ABSTRACT
This study was conducted to determine the total phenol content (TPC) and free radicals scavenging activity of methanolic and ethanolic extracts of wheat (WH-711) fractions (grain, bran and flour) fermented with Aspergillus oryzae (MTCC 3107). Maximum TPC (1469.35 ± 4.5, 1426.44 ± 5.5 and 1268.91 ± 5.3) was obtained in methanolic extracts as compared to ethanolic extracts (1368.65 ± 3.5, 1155.46 ± 4.7 and 1314.17 ± 5.6 respectively) of wheat bran, grain and flour on the 5th day of fermentation. The free radical-scavenging activity of ethanolic extracts of fermented wheat grain, flour and bran was found to be maximum (62.68 ± 0.49, 32.87 ± 0.71 and 43.89 ± 0.88 %, respectively) whereas methanolic extracts of wheat grain and flour was 59.67 ± 0.78 and 28.52 ± 0.56 %, respectively on 5th day of incubation using DPPH assay. Free radical-scavenging activity (71.30 ± 0.36%) of wheat bran was significantly higher than that of fermented grain and flour on the 2nd day of incubation. Likewise in ABTS assay, wheat bran extracted with ethanol and methanol showed higher inhibition (28.48 ± 0.62 and 43.55 ± 0.72%) on 5th day of incubation than to the fermented grain and flour. Among all the three fractions, methanolic extract of fermented wheat bran has the highest TPC and hence has highest free radical scavenging activity. Total phenolic contents, DPPH and ABTS+ radicals scavenging activity of wheat fractions were significantly correlated (P<0.01) which provide the strong evidence that the antioxidant activity in wheat fractions was derived from phenolic compounds.
Key words: Antioxidant activity, wheat, solid state fermentation, Aspergillus oryzae.
Abbreviation:
DPPH, 2, 2-diphenyl-1-picrylhydrazyl; TPC, Total phenolic content; ABTS, 2, 2-azinobis-3 ethylbenzothiazoline-6-sulphonic acid; MTCC, Microbial type culture collection.
Cancer and cardiovascular diseases are ranked as the first two leading causes of death in many developed countries (Doll and Peto, 1981). Unhealthy dietary habits, living habits and exposure to dangerous chemicals in the environment could lead to the production of more free radicals. It has been reported that oxygen free radicals and other reactive oxygen species can cause oxidative injury to living organisms and thus play an important role in many lifestyle-related diseases such as arthritis, atherosclerosis, emphysema and cancer (Halliwell et al., 1995). Antioxidant is a substance has the ability to delay the oxidation of a substrate by inhibiting the initiation or propagation of oxidizing chain reactions caused by free radicals. It plays important roles to prevent fats and oils from becoming rancid and protects human body from detrimental effects of free radicals. With an insufficient antioxidant system or under severe oxidative stress, reactive oxygen species (ROS) are overproduced and can damage biomolecules such as DNA, proteins, lipids and carbohydrates, leading to the development of several diseases (Halliwell, 1996). The majority of deaths due to cancer are related to diet. Therefore, current trends in dietary modifications have shifted to focus on the prevention of disease (Willet, 1994). Epidemiological studies have strongly suggested that consumption of whole grain and whole grain products rich in antioxidants is protective against certain chronic diseases, cardiovascular diseases, diabetes, obesity and cancer (Wojdy and Oszmai, 2007). These protective effects are mainly due to the presence of several important constituents of the whole grains such as polyphenols, dietary fibre, resistant starch, proteins, lipids, lignans, vitamins, and minerals (Stratil et al., 2007) as they are involved in inhibition of the formation of free radicals (Halliwell, 1996).
Synthetic antioxidants are widely used because they are effective and cheaper than natural types. However, the safety and toxicity of synthetic antioxidants have important concerns (Imaida et al., 1983). Much attention has been focused on the use of antioxidants, especially natural antioxidants to inhibit lipid peroxidation or to protect the human body from the oxidative damage by free radicals (Yang et al., 2000; Duhan et al., 2011a; Duhan et al., 2011b; Saharan et al., 2012; Saharan and Duhan, 2013; Rana et al., 2014; Duhan et al., 2015). In recent years much efforts has been devoted to natural antioxidant because of their association with health benefits (Arnous et al., 2001).
Plants are potential sources of natural antioxidants. It produces various antioxidative compounds to counteract reactive oxygen species (ROS) in order to survive. Cereals, in general, play an important role in human nutrition. Botanically, cereals are classified as Poaceae, the grass family that includes wheat, rice, barley, oats, rye, maize, sorghum and millets. Cereal grains are known to contain phenolic acids, saponins and phytoestrogens as well as small amounts of flavonoids (Zienlinski and Kozowska, 2000). According to Cassidy (1996) cereals serve as a major source of lignans in the human diet. Lignans may function as potent antioxidants by decreasing the production of ROS thereby exerting anticarcinogenic effects. Solid-state fermentation (SSF) process is an alternative way to improve the phenolic content and antioxidant potential in fermented foods. Lateef et al. (2008) showed that the nutritional qualities and antioxidant activities of different agro-solid wastes were enhanced by SSF. In the SSF process, different hydrolytic enzymes could be produced directly from solid substrate and simultaneously be utilized to release the phenolics.
Wheat is the most important cereal in the temperate zones. The importance of wheat for production of flour and semolina, which form the basic ingredients of bread and other bakery products and pasta, has been well recognized (Belderok, 2000). According to Yu et al. (2002) bran extracts of three different wheat varieties exhibited significant antioxidant properties against free radical scavenging and metal ion chelation. Wheat, together with maize and rice, supplies most of the dietary saccharides and proteins for human nutrition but is also a relevant source of antioxidants (Liu, 2007) together with other particular sources such as tartary buckwheat-enriched bread (Bojnanska et al., 2009).
(Einkorn) Triticum monococcum L. subsp. monococcum, a diploid wheat related to T. turgidum L. and T. aestivum L., shows higher protein, carotenoid and tocol contents (Hidalgo et al., 2006; Hejtmankova et al., 2010) than durum and bread wheat’s, being therefore a potential food source with high nutritional properties (Lavelli et al., 2009). Keeping in view the importance of wheat, the present study was planned to improve the nutrition value of wheat grain, bran and flour by solid state fermentation with GRAS fungus.
Fungal strain
The fungal strain that is Aspergillus oryzae (MTCC 3107) used for fermentation was procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh. The fungal strain was cultured and maintained on potato dextrose agar media.
Medium and chemicals
For this investigation, wheat (WH-711) grain, flour and bran were used as the substrate for the preparation of koji by using A. oryzae. The organic solvents (ethanol, methanol and hexane) were from Qualigens. All other chemicals used (DPPH, ABTS, gallic acid, Folin reagent, L-ascorbic acid, sucrose, sodium carbonate etc.) were of Hi Media.
Culture conditions
Substrates (except flour) were first washed and dried before use. Fifty grams of each substrate was taken in 500 mL Erlenmeyer flasks and then soaked in 50 mL Czapek-dox medium [NaNO3 (2.5 g/L), KH2PO4 (1.0 g/L), KCl (0.5 g/L) and MgSO4.2H2O (0.5 g/L)] overnight at room temperature. After decanting the excess media, the substrates were autoclaved (121°C, 15 min) and subsequently cooled before inoculation. The autoclaved substrates were inoculated with 5.0 mL spore suspension (1×106 spores/mL) of A. oryzae, mixed properly and incubated for 6 days at 30°C. The substrate without spore suspension was taken as control.
Extraction of enzymes
Fermented samples were taken at every 24 h of interval. The enzyme was extracted from fermented wheat grain, flour and bran with distilled water (1:10 (w/v)). The extracted solution was filtered through Whatman filter paper No.1. The supernatant was assayed for α-amylase activity.
Extraction of phenolic compounds
The samples from fermented wheat grain, flour and bran were taken out from the Erlenmeyer flask at every 24 h of interval and dried in oven at 60°C for 24 h. The dried substrates (fermented and non-fermented) were ground in an electric grinder. All samples were defatted by blending the ground material with hexane (1:5 w/v, 5 min, thrice) at room temperature. Defatted samples were air dried for 24 h and stored at -20°C for further analysis. Defatted samples were extracted with 54% ethanol and 54% methanol at 61°C for 64 min (Liyana-Pathirana and Shahidi, 2006). The extracted samples were filtered through Whatman filter paper No.1.The filtrate was used for determination of total phenolic content and antioxidant properties.
Determination of total phenolic content (TPC)
TPC was determined by using Folin-Ciocalteu method. Two hundred µL each of ethanolic and methanolic extract was mixed separately with 1.0 mL of Folin-Ciocalteu reagent (Sigma, Aldrich) and 0.8 mL of sodium carbonate (7.5%) (Singleton and Rossi, 1965).The contents were mixed and allowed to stand for 30 min at room temperature. Absorbance was measured at 765 nm using spectrophotometer (Singh et al., 2007).
The total phenolic content was expressed as mg GAE/g by using the formula (Afolabi et al., 2007):
C = c.V / M
Where C = total content of phenolic compounds in mg/g gallic acid equivalent; c = the concentration of gallic acid (mg/mL) established from the calibration curve; V = volume of extract and M = the weight of pure ethanol extract (g).
Amylase assay
Amylase activity was determined by mixing 0.25 mL of appropriately diluted enzyme (1:5 v/v) with 0.5 mL of 0.2 M acetate buffer (pH 5.0) and 1.25 mL of soluble starch (1%). After 10 min of incubation at 50 0C, the concentration of glucose liberated from starch by the action of α-amylase was estimated spectrophotometrically at 575 nm according to Miller (1959). One unit (U) of amylase activity is defined as the amount of enzyme that liberates one micromole of reducing sugar (glucose) per min under the assay conditions. Results were expressed as EU (µM/mL).
Determination of antioxidant activity
DPPH (1, 1-Dipheny l-2-picrylhydrazyl) radical scavenging assay
The free radical scavenging activity was measured by DPPH assay. Four mg of DPPH (0.1 mM) was dissolved in 100 mL of methanol to obtain working solution. One mL of ethanolic and methanolic extract
was mixed separately with 2.0 mL of 0.1 mM DPPH followed by 30 min incubation in dark. The reduction of the DPPH free radical was measured by taking the absorbance at 517 nm (Williams et al., 1995). Colour of DPPH was reduced from purple to yellow.
The antioxidant activity of both ethanolic and methanolic extracts was evaluated by calculating the inhibition % of free radical formation using the formula:
% inhibition = [(A-A1)/A] x 100; A= absorbance of the blank (DPPH); A1= absorbance of the extract (DPPH+ extract).
ABTS (2, 2-azinobis-3-ethylbenzothiazoline-6-sulphonic acid) assay
In ABTS assay, antioxidant activity was measured using 7.6 mM (19.0 mg/5.0 mL) ABTS+ solution and 2.6 mM potassium persulphate (3.5 mg/5.0 mL) solution in 5.0 mL of distilled water. The resulting solution was left to stand for 16 h in dark at room temperature. Working solution was prepared by mixing 1.0 mL of this reaction mixture with 60.0 mL water. 3 mL of ABTS solution was mixed with 30 µL of ethanolic and methanolic extract and optical density was measured spectrophotometrically at 734 nm after 1 min of incubation (Re et al., 1999; Arnao et al., 2001). The reduction of ABTS was measured by evaluating the inhibition % using the formula:
% inhibition = [(A-A1)/A] x 100; A= absorbance of the blank (DPPH); A1= absorbance of the extract (DPPH+ extract).
Statistical analysis of data
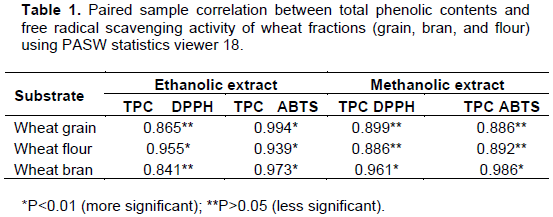
The mean value and standard deviation was calculated from the data obtained from the three replicates. Analysis of data was performed by paired sample t-test by using PASW statistics viewer 18. Statistical differences at P<0.01 and P<0.05 were considered as significant values.
Total phenol content (TPC)
In this investigation, the total phenolic content of ethanolic and methanolic extracts of fermented and non-fermented wheat fractions were carried out. Results show that the TPC was higher in fermented samples as compared to the non-fermented ones. Maximum phenolic content with ethanolic extract was observed on the 5th day of incubation for wheat bran, flour and grain (1368.65 ± 3.55, 1314.17±5.65 and 1155.46 ± 4.75 µM/g gallic acid equivalents (GAE) respectively). Likewise maximum TPC content of wheat bran, grain and flour (1469.35 ± 4.5, 1426.44 ± 5.5 and 1268.91 ± 5.3 µM/g GAE respectively) was observed as compared to their respective controls (Figure 1).
Antioxidant assays
DPPH assay
In the DPPH assay, various extracts of wheat grain, flour and bran showed potent free radical scavenging activity. The percent inhibition of free radicals formation was maximum (62.68%) in fermented ethanolic extract on the
5th day of incubation as compared to non-fermented ethanolic extract of wheat grain (41.52%). Similarly, in fermented wheat flour and bran, maximum inhibition (32.87 and 43.89%) was observed on 5th day of incubation which was significantly higher than their respective controls that is 11.45 and 12.68%, respectively (Figure 2).
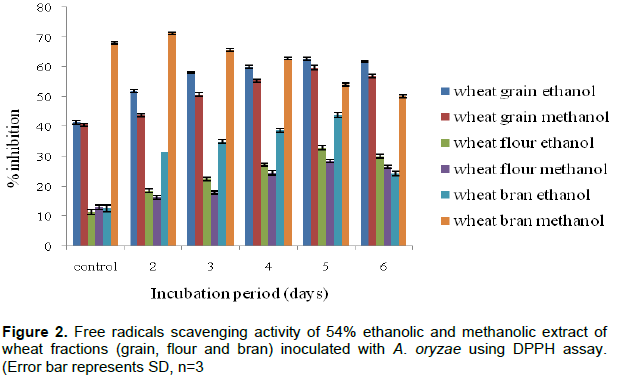
Inhibition percentage of free radicals formation was also studied for methanolic extract of fermented and non-fermented wheat grain, flour and bran with same fungus. Maximum percentage of inhibition (59.67 and 28.52%) was observed in wheat grain and flour on 5th day of incubation which was significantly different from their respective control (40.75 and 13.13 %). After 5th day, the inhibition percentage of free radical formation was decreased. Methanolic extract of fermented wheat bran showed maximum inhibition (71.30 %) on 2nd day of incubation as compared to control (68.04%) and further increase in incubation period lead to sharp decrease in free radicals inhibition. It has been observed that fermented wheat fractions have more antioxidant components than the non-fermented ones.
ABTS assay
Another antioxidant assay used was ABTS+ radical cation decolourisation assay which is widely used for the assessment of free radical scavenging activity of various substrates. The ABTS assay also showed quite similar results to those obtained in DPPH assay. The highest ABTS+ scavenging activity for ethanolic extract of wheat bran (28.48%) was observed on 5th day of inoculation. Subsequently slight decrease in inhibition was recorded after the 5th day. Almost similar inhibition was noticed on the 5th day in wheat flour and grain extracts (26.03 and 21.77 % respectively). Likewise maximum ABTS+ scavenging activity was recorded in methanolic extract of wheat bran (43.55%) followed by wheat grain (27.52%) and least in wheat flour (19.50%) where the respective values in controls were 20.81, 17.61 and10.99% respectively (Figure 3).
Amylase assay
Amylase is a classical calcium containing enzyme that catalyze the hydrolysis of starch and related carbohydrates by randomly cleaving internal αâ€Dâ€(1â€4) glycosidic linkage, yielding glucose, maltose, maltotriose and other oligosaccharides. This investigation focused on the activity of amylase under solid substrate fermentation for the enhanced release of polyphenol from wheat grain, flour and bran.
In case of wheat grain fermented by A. oryzae, maximum amylase activity (5.46 ± 0.04 µM/mL) was observed on 5th day of incubation, which was higher than its control (4.37 ± 0.02). However enzyme activity decreased with further increase in incubation period. Similarly amylase activity was increased with increase in incubation time in wheat flour and becomes highest on 4th day (7.84 ± 0.64 µM/mL). In case of wheat bran, maximum enzyme activity (5.54 ± 0.03 µM/mL) was noticed on 4th day of incubation (Figure 4). These results show that an amylase activity increase with increase in incubation period and becomes highest on the 4th and 5th day of incubation and then after declines.
In this study, solvents with different polarities including methanol and ethanol were used to extract antioxidants from wheat (WH-711) after fermentation with A. oryzae and it has been suggested that no single solvent can extract all the antioxidants from food because of its variation in solubility and polarity (Garcia-Alonso et al., 2004; Sun and Ho, 2005; Iqbal and Bhanger, 2007). Maximum phenolic content was observed in fermented wheat bran followed by wheat grain and flour. Enrichment of phenolic compound through SSF has also been reported in black bean (Lee et al., 2007), soybean (Mccue and Shetty, 2003), cranberry pomace (Vattem and Shetty, 2002) fava bean (Randhir et al., 2004) and wheat (Bhanja et al., 2009). Solvent extraction is a commonly used method to obtain anti-oxidants from plant materials. Moreover Yao et al., (2004) suggested that methanolic extraction gave higher phenolic content in wheat bran and grain than ethanolic extraction. Methanol is said to be the most suitable solvent in the extraction of phenolic compounds due to its ability to inhibit the reaction of polyphenol oxidase that causes the oxidation of phenolics. Several studies reported that methanol extracted samples had high phenolics and antioxidant activities (Ara and Nur, 2009; Jayasri et al., 2008; Chew et al., 2008; Mothana et al., 2010). The lysis ability of the solvent and the use of additional cell wall disruption techniques favour the extraction yield. To extract more polar pigments, such as carotenoids, hydrophilic solvents like methanol play the main role in extraction, which is not so influenced by the use of physical or mechanical disruption methods (Henriques et al., 2007).
DPPH and ABTS were used to determine the anti-oxidant activity of a specific compound or plant extracts (Koleva et al., 2002). In the DPPH assay, free radical accepts hydrogen and gets reduced by an antioxidant. The DPPH radical solution possesses a distinct purple color which disappears as antioxidants react with DPPH and it breaks the radical chain reaction by donating a hydrogen atom (Bhanja et al., 2008). Increase in antioxidant activity in the fermented wheat fractions are in conformity with the finding of Brand- Williams et al. (1995) which reveals that there were more antioxidant components in fermented okara than in non-fermented ones. Fermented okra are free radical inhibitors or scavengers, acting possibly as primary antioxidants. These results are similar with that of soybean kojis by various organisms (Lin et al., 2006) and fermented okra by Bacillus subtilis B2 (Zhu et al., 2008). Total phenolic contents and DPPH radical scavenging activity of wheat fractions were significantly co-related (P<0.01) which provide the strong evidence that the antioxidant activity in wheat fractions is derived from phenolic compounds (Table 1). Increased inhibition percentage of free radical formation in fermented samples than the non-fermented ones was supported by many other findings (Duenas et al., 2005; Jonnalagadda et al., 2011). In mung beans and black beans similar results were obtained, where SSF significantly increased the phenolic content, thus enhancing the antioxidant activity of the beans (Randhir and Shetty, 2007). Similar to DPPH assay, total phenolic content and ABTS+ scavenging activity was also significantly correlated (P<0.01) (Table 1). Phenolic compounds have been established to exert a scavenging effect for free radicals (Shahidi et al., 1992). High antioxidant activity observed in wheat fractions could be correlated to their high total phenolic contents. Similar results have been reported by several researchers.
Amylase production reached its maximum level after 120 h of incubation. On further incubation, the enzyme activity gradually decreased. This may be due to the depletion of essential nutrients required for the growth and enzyme production (Kumar and Duhan, 2011; Kumar
et al., 2013). Amylase hydrolyzes starch, glycogen and related polysaccharides by randomly cleaving internal glucosidic linkages to produce different sizes of oligosaccharides (Bhanja et al., 2007). Total phenolic contents and amylase activity of wheat fractions were significantly (P<0.01) correlated (Table 2). It was evident in this study that phenolic contents increased after fermentation, which may be due to the hydrolytic enzymes produced by fungi that catalyze the release of aglycones from the substrate and hence increase the phenolic content and antioxidant potential as well.
This investigation showed that the solid state fermentation carried out with A. oryzae was quite effective for the improvement of phenolic contents and free radicals scavenging activity of wheat fractions (grain, bran and flour). Maximum antioxidant activity was noticed in bran in both the extracts followed by grain and flour in methanolic extracts and flour and grain in ethanolic extracts. Amylase also played an important role in release of phenolics during the fermentation with the fungus. So it can be concluded that solid state fermentation can be used to improve the neutraceutical properties of wheat fractions. These results have shown that the fermented wheat fractions are antioxidant rich and may be used for the preparation of healthy food supplement as compared to non-fermented wheat fractions.
No conflict of interest among the authors.
DPPH, 2, 2-diphenyl-1-picrylhydrazyl; TPC, Total phenolic content; ABTS, 2, 2-azinobis-3 ethylbenzothiazoline-6-sulphonic acid; MTCC, Microbial type culture collection.
REFERENCES
|
Afolabi C, Akinmoladun EO, Ibukun EA, Akinrinlola BL, Onibon TR, Akinboboye AO, Obuotor EM, Farombi EO (2007). Chemical constituents and antioxidant activity of Alstonia boone. Afr. J. Biotechnol. 6(10):1197-1201.
|
|
|
|
Ara N, Nur H (2009). In vitro Antioxidant activity of methanolic leaves and flowers extracts of Lippia Alba. Res. J. Med. Med. Sci. 4(1):107-110.
|
|
|
|
Arnao MB, Cano A, Acosta M (2001). The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 73:239-244.
Crossref
|
|
|
|
Arnous A, Makris DP, Kefalas P (2001). Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 49:5736-5742.
Crossref
|
|
|
|
Belderok B (2000). Developments in bread-making processes. Plant Foods Hum. Nutr. 55:1-14.
Crossref
|
|
|
|
Bhanja T, Kumari A, Banerijee R (2009). Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filmentous fungi. Bioresour. Technol. 100(11):2861-2866.
Crossref
|
|
|
|
Bhanja T, Rout S, Banerjee R, Bhattacharyya BC (2007). Comparative profiles of a-amylase production in conventional tray reactor and GROWTEK bioreactor. Bioprocess. Biosyst. Eng. 30(5):369-376.
Crossref
|
|
|
|
Bhanja T, Rout S, Banerjee R, Bhattacharyya BC (2008). Studies on the performance of a new bioreactor for improving antioxidant potential of rice. LWT-Food Sci. Technol. 41(8):1459-1465.
|
|
|
|
Bojnanska T, Francakova H, Chlebo P, Vollmannova A (2009). Rutin content in buckwheat enriched bread and influence of its consumption on plasma total antioxidant status. Czech J. Food Sci. 27:236-240.
|
|
|
|
Brand W, Cuvelier ME, Berset C (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28:25-30.
|
|
|
|
Cassidy A (1996). Physiological effects of phyto-estrogens in relation to cancer and other human health risks. Proc. Nutr. Soc. 55:399-418.
Crossref
|
|
|
|
Chew YL, Lima YY, Omar M, Khoo KS (2008). Antioxidant activity of three edible seaweeds from two areas in South East Asia. Food Sci. Technol. 41(6)1067-1072.
Crossref
|
|
|
|
Doll R, Peto R (1981). The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 66:1197-1265.
|
|
|
|
Duenas M, Fernández D, Hernandez T, Estrella I, Munoz R (2005). Bioactive phenolic compounds of cowpeas (Vigna sinensis L.). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 85:297-304.
Crossref
|
|
|
|
Duhan JS, Bhardwaj M, Surekha (2011a). Free radical-scavenging and antimutagenic potential of acetone, chloroform and methanol extracts of leaf of Argemone mexicana. Int. J. Pharma. Bio. Sci. 2(1):B455-B464.
|
|
|
|
Duhan JS, Bhardwaj M, Surekha (2011b). Free radical-scavenging and antimutagenic potential of acetone, chloroform and methanol extracts of fruit of Argemone maxicana, Afr. J. Biotechnol. 10(43):8654-8661.
|
|
|
|
Duhan JS, Rana A, Sadh PK, Saharan P, Surekha (2015).Antimicrobial and free radical scavenging activity of selective medicinal plants combination. World J. Pharm. Pharm. Sci. 4(3):1202-1216.
|
|
|
|
Garcia-Alonso M, de Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC (2004). Evaluation of the antioxidant properties of fruits. Food Chem. 84:13-18.
Crossref
|
|
|
|
Halliwell B (1996). Oxidative stress, nutrition and health: Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic. Res. 25:57-74.
Crossref
|
|
|
|
Halliwell B, Murcia HA, Chirco S, Aruoma OI (1995). Free radicals and antioxidants in food an in vivo: what they do and how they work. CRC Crit. Rev. Food Sci. Nutr. 35:7-20.
Crossref
|
|
|
|
Hejtmankova K, Lachman J, Hejtmankova A, Pivec V, Janovska D (2010). Tocols of selected spring wheat (Triti¬cum aestivum L.),einkorn wheat (Triticum moonococcum L.) and wild emmer (Triticum dicoccum Schuebl) varieties. Food Chem. 123:1267-1274.
Crossref
|
|
|
|
Henriques M, Silva A, Rocha J (2007). Extraction and quantification of pigments from a marine microalga: a simple and reproducible method. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 586-593.
|
|
|
|
Hidalgo A, Brandolini A, Pompei C, Piscozzi R (2006). Carot¬enoids and tocols of einkorn wheat (Triticum monococcum ssp. monococcum L.). J. Cereal Sci. 44:182-193.
Crossref
|
|
|
|
Imaida K, Fukushima S, Shivai T, Ohtani M, Nakanishi K, Ito N (1983). Promoting activities of butylated hydroxyanisole and butylated hydroxytoluene on 2-stage urinary bladder carcinogensis and inhibition of -glutamyl transpeptidase-positive foci development in the liver of rats. Carcinog. 4:895-899.
Crossref
|
|
|
|
Iqbal S, Bhanger MI (2007). Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem.100:246-254.
Crossref
|
|
|
|
Jayasri MA, Mathew L, Radha A (2009). A report on the antioxidant activity of leaves and rhizomes of Costus pictus D. Don. Int. J. Integr. Biol. 5(1):20-26.
|
|
|
|
Jonnalagadda SS, Harnack L, Liu RH, Mckeown N, Seal C, Liu S, Fahey GC (2011). Putting the whole grain puzzle together: Health benefits associated with whole grains-summary of American society for nutrition 2010 satellite symposium. J. Nutr. 141:1011S-1022S.
Crossref
|
|
|
|
Koleva II, Van beek TA, Linssen JPH, De Groot A, Evstatieva LN (2002). Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal. 13:8-17.
Crossref
|
|
|
|
Kumar A, Duhan JS (2011). Production and characterization of amylase enzyme isolated from Aspergillus niger MTCC-104 employing solid state fermentation. Int. J. Pharm. Bio. Sci. 2(3):250-258.
|
|
|
|
Kumar A, Duhan JS, Tanwar SK (2013). Screening of Aspergillus spp. for extra cellular α-amylase activity. In: Khanna DR, Chopra AK, Matta G, Singh V, Bhutiani R. (Eds). Impact of Global Climate Change on Earth Ecosystem. Biotech Books, New Delhi. pp. 205-214.
|
|
|
|
Lateef A, Oloke JK, Gueguim kana EB, Oyeniyi SO, Onifade OR, Oyeleye AO, Oladosu OC, Oyelami AO (2008). Improving the quality of agro-wastes by solid-state fermentation: enhanced antioxidant activities and nutritional qualities. World J. Microbiol. Biotechnol. 24:2369-2374.
Crossref
|
|
|
|
Lavelli V, Hidalgo A, Pompei C, Brandolini A (2009). Radical scavenging activity of einkorn (Triticum monococcum L. subsp. monococcum) wholemeal flour and its relationship to soluble phenolic and lipophilic antioxidant content. J. Cereal Sci. 49:319-321.
Crossref
|
|
|
|
Lee IH, Hung YH, Chou CC (2008). Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 121:150-156.
Crossref
|
|
|
|
Lin CH, Wei YT, Chou CC (2006). Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 23:628-633.
Crossref
|
|
|
|
Liu RH (2007). Whole grain phytochemicals and health. J. Cereal Sci. 46:207-219.
Crossref
|
|
|
|
Liyana-Pathirana C, Shahidi F (2006). Importance of insoluble bound phenolics to antioxidant properties of wheat. J. Agri. Food Chem. 54:1256-1264.
Crossref
|
|
|
|
Mccue P, Shetty K (2003). Role of carbohydrate-cleaving enzymes in phenolic antioxidant mobilization from whole soybean fermented with Rhizopus oligosporus. Food Biotechnol. 17(1):27-37.
Crossref
|
|
|
|
Miller GL (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31:426-429.
Crossref
|
|
|
|
Ramzi AA Mothana, Salah AA Abdo, Sidgi Hasson, Faisal MN Althawab, Sama A Z Alaghbari, Ulrike Lindequist (2010). Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some yemeni medicinal plants. Evid. Based Complementary Altern. Med. 7(3):323-330.
Crossref
|
|
|
|
Rana A, Saharan P, Sadh PK, Surekha, Duhan JS (2014). Free radical scavenging and antimicrobial potential of mixture of selective medicinal plants. Asian J. Pharm. Clin. Res. 7(4):27-32.
|
|
|
|
Randhir R, Shetty K (2007). Mung beans processed by solid-state bioconversion improve phenolic content and functionality relevant for diabetes and ulcer management. Innov. Food Sci. Emerg. Technol. 8:197-204.
Crossref
|
|
|
|
Randhir R, Vattem D, Shetty K (2004). Solid-state bioconversion of fava bean by Rhizopus oligosorus for enrichment of phenolic antioxidants and L-DOPA. Innov. Food Sci. Emerg. Technol. 5:235-244.
Crossref
|
|
|
|
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Evans C (1999). Antioxidant activity applying an improved ABTS free radical cation decolorization assay. Free Radic. Biol. Med. 26:1231-1237.
Crossref
|
|
|
|
Saharan P, Duhan JS (2013). Studies on antioxidant activity, total phenolic and flavanoid contents of leaf extracts of Thuja orientalis. In: Khanna DR, Chopra AK, Matta G, Singh V, and Bhutiani R. (Eds). Impact of Global Climate Change on Earth Ecosystem. Biotech Books, New Delhi. 193-203.
|
|
|
|
Saharan P, Duhan JS, Gahlawat SK, Surekha (2012). Antioxidant potential of various extracts of stem of Thuja orientalis: In vitro study. Int. J. Applied Biol. Pharm. Technol. 3(4):264-271.
|
|
|
|
Shahidi F, Janitha PK, Wanasundara PD (1992). Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 32:67-103.
Crossref
|
|
|
|
Singh R, Singh S, Kumar S, Arora S (2007). Studies on antioxidant potential of methanol extract/fractions of Acacia auriculiformis. A. Cunn. Food Chem. 103:505-511.
Crossref
|
|
|
|
Singleton VL, Rossi JA (1965). Colorimetry of total phenolics with phospho molibdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144-158.
|
|
|
|
Stratil P, Klejdus B, Kuban V (2007). Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 71:1741-1751.
Crossref
|
|
|
|
Sun T, Ho CT (2005). Antioxidant activities of buck wheat extracts. Food Chem. 90:743-749.
Crossref
|
|
|
|
Vattem D, Shetty K (2002). Solid-state production of phenolic antioxidants from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 16:189-210.
Crossref
|
|
|
|
Willet WC (1994). Diet and health: What should we eat? Sci. 264:532-537.
Crossref
|
|
|
|
Wojdy OA, Oszmai SJ (2007). Comparison of the content phenolic acid, atochopherol and the antioxidant activity in oat naked and weeded. Elec. J. Env. Agric. Food Chem. 6:1980-1988.
|
|
|
|
Yang JH, Mau JL, Ko PT, Huang LC (2000). Antioxidant properties of fermented soybean broth. Food Chem. 71:249-254.
Crossref
|
|
|
|
Yao LH, Jiang YM, Datta N, Singanusong R, Liu X, Duan J, Raymont K, Lisle A, Xu Y (2004). HPLC analyses of flavanols and phenolic acids in the fresh young shoots of tea (Camellia sinensis) grown in Australia. Food Chem. 84(2):253-263.
Crossref
|
|
|
|
Yu L, Haley S, Perret J, Harris M (2002). Antioxidant properties of hard winter wheat extracts. Food Chem. 78:457-462.
Crossref
|
|
|
|
Zhu YP, Fan JF, Cheng YQ, Li LT (2008). Improvement of the antioxidant activity of Chinese traditional fermented okara (Meitauza) using Bacillus subtilis B2. Food Control.19:654-661.
Crossref
|
|
|
|
Zielinski H, Kozowska H (2000). Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 48:2008-2016.
Crossref
|