ABSTRACT
The aim of this study was to assess the level of pesticide residues in locally produced grape wine in Tanzania. Fifty samples of grape wine from different locations in Dodoma urban and Bahi district were analyzed to determine the presence of 49 pesticides using the quick, easy, cheap, effective, rugged and safe (QuEChERS) multi-residue extraction, followed by gas chromatography-tandem mass spectrometry (GC-MS/MS). Twenty-two pesticides were detected among the 49 pesticides analyzed. The selected 49 pesticides was based on its use in grape cultivation which were reported by grape farmers in the study location which includes fungicides, insecticides and herbicides. The pesticides whose concentrations exceeded the maximum residue levels (MRL) were: Pyroquilon, ethofumasate, chlorobeb, azobenzene and cycloate in 38, 33, 46, 14 and 1 wine samples, respectively. Of the samples analyzed 9 (18%) contained one pesticide, 8 (16%) contained two different pesticides, 23 (46%) contained three different pesticides 8 (16%). The results indicated the occurrence of pesticide residues in grape wine produced in Dodoma urban and Bahi districts, Tanzania, and pointed to an urgent need to develop comprehensive intervention measures to reduce potential health risk to consumers.
Key words: Pesticides residues, grape wine, food safety, maximum residue levels (MRL).
Grapes (Vitis vinifera) belong to the Vitaceae family and are one of the world’s most important economic fruit crops (Kocher and Nikhanj, 2019). This crop has many uses as it can be eaten raw or can be used for the manufacture of wine, jam, juice, jelly, grape seed-extracts, raisins, vinegar and grape-seed oil (Kalimang’asi et al., 2014; Grimalt and Dehouck, 2016). Approximately 4,744 tons of grapes were produced in Tanzania in the year 2018 while the largest producer of grapes in the world for the year 2018 was China with 13,494,811 tons (FAOSTAT, 2020). Grape production is the mainstay for many farmers in the Dodoma region, including Dodoma city, Bahi, Chamwino and Kongwa districts (Kalimang’asi et al., 2014). The excellence of wine depends on the quality of grapes and to obtain high-quality wines, grapes at the correct stage of ripeness and free from parasites must be used (Caboni and Cabras, 2010). In addition, the quality of wine depends on the vinification process, the geographical origin of the grapes as well as the varietal composition of the grape wort; therefore, grapes traceability is important in quality control and suppliers’ information (Espineira and Santaclara, 2016).
There is increasing interest in health and safety aspects associated with pesticide use and the presence of their residues in processed foods and beverages (Lian et al., 2010). Pesticide use in viticulture is a major issue for grape protection, increased productivity and wine quality (Martins et al., 2011). Wine has beneficial effects on human health, if moderately consumed, including prevention of heart and circulatory diseases, favorable to the fight against obesity, provides greater longevity and quality of life, creates barriers to the development of dementia, the meal accompanied by wine results in a better digestion, anti-infective effect, can prevent blindness, have anti-inflammatory action and can alleviate lung diseases (Wurz, 2019).
However, wine may also contain components, which have detrimental effects on human health such as pesticide residues, as a result of their use in viticulture and those that can remain on grapes after harvest and may be transferred to the wine (Grimalt and Dehouck, 2016; Cepo et al., 2018). Grey mould (Botrytis cinerea), powdery mildew (Erysiphe necator) and downy mildew (Plasmopara viticola) are responsible for serious yield loss in the wine sector resulting in significant commercial losses (Weng et al., 2014). In addition to an important crop yield loss, these diseases can also reduce the wine quality by providing an unstable colour, oxidative damages, premature aging, unpleasant flavours, and clarification difficulties (Rodriguez et al., 2020). The correct use of these phytosanitary products has no adverse effects on public or environmental health, but indiscriminate treatment applied without respecting the safety periods or the recommended doses may result in residues being present in the grapes used for winemaking these fungicides may be passed on to the must and then to the wine during fermentation (Vaquero-Fernandez et al., 2012).
Grapes are usually harvested and directly used for follow-up fermentation without washing or other treatment to reduce pesticides residues, this may lead to their presence in wine offered commercially or public consumption, therefore, their determination in grape wine is designed to ensure the safe consumption of the important beverage in the community (Jiang et al., 2009).
In order to ensure food safety for consumers and protect human health, many organizations and countries around the world have established Maximum Residue Limits (MRLs) for pesticides in food commodities (Cepo et al., 2018). The MRL is the maximum level of a pesticide residue (expressed in mg/kg) which is legally permitted in food or animal feed (Jallow et al., 2017). The MRLs which specify the maximum concentration of a pesticide that can exist in certain agricultural commodities were regulated by many nations to promote good agricultural practice (GAP) (Lekei et al., 2016). Furthermore, current agricultural practices are based on the wide use of chemical pesticides that have been associated with negative impacts on human health, wildlife and natural environment (Goulson, 2013; Nicolopoulou-Stamati et al., 2016). This, in turn, raises concerns among consumers and producers due to possible health hazards as well as the impact of these residues on the sensory quality of wines. These facts emphasized the need for continuous monitoring of pesticide residues in wine (Cepo et al., 2018).
The study aims to establish the levels of pesticide residues in locally manufactured wine, particularly in the leading producer region Dodoma, in order to determine its quality and safety. This study will serve as a basis for awareness creation to farmers, consumers, processors and other stakeholders and enable the government to regulate the sector and advocate use of best practices and prevent economic losses.
Sample collection
Fifty bottled grape wine samples were collected from all 15 wine processors, branded (with label) and unbranded (without label sold in bulk) in Dodoma urban (capital of Tanzania) and Bahi district in Dodoma region, these districts were selected purposively due to high production of grapes. Approximately one liter of each branded and unbranded wine 36 red wines and 14 white wine from small, medium and large scale industries was packed into sterile amber plastic containers, sealed, coded, stored at -2°C and transported to the Tanzania Bureau of Standards laboratory in Dar es Salaam. The samples were stored at -2°C for further analysis.
Sample extraction
Quick, easy, cheap, effective, rugged and safe (QuEChERS) method (Anastassiades et al., 2003; Jiang et al., 2009; Pazzirota et al., 2013; Wang and Telepchak, 2013) with some modifications were used to extract pesticide residues from the samples. 10 ml of sample was transferred into an empty 50 ml centrifuge tube polytetrafluoroethylene (PTFE), followed by 10 ml of acetonitrile (Romil Ltd The Source Convent Drive Waterbeach Cambridge Gb-Cb259QT) and vortexed for 1 min using Stuart auto vortex Mixer (Stuart Scientific Co. Ltd-England). A blank sample was fortified with 10 ml of standard pesticides.
A sachet made up of 4 g of magnesium sulfate anhydrous, 1 g of sodium chloride 1 g of trisodium citrate dihydrate, and 0.5 g of disodium hydrogen citrate sesquihydrate was added (Quercher Extract Pouch, En Method shaked and vortexed for 1 min, then centrifuged for 5 min at 4,000 rpm using Centrifuge 5810R (Eppendorf Ag Co. Ltd Harmburg, Germany).
Sample clean up
Exactly 7.5 ml of the supernatant (upper layer) was transferred into 15 ml PTFE, then 750 mg of MgSO4 (Surechem Products Ltd Suffolk, England) and 150 Primary Secondary Amine (PSA) was added into PTFE tube. The mixture was then vortexed for one minute then centrifuged at 4,000 rpm for 5 min. Then 5 ml of extracted solution was added into a test tube and evaporated to near dryness under nitrogen at the temperature below 42°C. Then reconstituted to 1 ml with toluene and homogenized for 5 s and transferred into autosampler vials.
Blank sample preparation
A blank sample was prepared using distilled water which was prepared using Evoqua Water Technologies PTE LTD Farrernberg-Germany) and also wine from South Africa which was free from pesticide residues used as black and the same procedures for extraction was followed.
Standard preparation
Individual pesticides solution was prepared by 10 mg of each standard pesticides purchased from (Sigma- Aldrich Co. Ltd. Steinheim am Albuch - Germany) were dissolved in 10 ml acetone and stored at -20°C, then intermediate Standard solution was prepared by 100 µl stock standard solution which was brought to 10 ml volumetric flask mixed with acetone and stored at 2°C. Working solution was prepared by 10 µl which was dissolved to 10 ml acetone and six levels of an intermediate standard solution of each pesticide were prepared to maintain the same matrix concentration for the preparation of calibration curve and stored at 2°C.
Gas chromatography-tandem mass spectrometry (GC-MS-MS) conditions
An Agilent 7010 GC/MS Triple Quad (Agilent Technologies Co. Ltd. Waldbronn-Germany) with a 7693 Autosampler with a capacity of 150 samples at a time was used for the analysis of pesticide. GC-Column –J&W HP-5MS UI (15 m × 0.25 mm i.d. × 0.25 mm film thickness) was used for GC separation, with helium (99.99%) as the carrier gas at a constant flow rate of 1.5 ml/min. The column inlet temperature was initially at 60°C (hold for 1 min), increased to 120°C at rate of 40°C/min, then to 310°C at a rate of 5°C/min, the total holding time was 40.50 min. Quantification and results calculation were done by mass hunter software using the following formula.
Concentration of each analyte (mg/L) = Concentration from curve × dilution factor
Where by, concentration from curve = Peak area of the analyte/Peak area of internal standard.
Linearity was studied in the range 0.5 - 100 mg/L with five calibration points (0.5, 10, 25, 50, 75 and 100 mg/L) by matrix-matched standard calibration which were spiked with the corresponding volume of the working solution. Linear calibration graphs were constructed by least-squares regression of concentration versus relative peak area (analyte/IS) of the calibration standards. Linearity values, calculated as determination correlation coefficients (R2), were in the range 0.986-0.999 (Table 1).
Pesticides analysis
Wine samples were analyzed at the Tanzania Bureau of Standards (TBS) quality control laboratory, Dar es Salaam. The samples which were stored at -2°C were equilibrated to room temperature (25°C) before analysis.
Statistical analysis
Data were analyzed using R- version 3.5.0 (2018). Analysis of variance (ANOVA) was used to test significant differences on the pesticide residues concentration (mg/L) amongst the brand, color of wine and among the categories of processors (Large, Medium and Small Scale). Statistical Package for Social Sciences (IBM SPSS® Version 20) was used to test compliance of pesticide residues detected to European Union Database MRL.
Method validation
Percentage recovery of pesticides
Recovery studies were performed to examine the efficacy of extraction and clean up. Grape wine samples were spiked with known concentration of the pure pesticides standard solution and extraction and clean-up were performed as described earlier in the process of sample extraction.
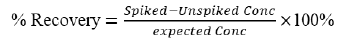
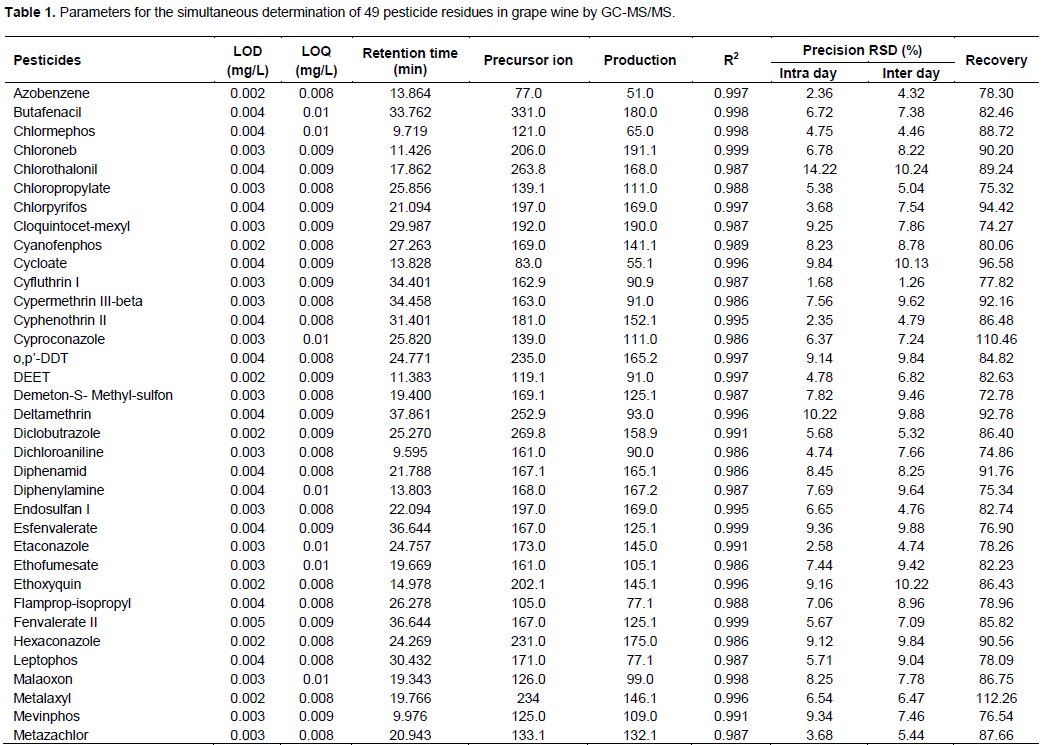
The sensitivity of the method was obtained by determining the percent recovery of pesticides by calculating the percentage recovery of pesticides spiked samples and unspiked samples. Accuracy was evaluated in terms of recovery, and the satisfactory recoveries were from 70 to 110%) indicating the suitability and good performance of GC-MSMS). To test the performance of an analytical method, the following criteria have to be considered: pesticide recoveries should be in the range 70-120%. The precision of the method was studied; intra- and inter-day variations were estimated and expressed as relative standard deviation (RSD) of the signals or peak areas for each analyte following an analysis of 0.1 mg/L standard working solution injected five times consecutively on the same day and injected five times over four consecutive days. The results in Table 1 showed that inter-day variation of peak areas for 49 pesticides were in the range of 1.26- 10.24%, and intra-day variations of 1.68-14.22% (Vaquero-Fernandez et al., 2012; Machado et al., 2016).
Limit of detection and quantification
The qualitative pesticides were based on the retention time of peak and abundance ratios of the selected ions for each pesticides. Limit of detection (LOD) and limit of quatification (LOQ) was determined based on the signal to noise ratio of quantifier transition of all analyte. The retention time, precusor and product ion, LOD, LOQ of the analyzed pesticides on the samples are presented in Table 1.
Pesticide residues in grape wine
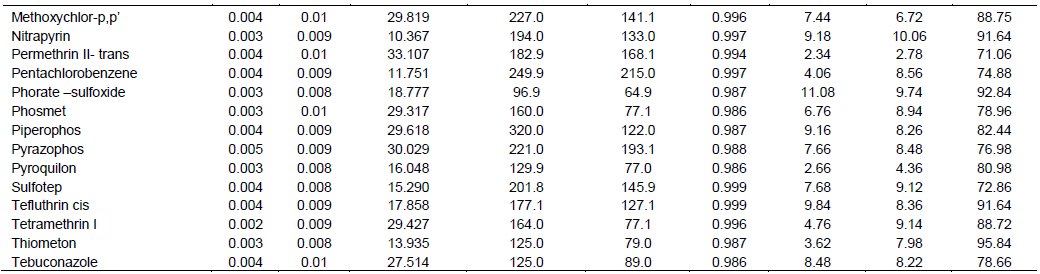
Results indicated that, twenty-two pesticides were detected among the 49 pesticides analyzed (Figure 1).
These included: mevinphos, thiometon, diethyl-m-toluene (DEET), chloroneb, dipnenylamine, pyroquilon, metalaxyl, Chlorpyrifos, endosulfan 1, o, p´-DDT, cryproconazole, tebuconazole, deltamethrin, cypermethrin-beta, cyanofenophos, ethofumesate, metazachlor, azobenzene, cycloate, phosmet, chloropropylate and chlormephos. Among the detected pesticides, there were five pesticides that exceeds the MRL according to the European Union pesticides specification of grape wine. These pesticides included pyroquilon whose concentation exceeded MRL in 38 wine samples, ethofumesate exceeded MRL in 33 samples, chloroneb exceeded MRL in 46 sample, azobenzene exceeded MRL in 14 samples and cycloate exceeded MRL in 1 sample.
Among the 50 wine samples analysed 9 (18%) contained one pesticide, 8 (16%) contained two different pesticides, 23(46%) contained three different pesticides 8 (16%) contained four diffent pesticides among those exceeds MRL (pyroquilon, ethofumesate, chloroneb, azobenzene and cycloate). The incidence of having multiple pesticide residues in grape wine sample was reported in different studies. The study conducted by Cus et al. (2010) on pesticide residues and microbiological quality of bottled wine indicated that, among 25 wines, two wines did not contain residues of pesticides analyzed, eight wine samples contained residues of one pesticide, four wine samples contained the residues of two pesticides, seven wine samples contained the residues of three, and four wine samples contained the residues of four pesticides.
Also the study conducted by European Food Safety Authority in the year 2018, on pesticide residues in food, it was reported that, 1,317 samples of wine (red or white) made from grapes were analyzed, where 768 samples (58.3%), had no quantifiable pesticide residues, while 549 samples (41.7%) contained one or several pesticides in quantified concentrations and multiple residues were reported in 263 samples (20%); up to 10 different pesticides were reported in an individual wine sample (EFSA, 2018). The incidence of having multiple pesticide in grape wine were also reported by (Duca et al., 2012; Esteve-Turrillas et al., 2016).
Presence of multiple pesticides in grape wine might be due to unfavourable weather conditions most commonly occurring in geographical area which favours the development of pests and diseases in the vineyards, so more intense application of different types of pesticides are generally required in order to safeguard grape quality, which eventually may result in greater fungicide residues in wines (Esteve-Turrillas et al., 2016; Jallow et al., 2017).
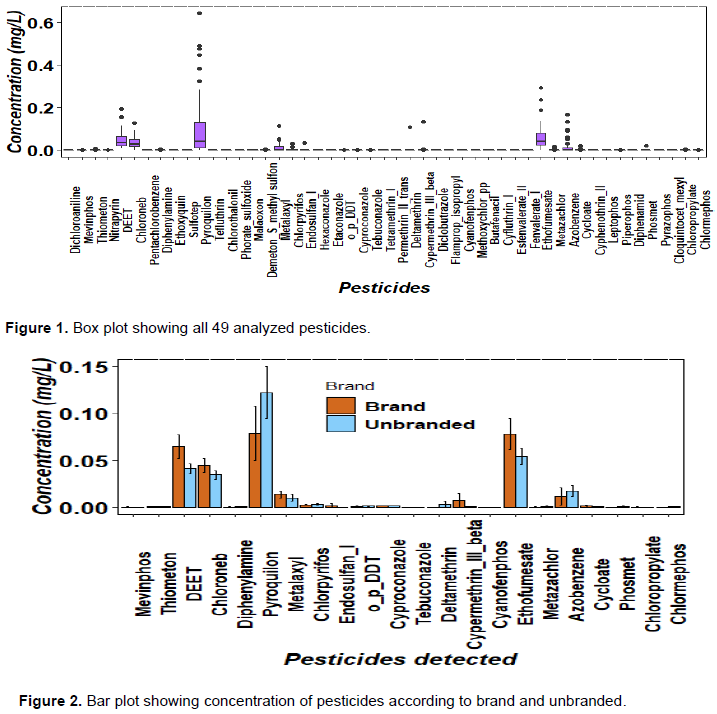
All pesticides that were not detected were removed during data analysis therefore twenty-two pesticides was used for further statistical analysis such as ANOVA. The study revealed that there was significant difference at (p<0.05) on pesticide concentration when comparing according to branded and unbranded grape wine (Figure 2). There was high concentration in unbranded wine compared to branded wine especially for Pyroquilon, Chlorpyrifos, o,p’-DDT, Deltamethrin and Azobenzene. According to the survey conducted to wine processors with the branded wine (labeled) they have high production capacity and they undergoes wine clarification and fermentation take long time compared to unbranded wine processors which help to reduce pesticide residues in grape wine. Wine processors of unbranded wine they usually depend on spontaneous fermentation by using natural fermenting yeasts present in grapes and they usually sell their products in bulk without clarification stage. Wine clarification help to reduce the level of pesticides residues. Among the clarifying substances commonly used in wine (bentonite, charcoal, gelatin, polyvinylpolypyrrolidone, potassium caseinate, and colloidal silicon dioxide), charcoal allowed the complete elimination of most pesticides, especially at low levels, whereas the other clarifying substances were ineffective (Cabras and Angioni, 2000). Also during the fermentative process, yeasts can cause the disappearance of pesticide residues by degradation or absorption at the end of the fermentation when yeasts are deposited as lees (Caboni and Cabras, 2010). It was also observed that deltametrin, permethrin and fenvalerate were completely degraded after fermentation with Saccharomyces cerevisiae that can be attributable to the yeast activity, while the fungicides benalaxyl, folpet, furalaxyl, metalaxyl, iprodione, procymidone, and ofurace remained unaffected (Regueiro et al., 2014). Malolactic fermentation using Oenococcus oeni resulted in significant reduction in chlorpyrifos and dicofol concentrations which were reduced by 70 and 30%, respectively, where as the concentrations of chlorothalonil and procymidone diminished only slightly (Bajwa and Sandhu, 2011).
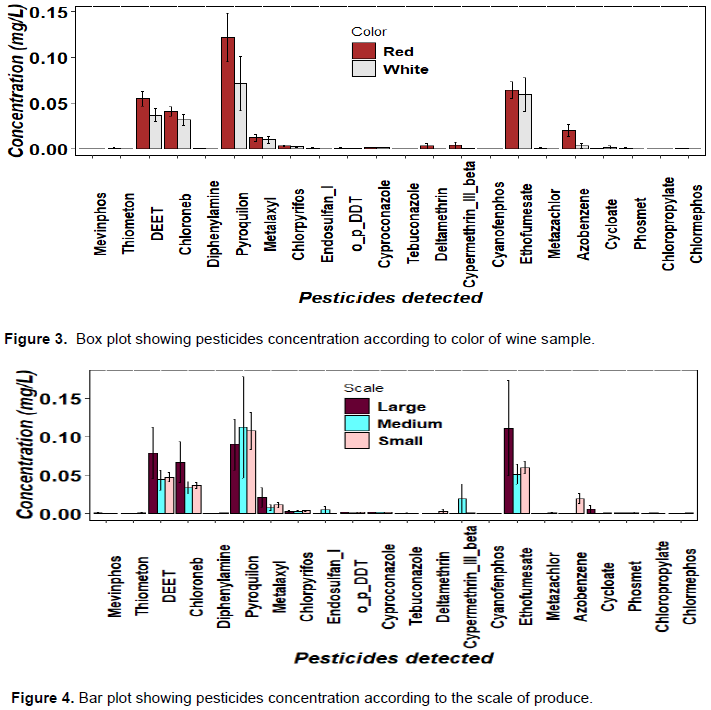
There was a significance difference (p<0.05) in pesticides concentration with colour of grape wine (Figure 3). The study showed that red wine has high concentration of pesticide residues compared to white wine this suggest the idea that there might be a correlation between applied winemaking technology, red or white the skin maceration could contribute to higher degree of pesticide residues transferred to wine. Fermentation on the skins, as carried out in red wine production, is likely to lead to higher residue levels in raw wine (Pazzirota et al., 2013). This was also observed in another study which was conducted in Moldovan wine products originated from traditional agriculture which showed that red wine had higher levels of pesticide residues than white wine due to grape skin maceration (Duca et al., 2012; Regueiro et al., 2014). The study revealed that there was no significance difference (p<0.05) on pesticides concentration when comparing to scales of grape wine producers (Figure 4). It was expected that there would be a large amount of pesticides in the wine produced by small producers due to the poor production system. But it was different because nothing was done to reduce the amount of pesticides before they started production, this was observed during survey conducted to different scale of wine producers.
This study investigated the levels of pesticide residues in grape wine produced in Dodoma urban and Bahi districts (Tanzania). The results indicated that, majority of grape wine samples were contaminated with pesticide residues some of which had their concentrations above the MRL. According to the public health perspective, the observed levels of pesticide residues would pose potential health risks to the public. In order to reduce health and environmental problems there is a need for sensitization to grape farmers, on better pesticide safety use and handling practices and the need for continuous pesticide residues through regular monitoring.
The authors have not declared any conflict of interests.
The authors appreciate the financial support from Tanzania Medicine and Medical devices formerly known as Tanzania Food and Drugs Authority and also grateful to Mr. Roman Fortunatus and Mr. Wiseborn Makaka of Tanzania Bureau of Standards (TBS) for technical assistance during samples extraction and analysis of pesticides residues.
REFERENCES
|
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and 'dispersive solid-phase extraction' for the determination of pesticide residues in produce. Journal of Official Analytical Chemists International 86:412-431.
Crossref
|
|
|
|
Bajwa U, Sandhu KS (2011). Effect of handling and processing on pesticide residues in food- a review. Journal of Food Science and Technology 51(2):201-220.
Crossref
|
|
|
|
|
Caboni P, Cabras P (2010). Pesticides influence on wine fermentation. Advances in Food and Nutrition Research 59:43-62.
Crossref
|
|
|
|
|
Cabras P, Angioni A (2000). Pesticide Residues in Grapes, Wine, and Their Processing Products. Journal of Agricultural and Food Chemistry 48(4):967-973.
Crossref
|
|
|
|
|
Cepo VD, Pelajic M, Vrcek IV, Krivohlavek A, Zuntar I, Karoglan M (2018). Differences in the levels of pesticides, metals, sulphites and ochratoxin A between organically and conventionally produced wines. Journal of Food Chemistry 246:394-403.
Crossref
|
|
|
|
|
Cus F, Cesnik HB, Bolta SV, Gregorcic A (2010). Pesticide residues and microbiological quality of bottled wines. Journal of Food Control 21:150-154.
Crossref
|
|
|
|
|
Duca G, Sturza R, Siretanu L (2012). Estimation of organic pesticide residues in wines of Moldova. Clean Soil Air Water 40(6):661-666.
Crossref
|
|
|
|
|
Espineira M, Santaclara FJ (2016). The use of molecular biology techniques in food traceability. Advances in Food Traceability Techniques and Technologies 2016:91-118.
Crossref
|
|
|
|
|
Esteve-Turrillas FA, Agullo C, Abad-Somovilla A, Mercader JV, Abad-Fuentes A (2016). Fungicide multiresidue monitoring in international wines by immunoassays. Food Chemistry 196:1279-1286.
Crossref
|
|
|
|
|
European Food Safety Authority (EFSA) (2018). The 2016 European Union report on pesticide residues in food. European Food Safety Authority Journal 16(7):3-139.
Crossref
|
|
|
|
|
FAOSTAT (2020). Grape production in Tanzania 2018.
|
|
|
|
|
Goulson D (2013). An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology 50(4):977-987.
Crossref
|
|
|
|
|
Grimalt S, Dehouck P (2016). Review of analytical methods for the determination of pesticide residues in grapes. Journal of Chromatography 1433:1-23.
Crossref
|
|
|
|
|
Jallow MFA, Awadh DG, Albaho MS, Devi VY, Ahmad N (2017). Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. International Journal of Environmental Research and Public Health 14(833):1-12.
Crossref
|
|
|
|
|
Jiang Y, Lia X, Xua J, Pana C, Zhang J, Niu W (2009). Multiresidue method for the determination of 77 pesticides in wine using QuEChERS sample preparation and gas chromatography with mass spectrometry. Food Additives and Contaminants 26(6):859-866.
Crossref
|
|
|
|
|
Kocher GS, Nikhanj P (2019). Development of red and white wines from locally adapted grape cultivars using indigenous yeast. Fermented Beverages 5:147-170.
Crossref
|
|
|
|
|
Kalimang'asi N, Majula R, Kalimang'asi NN (2014). The economic analysis of the smallholders grape production and marketing in Dodoma Municipal: A case study of Hombolo Ward. International Journal of Scientific and Research Publications 4(10):1-8.
|
|
|
|
|
Lekei EE, London L, Ngowi AV (2016). Underreporting of acute pesticide poisoning in Tanzania: modelling results from two cross-sectional studies. Environmental Health 15(1):1-9.
Crossref
|
|
|
|
|
Lian Y, Pang G, Shu H, Fan C, Liu Y, Feng J, Wu Y, Chang Q (2010). Simultaneous Determination of 346 Multiresidue Pesticides in Grapes by PSA- MSPD and GC-MS-SIM. Journal of Agricultural and Food Chemistry 58:9428-9453.
Crossref
|
|
|
|
|
Machado AF, Militao S, Sarubbi MM, Brandelli A (2016). Pesticide residues screening in wine by mass spectrometry. Bio Web of Conferences 7:1-4.
Crossref
|
|
|
|
|
Martins J, Esteves C, Simoes T, Correia M, Delerue-Matos C (2011). Determination of 24 pesticide residues in fortified wines by solid-phase micro extraction and gas chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry 5(13):6847-6855.
Crossref
|
|
|
|
|
Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L (2016). Chemical pesticides and human health: The urgent need for a new concept in agriculture. Frontiers in Public Health 4(148):1-9.
Crossref
|
|
|
|
|
Pazzirota T, Martin L, Mezcua M, Ferrer C, Fernandez-Alba AR (2013). Processing factor for a selected group of pesticides in a wine-making process: distribution of pesticides during grape processing. Food Additives and Contaminants: Part A 30(10):1752-1760.
Crossref
|
|
|
|
|
Regueiro J, Lopez-Fernandez O, Rial-Otero R, Cancho-Grande B, Simal-Gandara J (2014). A Review on the Fermentation of Foods and the Residues of Pesticides Biotransformation of Pesticides and Effects on Fermentation and Food Quality. Critical Reviews in Food Science and Nutrition 55(6):839-863.
Crossref
|
|
|
|
|
Rodriguez JAC, Fernandez-Gonzalez E, Fernandez-Gonzalez M, Vazquez-Ruiz RA, Aira MJ (2020). Fungal Diseases in Two North-West Spain Vineyards: Relationship with Meteorological Conditions and Predictive Aerobiological Model. Agronomy 10(219):1-19.
Crossref
|
|
|
|
|
Vaquero-Fernandez L, Sanz-Asensio J, Fernandez-Zurbano P, Lopez-Alonso M, Martinez-Soria M (2012). Determination of fungicide pyrimethanil in grapes, must, fermenting must and wine. Journal of the Science of Food and Agriculture 93(8):1960-1966.
Crossref
|
|
|
|
|
Wang X, Telepchak MJ (2013) .Determination of Pesticides in Red Wine by QuEChERS Extraction, Rapid Mini-Cartridge Cleanup and LC-MS-MS Detection. LC-GC Europe 2(26):66-76.
|
|
|
|
|
Weng K, Li ZQ, Liu RQ, Wang L, Wang YJ, Xu Y (2014). Transcriptome of Erysiphe necator-infected Vitis pseudoreticulata leaves provides insight into grapevine resistance to powdery mildew. Horticulture Research 1:1-49.
Crossref
|
|
|
|
|
Wurz DA (2019). Wine and health: A review of its benefits to human health. BIO Web of Conferences 12(04001):1-3.
Crossref
|
|