signal at 4.19 ppm with J=1.4, 3.2 Hz (H-2Ë‹Ë‹) together with a doublet signal at 0.91with J=5.9 Hz (H-6Ë‹Ë‹), were all characteristic for α-L-rhamnopyranoside moiety. In accordance with the earlier discussed data along with a comparison of the previous reported data (Agrawal and Bansal, 1989; Mahmoud et al., 2001)
|
, supporting evidence for the structure of glycoside was achieved by 13C-NMR spectrum which showed the characteristic 15 13C resonance for 3-0-substituted quercetin. The sugar moiety was confirmed as rhamnose from characteristic resonance at δ ppm 102.2 and 16.3 for anomeric carbon and CH3-6``, respectively, together with the rest of carbon resonances for rhamnose carbons. Compound 8 was confirmed as Quercetin 3-O-α-L-rhamnopyranoside (Quercetrin), which is isolated for the first time from genus Citharexylum. |
Cytotoxic activity of aqueous methanolic residue, chloroform extract and pure compounds (5 and 8) obtained from the aerial parts of C. spinosum L. were examined against A2780; a human ovarian cell line using MTT cell prolifiration assay. It was found that, aqueous methanolic residue and chloroform extract had weak cytotoxic activity, pure compound 8 (Quercetrin) had moderate cytotoxic activity, while pure compound 5 (Quercetin) had significant cytotoxic activity. Estimation of analgesic activity done using hot plate test showed that, aqueous methanolic residue and chloroform extract had no analgesic activity. The antipyretic activity of aqueous methanolic residue and chloroform extract were evaluated using Brewer's yeast-induced pyrexia in rats, which found that aqueous methanolic residue at 300 mg/kg had antipyretic activity, while chloroform extract had weak antipyretic activity.
In the present study, the antimicrobial activity was evaluated using agar well diffusion method. For aqueous methanolic residue of C. spinosum, results were almost the same against the tested Gram positive and negative bacteria while chloroform extract showed stronger antimicrobial activity against Gram positive than negative bacteria. This is in contrast to the study made by Shalaby and Bahgat (2003), who reported stronger antimicrobial activity against Gram negative bacteria and positive bacteria tested by disc diffusion method. Different species of the genus Citharexylum were reported to have antiulcer, antihypertensive and hepatoprotective effects, immunomodulatory, antimicrobial, anti-Schistosoma mansoni activities, antioxidant nephroprotective, radical scavenging, cytotoxic activities and regulating immediate type of allergic reaction (Shin et al., 2000; Khalifa et al., 2002; Shalaby and Bahgat, 2003; Bahgat et al., 2005; Khan and Siddique, 2012; Kadry et al., 2013; Allam, 2014).
The authors have not declared any conflict of interests.
REFERENCES
|
Ademola IO, Akanbi AI, Idowu SO (2005). Comparative nematocidal activity of chromatographic fractions of Leucaena leucocephala seed against gastrointestinal sheep nematodes. Pharm. Biol. 43:599-604.
Crossref
|
|
|
|
Adjanahoun E, Ahyi MRA, Ake-Assi L, Elewude JA, Fadoju SO, Gbile ZO, Goudole E, Johnson CLA, Keita A, Morakinyo O, Ojewole JAO, Olatunji AO, Sofowora EA. (1991). Traditional medicine and pharmacopoeia. Contribution to ethnobotanical floristic studies in Western Nigeria, Pub. Organization of African Unity, Scientific Technical and Research Commission Lagos, Nigeria P 420.
|
|
|
|
|
Alonzo-Diaz MA, Torres-Acosta JFJ, Sandoval-Castro CA, Capetillo Leal C, Brunet S, Hoste H (2008). Effects of four tropical tanniniferous plants on the inhibition of larval migration and the exsheathment process of Trichostrongylus colubriformis infective stage. Vet. Parasitol. 153:187-192.
Crossref
|
|
|
|
|
Arbonnier M (2004). Trees, Shrubs and Lianas of West African dry zones. CIRAD, Margraf, Weikersheim, Germany; MNHN, Paris, France. Editions Quae P 573.
|
|
|
|
|
Azando EVB, Hounzangbé-Adoté MS, Olounladé AP, Brunet S, Fabre N, Valentin A, Hoste H (2011). Involvement of tannins and flavonoids in the in vitro effects of Newbouldia laevis and Zanthoxylum zanthoxyloides extracts on the exsheathment of third-stage infective larvae of gastrointestinal nematodes. Vet. Parasitol. 180(3):292-297.
Crossref
|
|
|
|
|
Bahuaud D, Martinez-Ortiz De Montellano C, Chauveau S, Prevot F, Torres-Acosta F, Fouraste I, Hoste H (2006). Effects of four tanniferous plant extracts on the in vitro exsheathment of third-stage larvae of parasitic nematodes. Parasitology 132:545-554.
Crossref
|
|
|
|
|
Bar NG, Epshtein V, Ruckenstein AE, Rafikov R, Mustaev A, Nudler E (2005). A ratchet mechanism of transcription elongation and its control. Cell 120:183-193.
Crossref
|
|
|
|
|
Barrau E, Fabre N, Fouraste I, Hoste H (2005). Effect of bioactive compounds from sainfoin (Onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: role of tannins and flavonol glycosides. Parasitology 131:531-538.
Crossref
|
|
|
|
|
Bizimenyera ES, Githiori JB, Eloff JN, Swan GE (2006). In vitro activity of Peltophorum africanum Sond. (Fabaceae) extracts on the egg hatching and larval development of the parasitic nematode Trichostrongylus colubriformis. Vet. Parasitol. 142:336-343.
Crossref
|
|
|
|
|
Bizimenyera ES, Swan GE, Chikoto H, Eloff JN (2005). Rationale for using Peltophorum africanum (Fabaceae) extracts in veterinary medicine. J. S. Afr. Vet. Assoc. 76:54-58.
Crossref
|
|
|
|
|
Brunet S, Jackson F, Hoste H (2008). Effects of sainfoin (Onobrychis viciifolia) extract and monomers of condensed tannins on the association of abomasal nematode larvae with fundic explants. Int. J. Parasitol. 38:783-790.
Crossref
|
|
|
|
|
Brunet S, Aufrere J, Elbabili F, Fourasté I, Hoste H (2007). The kinetics of exsheathment of infective nematode larvae is disturbed in the presence of a tannin-rich plant extract (sainfoin) both in vitro and in vivo. Parasitology 135:1-10.
Crossref
|
|
|
|
|
Brunet S, Hoste H (2006). Les monomères des tannins condensés affectent le dégainement des larves des nématodes parasites des ruminants. J. Agric. Food Chem. 54:7481-7487.
Crossref
|
|
|
|
|
Chiejina SN (2001). The epidemiology of helminth infections of domesticated animals in the tropics with emphasis on fascioliasis and parasitic gastroenteritis. In: Chowdhury, N., Tada, I. (Eds.), Perspectives on Helminthology. Science Publishers Inc., Enfield. pp. 41-87.
|
|
|
|
|
Cronquist A (1988). The evolution and classification of flowering plants. New York: New York Botanic Gardens. P 555.
|
|
|
|
|
Dieguez-Hurtado R, Garrido G, Prieto Gonzalez S, Iznaga Y, Gonzalz L, Molina Tores J, Curini, M, Epifano F, Marcotullio MC (2003). Antfungal activity of some Cuban Zanthoxylum species. Fitoterapia 74:384-386.
Crossref
|
|
|
|
|
Eloff JN (1998). Which extractant should be used for screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 60:1- 8.
Crossref
|
|
|
|
|
Eyong KO, Folefoc GN, Kuete V, Beng PV, Krohn K, Hussain H, Nkengfack EA, Saeftel M, Sarite SR, Hoerauf A (2006). Newbouldiaquinone A: a naphthoquinone-anthraquinone ether coupled pigment, as a potential antimicrobial and antimalarial agent from Newbouldia laevis. Phytochemistry 67:605-609.
Crossref
|
|
|
|
|
Eyong KO, Krohn K, Hussain H, Folefoc GN, Nkengfack AE, Schulz B, Hu Q (2005). Newbouldiaquinone and newbouldiamide: a new naphthoquinone-anthraquinone coupled pigment and a new ceramide from Newbouldia laevis. Chem. Pharm. Bull. 53:616-619.
Crossref
|
|
|
|
|
Hammond JA, Feiling D, Bishop SC (1997). Prospects for plant anthelmintics in tropical veterinary medecine. Vet. Res. Comm. 21:213-228
Crossref
|
|
|
|
|
Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO (2006). The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 22:253-261.
Crossref
|
|
|
|
|
Hounzangbé-Adoté MS (2004). Propriétés anthelminthiques de 4 plantes tropicales testées in vitro et in vivo sur les nématodes gastro-intestinaux chez les petits ruminants Djallonké. PhD dissertation, University of Abomey-Calavi, Abomey-Calavi, Benin.
|
|
|
|
|
Hounzangbé-Adoté MS (2000). La pharmacopée en médecine vétérinaire au sud Bénin (cas des ovins et caprins). Colloque Européen d'Ethnopharmacologie. Société Française d'ethnopharmacologie. Metz du 11 au 13 mai 2000.
|
|
|
|
|
Jackson F, Hoste H (2010). In vitro methods for the primary screening of plant products for direct activity against ruminant gastrointestinal nematodes. In: "In vitro screening of plant resources for extra nutritional attributes in ruminants nuclear and related methodologies" P Vercoe, HPS Makkar and AC Schlink Eds, Springer/International Atomic Energy Agency Edition Dordrecht. pp. 25-45.
Crossref
|
|
|
|
|
Makkar HPS (2003). Effects and fate of tannins in ruminant animals, adaptation to tannins and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 49:241-256.
Crossref
|
|
|
|
|
Makkar HPS (2000). In Quantification of tannins in tree foliage: Working document, FAO/IAEA, Vienna.
|
|
|
|
|
Mara SP, Arruda Jao B, Fernandes Paulo C, Viera M, Fatima Das GF, Da Silva Jose RP (1992). Chemistry of Zanthoxylum rhoifolium. A new secofuraquinoline alkaloids. Biochem. Syst. Ecol. 20:173-178.
Crossref
|
|
|
|
|
Olounladé AP (2005). Effets anthelminthiques des feuilles de Newbouldia laevis testées in vivo sur les nématodes gastro-intestinaux (Haemonchus contortus et Trichostrongylus colubriformis) chez les moutons Djallonké.DEA, Université de Lomé, Lomé,Togo. P 65.
|
|
|
|
|
Porter LJ, Hrstich LN, Chan BG (1986). The conversion of procyanidins and prodelphinidins to cyanidins and delphinidin. Phytochemistry 25:223-230.
Crossref
|
|
|
|
|
Rabel B, McGregor R, Dough PGC (1994). Improved bioassay for estimation of inhibitory effects of ovine gastrointestinal mucus and anthelmintic on nematode larval migration. Int. J. Parasitol. 24:671-676.
Crossref
|
|
|
|
|
Sanyal PK (2001). Integrated management of parasitic gastroenteritis in ruminants. In: Chowdhury, N., Tada, I. (Eds.), Perspectives on Helminthology. Science Publishers Inc., Enfield pp. 439-460.
|
|
|
|
|
Schofield P, Mbugua DM, Pell AN (2001). Analysis of condensed tannins: a review. Anim. Feed Sci. Technol. 91:21-40.
Crossref
|
|
|
|
|
Thompson A (1997). As patients embrace herbal remedies, dearth of scientific evidence frustrates clinicians. Am. J. Health Syst. Pharmacol. 54:2656-2664.
|
|
|
|
|
Whitney TR, Lee AE, Klein DR, Scott CB, Craig TM, Muir JP (2011). A modified in vitro larvae migration inhibition assay using rumen fluid to evaluate Haemonchus contortus viability. Vet. Parasitol. 176:217-225.
Crossref
|
|
|
|
|
Wolstenholme AJ, Fairweather I, Prichard RK, Von Samson-Himmelstjerna G, Sangster NC (2004). Drug resistance in veterinary helminths. Trends Parasitol. 20:469-476.
Crossref
|
|