ABSTRACT
Kilum-Ijim forest is a montane forest in the North West Region of Cameroon. Wild edible mushrooms are mostly consumed by the communities of Kilum-Ijim as substitute of meat to obtain protein, hence the need to evaluate the nutrient and mineral components of the species consumed in these communities. The most eight preferred wild mushroom species from ethnomycological studies are: Polyporus tenuiculus, Termitomyces striatus, Termitomyces macrocarpus, Auricularia polytricha, Laetiporus sulphureus, Termitomyces sp.1, Termitomyces sp.2 and Polyporus dictyopus were identified by ITS gene region. These species were analysed for nutrient and mineral contents using standard protocols. Significant differences in nutrient values were demonstrated among these mushroom species. The study results on dry weight basis range from 43.49 to 64.88 for carbohydrates, 6.60 to 30.69 for crude protein, 7.74 to 14.10 for ash, 2.17-3.22 g for fat and 11.60 to 20.69 g per 100 g for crude fibres with significant differences (P? 0.05) between species for each nutrient. The dry matter content ranged from 12.69-17.77 g per 100 g while the total calorie values ranged from 285.16-319.27 Kcal per 100 g. Mineral nutrient analyses also showed that these mushrooms are rich in both macro and micro nutrients. In conclusion, the study revealed that soil inhabiting mushrooms especially the Termitomyces species have nutritional values which can greatly supplement diets especially in rural communities.
Key words: Cameroon, Kilum-Ijim Forest, Macrofungi, Mineral content, nutritional analysis.
Edible mushrooms are mostly growing in forests in association with woody parts of trees either as parasite, saprophyte or as symbionts in the soil (Chamberlain et al., 1998). Macrofungi have several ecological functions in both natural and agroecosystems, and are widely exploited by humans for food and medicinal purposes (Mueller et al., 2007; Osemwegie et al., 2006; Boa, 2004). Mushrooms represent one of the world’s greatest untapped resources of nutrition (Wani et al., 2010). More than 2,000 species of mushrooms exist in nature; however, less than 25 species are widely accepted as food and only a few have attained the level of an item of commerce (Lindequist et al., 2005). Mushrooms have probably been a part of the human omnivore diet ever since humans have evolved as a species. Actually, it is quite possible that many fungal species developed the highly nutritious sporocarps concurrently with the evolution of omnivores, as a very small number of animal species has been reported to be strictly mycophagous (Witte and Maschwitz, 2008).
Macrofungi play important roles in the lives of many people around the world. They provide two main benefits; they are a source of food, income and also have medicinal properties. The awareness of wild edible fungi and their importance to people are generally poor. Subsistence uses in developing countries have often been ignored. The importance of wild edible fungi to people in developing countries may also have gone unremarked for the simple reason that many of the collections are for personal use (Yorou and Kesel, 2002).
The most cultivated mushroom worldwide is Agaricus bisporus, followed by Lentinula edodes, Pleurotus spp. and Flammulina velutipes (Aida et al., 2009; Chang and Miles, 2004). Newer species or varieties of wild mushrooms like Tricholoma spp. (Spain), Cantharellus spp., Hydnum spp., Lactarius spp., Xerocomus spp., Amanita spp. and Hygrophorus spp. (Greece), Lactarius spp., Tricholoma spp., Leucopaxillus spp., Sarcodon spp. and Agaricus spp. (Portugal), Ramaria spp., Psathyrella spp. and Termitomyces spp. and Agaricus spp., Amanita spp., Boletus spp., Hydnum spp., Hypholoma spp., Lactarius spp., Pleurotus spp., Russula spp. and Tricholoma spp. from various countries have been investigated for their nutritional values and antioxidant activity (Aletor, 1995; Barros et al., 2007; Ouzouni et al., 2007). Despite these advances in mushroom cultivation (Manjunathan and Kaviyarasan, 2011), over 95% of edible mushrooms are still collected from the wild in most African countries.
The people of West African sub-region still rely on wild edible mushrooms for their livelihood especially as a low-cost alternative for animal proteins and flavouring in diets. In addition, they represent a venerable source of subsistent income and incontrovertible raw material in local traditional medicine practice (Osarenkhoe et al., 2014).
In Cameroon, edible and medicinal mushrooms are ubiquitous and constitute a substantial volume of internal trade especially by women in rural areas (Kinge et al., 2014; Teke et al., 2018). Mushrooms have good nutritional value particularly as a source of protein that can enrich human diets, especially in some developing countries where animal protein may not be readily available and are expensive (Heleno et al., 2010). Edible mushrooms have high nutritional values since they are quite rich in protein, vitamins, mineral, fibers and various amino acids (Hyde et al., 2010; Luangharn et al., 2014; Bandara et al., 2015), with an important content of essential amino acids, and low in fat contents. Edible mushrooms also provide a nutritionally significant content of vitamins (B1, B2, B12, C, D and E) and have high antioxidant abilities (Manjunathan and Kaviyarasan, 2011; Mattila et al., 2001), although the total nutrient contents vary significantly among species. Hence, due to their high content of nutritional values, edible wild mushrooms are considered in many parts of tropical Africa as “meat” for the poor communities (Kinge et al., 2014). Based on their chemical composition, mushrooms have also been reported as therapeutic foods, useful in preventing diseases such as hypertension, hypercholesterolemia, and cancer (Shashirekha and Rajarathnam, 2011).
Wild edible mushrooms are one of the important natural resources on which the local people of all nationalities rely heavily, and these mushrooms certainly play a role in improving the food nutrition (Yang, 2002). Most people eat mushrooms, mostly because of its flavour, meaty taste and medicinal value (Grangeia, 2011). Hence, this study set out to determine the nutrient and mineral components of some wild edible soil and wood inhabiting mushrooms in order to assess its nutritional value and enhance their cultivation. Thus the objective of this study was to evaluate and compare the nutrients and minerals from soil and wood inhabiting edible mushroom species which would increase our understanding of their nutritional potential and their possibility for cultivation using different substrates and development of new foods in the food industry.
Study area and sample collection
Fresh fruiting bodies for proximate and nutritional analysis were collected from five community forests in the Kilum-Ijim (Figure 1). Prior to entering into the Kilum-Ijim forest, visitations were made to the various chiefs and administrative authorities within the Kom and Oku districts to sought traditional and administrative permission to use the forest. Five community forests out of 18 were selected based on accessibility after a reconnaissance survey was carried out in the study area.
Nutritional analysis of edible mushrooms
The eight species used for nutritional and mineral analysis were identified using DNA barcoding of the ITS regions using ITS1/ITS 4 primers (Teke et al., 2017). The species were identified as: Termitomyces microcarpus, Laetiporus sulphureus, Auricularia polytricha, Termitomyces striatus, Polyporus tenuiculus, Polyporus dictyporus, Termitomyces sp. 1 and Termitomyces sp. 2. These eight mushroom species had also been identified as edible from an ethnomycological survey (Teke et al., 2018). One Kg each of dried fruiting bodies of the different samples were separately milled to powder using a blender and stored in air tight bottles at 4°C until use. The samples were then analysed for dry moisture content, crude protein, carbohydrates, energy values, total fat, crude fibre, total ash and mineral contents using standard protocols of Association according to Official and Analytical Chemists (AOAC, 2005).
Dry matter content determination
Dry matter content was determined by oven drying method, in which porcelain crucibles were oven-dried at 110± 5°C until a constant weight was attained. The dishes were cooled in a vacuum desiccator for 30 min and weighed (W1). This operation was done repeatedly until a constant weight was attained. 1 g of sample was put into the pre-weighed crucibles. The crucibles were then placed in a pre-heated oven and dried for 16 h at 110°C. The crucibles with the samples were removed and immediately transferred into a vacuum desiccator for 30 min and weighed. The heating/cooling weighing procedure was repeated until a constant weight was attained (W3). The moisture content was calculated using the following equation:
Nutrient contents of edible mushrooms
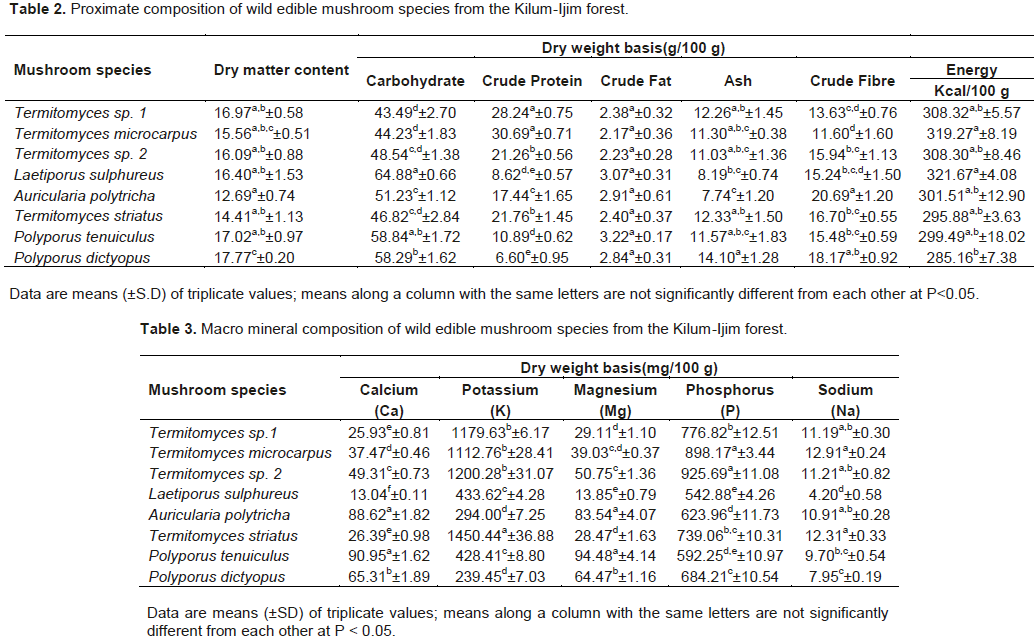
Table 1 and Figure 2 shows the properties and pictures of the eight mushroom species reported as edible from ethnomycological survey which were used in analysing for the proximate and mineral compositions.
Proximate contents of edible mushrooms
The proximate composition and calculated energy value of edible mushroom species from the Kilum-Ijim forest are shown in Table 2. Dry matter content ranged from 17.77% in P. dictyopus to 12.69% in A. polytricha. With the exception of P. dictyopus which showed significant difference in dry matter content of the species studied, no significant differences were observed in the dry matter contents amongst the other species. Crude protein content of studied mushrooms ranged from 6.6 g/100 g in P. dictyopus to 30.69 g/100 g in T. microcarpus. Carbohydrate content ranged from 43.49 g/100 g in Termitomyces sp. to 64.88 g/100 g in L. sulphureus. Crude fat content ranged from 2.17 g/100 g in T. microcarpus to 3.22 g/10 0g in P. tenuiculus. Ash content varied between 7.74 g/100 g in A. polytricha and 14.10 g/100 g in P. dictyopus while crude fibre content ranged from 11.60 g/100 g in T. microcarpus to 18.17g/100g in P. dictyopus. It was observed that the Termitomyces species differed significantly in crude protein content from all the other species. The mean content of crude fat showed no significant difference amongst all the species. However, significant differences were observed amongst species in ash and crude fibre contents. The studied mushroom species proved to be high in energy content ranging from 285.16 Kcal/100 g in P. dictyopus to 321.67 Kcal/100 g in L. sulphureus.
Some macro mineral nutrient contents of edible mushrooms
Macro mineral compositions of the edible mushrooms are presented in Table 3. Macro mineral contents were predominantly high in potassium and phosphorus when compared with Calcium, Magnesium and Sodium.
Phosphorus concentrations ranged from 542.88 mg/100 g in L. sulphureus to 898.17mg/100g in T. microcarpus. Calcium and Magnesium contents ranged from 13.04 mg/100 g and 13.85 mg/100 g in L. sulphureus to 90.95 mg/100 g and 94.48 mg/100 g in P. tenuiculus respectively recording significant differences among the species. However, P. tenuiculus and A. polytricha recorded no significant differences from each other in Calcium and Magnesium contents. Potassium ranged from 239.45 mg/100 g in P. dictyopus to 1450 mg/100 g in T. striatus. Termitomyces species recorded no significant differences from each other in Potassium content, but where significantly different from the other species. Sodium content was very low in all the mushrooms studied ranging from 4.2 mg/100 g in L. sulphureus to 12.91 in T. microcarpus. However, Termitomyces species recorded no significant differences from each other but were significantly different from the other species. Overall, L. sulphureus is very low in macromineral concentrations while T. microcarpus is very rich in macrominerals. Our results also showed that soil-inhabiting macrofungi species (Termitomyces sp.1, T. microcarpus, Termitomyces sp.2 and Termitomyces striatus) showed higher levels of Potassium and Phosphorus than the wood-inhabiting species (L. sulphureus, A.polytricha, P. tenuiculus and P. dictyopus).
Some micromineral nutrient contents of edible mushroom species
The mean values of micro mineral contents of Copper, Iron and Zinc of edible mushrooms are presented in Table 4. Micromineral contents for copper ranged from 0.14 mg/100 g in A.polytricha to 3.90 mg/100 g in Termitomyces microcarpus with significant differences from each other. Iron content ranged from 6.92 mg/100 g in P. dictyopus to 36.01 in Termitomyces sp. 2. Termitomyces sp. 2 recorded a very high iron content with significant difference from the other species analysed. Zinc concentrations ranged from 1.31 mg/100 g in P. dictyopus to 10.80mg/100g in Termitomyces sp 2. It was observed that soil inhabiting fungi were richer in micro minerals than their wood-inhabiting counterparts.
Mushrooms contribute enormously to the supply of nutrients in our diet. They are considered to be good sources of carbohydrates, proteins, fats and minerals. Results from our study revealed that the soil inhabiting mushrooms were higher in nutrient content than their wood inhabiting counterparts. The chemical composition of mushrooms varies depending on the substrate, species of mushroom, harvest time and storage conditions after harvest (Adejumo and Awosanya, 2005; Guillamón et al., 2010). The nutrient contents of the wild mushrooms studied were generally high. This may be due to the fact that the Kilum-Ijim forest is a humid zone. This is similar to the findings of (Colak et al., 2009), who reported that mushrooms from humid zones had high concentration of nutrients due to the high organic matter content of the soil. Different species of wild mushrooms had varied nutrient composition probably due to species or strain differences (Mattila et al., 2001; Mshandete and Cuff, 2007).
Dry matter content ranged from 17.77% in P. dictyopus to 12.69% in A. polytricha. This difference may have probably been caused by fluctuations in environmental factors during growth and storage therefore affecting metabolism (Mattila et al., 2001). Our study revealed that the dry matter contents of the wild mushroom studied were relatively high. Similar results in wild mushrooms have been reported by previous authors in other parts of the world (Sanmee et al., 2003; Saiqa et al., 2008). The protein content of wild mushrooms in this study ranged from 6.6 g/100 g in P. dictyopus to 30.69 g/100 g in T. microcarpus. Protein content of mushrooms may vary according to the genetic structure of species, the physical and chemical differences in the growing medium (Sanmee et al., 2003; Ragunathan and Swaminathan, 2003). Variations in protein contents in mushrooms may also be due to species/strain, stage of development, size of the pileus and the method of analysis (Bernas et al., 2006).
Results obtained from this study revealed that the wild mushrooms studied were found to be rich in proteins but with very low fat contents. This finding is similar to those of Barros et al. (2008) who reported that wild mushrooms were richer sources of protein and had a lower amount of fat than commercial mushrooms. The protein content of P. tenuiculus recorded in this study was 10.89±0.62 g/100 g. This results however differed from that obtained by Nakalembe et al. (2015) who had protein content values for P. tenuiculus species from Uganda ranging from of 11.56% for subhumid species to 16.86% for humid species. Mushroom protein is generally higher than those of green vegetables and oranges (Jonathan, 2002).
Proximate analysis of T. microcarpus revealed carbohydrate content of 44.23±1.83 g/100 g, crude protein content of 30.69±0.71 g/100 g, crude lipid content of 2.17±0.36, ash content of 11.30±0.38 g/100 g and crude fibre content of 11.60±1.60 g/100 g. All these results are closely similar with that of Nabubuya et al. (2010) who studied the nutritional properties of T. microcarpus in Uganda. The values of the polypore mushroom A. polytricha analyzed were compared with those carried out by Usha and Saguna (2014). Our study revealed slight variations for dry matter content, ash and crude fibre contents while high variations were noticed for carbohydrates, protein and fat. Nevertheless, our findings on protein and fat content were similar to those of Asaduzzaman et al. (2009) on their study on nutrient composition of A. polytricha mushroom. Based on ash content, (Varo et al., 1980) reported ash content of edible fungi ranging from 5 g/100 g to 13 g/100 g. Our findings revealed that the ash contents were within this range with the exception of P. dictyopus which had an ash content of 14.10 g/100 g.
Mushrooms are generally considered as low calorie diets. Calculated energy values of edible wild mushrooms studied varied from 285.16 kcal/100 g to 321.67 kcal/100 g on dry matter basis confirming them as low calorie source. These values fall slightly below that of cereals (millet; 341 kcal and maize 349 kcal) (FAO, 1972). Similar studies from different parts of the world have also revealed high energy values in mushrooms ranging from 367.9-450.2 kcal/100 g (32-33). Though P. dictyopus has relatively low crude protein content of 6.6 g/100 g, it is relatively rich in carbohydrate; 58.29 g/100 g; ash 14.1 g/100 g and crude fibre 18.17 g/100 g. It is also a very low source of fat 2.84 g/100 g and energy 285.16 Kcal/100 g. P. dictyopus was highly cherished as meat by the Kilum-Ijim inhabitants due to its taste and tender nature.
The wild mushrooms reported in this study were predominantly rich in potassium and phosphorus compared to the other macro minerals. This is in agreement with studies reported by different authors on mushrooms (Mattila et al., 2001; Colak et al., 2009; Barros et al., 2008; Palazzolo et al., 2012). Potassium is an important electrolyte in the body and is the major cation within cells. It functions in reducing the effect of salt on blood pressure. All the Termitomyces species studied showed high concentrations of mineral nutrients. This is in agreement with (Mattila et al., 2001) who reported that Termitomyces species were generally rich in minerals such as potassium, calcium magnesium and iron. Manzi et al. (1999) reported that calcium levels are not so high in mushrooms. Calcium level in this study, varied from 13.04 mg/100 g to 90.95 mg/100 mg. However, reported literature range for calcium in mushrooms varies from 1.8 mg/100 g to 59.0 mg/100 g (Falandysz et al., 2001). Magnesium levels in this study ranged from 13.85 mg/100 g to 94.48 mg/100 g. These results differ with those of Nakalembe et al. (2015) who reported magnesium values ranging from 7.14-31.9 mg/100 g in some wild mushroom species from Uganga. However, reported literature ranges magnesium contents in mushrooms from 60 mg/100 g to 250 mg/100 g (Bakken and Oslen, 1990). Sodium concentrations were relatively low in this study ranging from 4.2 mg/100 g to 12.91 mg/100 g. This supports previous findings that sodium is relatively less in mushroom species and therefore of great benefit to patients with hypertension (Feldman et al., 1986).
Among the trace elements studied, Fe content was higher (6.92 mg/100 g -36.01 mg/100 g) than other trace elements. Nevertheless, range of reported literature values vary between 1.46 mg/100 g-83.5 mg/100 g (Tuzen, 2003). Copper is the third most abundant trace element in the body and plays a role in protecting the cardiovascular, skeletal and nervous systems. The copper range in our study varied from 0.14 mg/100 g to 3.9 mg/100 g. The recommended daily intake of copper for all age groups is 2 mg/day. However, pregnant and lactating mothers need 1 mg/100 g of copper daily (Food and Nutrition Board, 2001). Copper contents in mushrooms might vary due to the habitat and substrate of the mushrooms. Very low copper contents were reported (Nakalembe et al., 2015). On the contrary, various studies from different parts of the world have reported high copper contents in mushrooms (Colak et al., 2009; Nabubuya et al., 2010). Zinc content in this study varied from 1.3 mg/100 g to 10.8 mg/100 g. Zinc is an important element in cellular metabolism involving cell division, wound healing and protein synthesis (Heyneman, 1996). The recommended daily intake of zinc is 15 mg/day (Food and Nutrition Board, 2001). Reported literature range of Zinc contents in mushrooms is between 2.98 and 15.8 mg (Islolu et al., 2001). Nevertheless, (Nakalembe et al., 2015) reported zinc content values of studied mushrooms in Uganda as low as 0.56 to 1.1 mg/100 g.
From the results obtained, it can be seen that all the mushroom species can be used as nutrient sources to upgrade the diet of the communities. These high nutritional qualities and unique flavors of the studied mushrooms are likely to be poorly known and to be lost if they are not documented, so it is imperative that a nutritional database of these mushrooms is set up to collect and improve the characteristics of these unique species and for their eventual domestication.
The authors have not declared any conflict of interests.
The authors gratefully appreciate the Rufford Small Grant award and the BecA-ILRI Hub through the Africa Biosciences Challenge Fund (ABCF) program towards the realization of this work. The ABCF Program is funded by the Australian Department for Foreign Affairs and Trade (DFAT) through the BecA-CSIRO partnership; the Syngenta Foundation for Sustainable Agriculture (SFSA); the Bill and Melinda Gates Foundation (BMGF); the UK Department for International Development (DFID) and; the Swedish International Development Cooperation Agency (SIDA).
REFERENCES
|
Adejumo TO, Awosanya OB (2005). Proximate and mineral composition of four edible mushroom species from South Western Nigeria. African Journal of Biotechnology 4(10):1084-1088.
|
|
|
|
Aida FMN, Shuhaimi M, Yazid M, Maaruf AG (2009). Mushroom as a potential source of prebiotics: A review. Trends in Food Science and Technology 20(11-12):567-575.
Crossref
|
|
|
|
|
Aletor V (1995). Compositional studies on edible tropical species of mushrooms. Food Chemistry 54(3):265-268.
Crossref
|
|
|
|
|
Asaduzzaman K, Liakot AK, Shahdat H, Mousumi T, Nazin U (2009). Investigation on the nutritional composition of common edible and medicinal mushrooms cultivated in Bangladesh. Bangladesh Journal of Mushrooms 3(1):21-28.
|
|
|
|
|
ASEAN Manual of Food Analysis (2011). Regional Centre of ASEAN Network of Food Data System.Institute of Nutrition, Mahidol University Thailand 188 p.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (2005). Official Methods of Analysis of the Association of Official Analytical Chemists International, 17th ed., Arlington U.S.A. Official Methods 945.16.
|
|
|
|
|
Bakken LR, Olsen RA (1990). Accumulation of radiocaesium in fungi. Canadian Journal of Microbiology 36(10):704-710.
Crossref
|
|
|
|
|
Bandara AR, Rapiord S, Bhat DJ, Kakumyana P, Chamyuang S, Jianchu XU, Kevin DH (2015). Polyporus umbellatus, an edible-medicinal cultivated mushroom with multiple developed health-care products as food, medicine and cosmetics: a review. Cryptogamie Mycologie 36(1):3-42.
Crossref
|
|
|
|
|
Barros L, Baptista P, Estevinho ML, Ferreira CF (2007). Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. Journal of Agricultural and Food Chemistry 55(21):8766-8771.
Crossref
|
|
|
|
|
Barros T, Cruz P, Baptista L, Estevinho M, Ferreira IC (2008). Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food and Chemical Toxicology 46(8):2742-2747.
Crossref
|
|
|
|
|
Bernas E, Jawarska G, Lisiewska Z (2006). Edible mushrooms as a source of valuable nutritive constituents. Acta Scientiarum Polonorum Technologia Alimentaria 5(1):5-20.
|
|
|
|
|
Boa ER (2004). Wild Edible Fungi: A Global Overview of their Use and Importance to People; Non Wood Forest Products 17. FAO Publishing Management Services, Rome 157 p.
|
|
|
|
|
Chamberlain J, Bush R, Hammett A (1998). Non-timber forest products: The other forest products. Forest Products Journal 48(10):10-19.
|
|
|
|
|
Chang ST, Miles PG (2004). Pleurotus - A Mushroom of Broad Adaptability. In: Mushrooms: Cultivation, Nutritional Value, Medicinal effect, and Environmental Impact (2nd Ed.). CRC Press pp. 315-325.
Crossref
|
|
|
|
|
Colak Y, Kolcuoglu E, Sesli O, Dalman (2009). Biochemical composition of some Turkish fungi. Asian Journal of Chemistry 19(3):2193-2199.
|
|
|
|
|
Falandysz JK, Szymczyk H, Ichihashi (2001). ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Additives and Contaminants 18(6):503-513.
Crossref
|
|
|
|
|
Feldman R, Lawton WJ, McArdie WL (1986). Low sodium diet corrects the defect in lymphocyte-adrenergic responsiveness in hypertensive subjects. Journal of Clinical Investigation 78:166.
|
|
|
|
|
Food and Agriculture Organization (1972). Food composition Table for use in East Asia .Food Policy and Nutrition Division FAO Rome.
|
|
|
|
|
Food and Agriculture Organization (2003). Food and Nutrition paper, 77, ISSN 0254-4725. Food energy-methods of analysis and conversion factor, Report of the technical workshop, Rome.
|
|
|
|
|
Food and Nutrition Board (2001). Dietary reference intakes for Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Institute of Medicine, National Academies Press, Washington, DC.
|
|
|
|
|
Grangeia C, Heleno SA, Barros L, Martins A, Ferreira IC (2011). Effects of tropism on nutritional and nutraceutical potential of wild edible mushrooms. Food Research International 44(4):1029-1035.
Crossref
|
|
|
|
|
Guillamón E, García-Lafuente A, Lozano M, D'Arrigo M, Rostagno MA, Villares A, Martínez JA (2010). Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 81(7):715-723.
Crossref
|
|
|
|
|
Heleno SA, Barros L, Sousa MJ, Martins A, Ferreira IC (2010). Tocopherols Composition of Portuguese wild mushrooms with antioxidant capacity. Food Chemistry 119(4):1443-1450.
Crossref
|
|
|
|
|
Heyneman CA (1996). Zinc deficiency and taste disorders. The Annals of Pharmacotherapy 30(2):186-187.
Crossref
|
|
|
|
|
Hyde KD, Bahkali AH, Moslem MA (2010). Fungi an unusual source for cosmetics. Fungal Diversity 43(1):1-9.
Crossref
|
|
|
|
|
Islolu M, Ylmaz F, Merdivan M (2001). Concentration of trace elements in mushrooms. Food Chemistry 73(2):169-175.
Crossref
|
|
|
|
|
Jonathan SG (2002). Vegetable growth requirements and antimicrobial activities of some higher fungi in Nigeria Ph.D. thesis, University of Ibadan.
|
|
|
|
|
Kinge TR, Nji TM, Ndam LM, Mih AM (2014). Mushroom research, production and marketing in Cameroon: A review. Issues in Biological Sciences and Pharmaceutical Research 2(7):069-074.
|
|
|
|
|
Lindequist U, Niedermeyer T, Jülich W (2005). The pharmacological potential of mushrooms. Evidence-Based Complementary and Alternative Medicine 2(3):285-299.
Crossref
|
|
|
|
|
Luangharn T, Hyde KD, Chukeatirote E (2014). Proximate analysis and mineral content of Laetiporus sulphureus strain MFLUCC 12-0546 from northern Thailand. Journal of Science 41(4):765-770.
|
|
|
|
|
Manjunathan J, Kaviyarasan V (2011). Nutrient composition in wild and cultivated edible mushroom, Lentinus tuberregium (Fr.) Tamil Nadu. India. International Food Research Journal 18(2):809-811.
|
|
|
|
|
Manzi P, Gambelli L, Marconi S, Vivanti V, Pizzoferrato L (1999). Nutrients in edible mushrooms: An interspecies comparative study. Food Chemistry 65(4):477-482.
Crossref
|
|
|
|
|
Mattila P, Könkö K, Eurola M, Pihlava JM, Astola J, Vahteristo L, Hietaniemi V, Kumpulainen, J, Valtonen M Piironen V (2001). Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. Journal of Agricultural and Food Chemistry 49(5):2343-2348.
Crossref
|
|
|
|
|
Mshandete MA, Cuff J (2007). Proximate and nutrient composition of three types of indigenous edible wild mushrooms grown in Tanzania and their utilization prospects. African Journal of Food, Agriculture, Nutrition and Development 7(6):230-238.
|
|
|
|
|
Mueller GM, Schmit, JP, Leacock PR, Buyck B, Cifuentes DJ, Kurt H, Teresa I, Karl-Henrik LD, Jean L, Tom WM, David M, Mario R, Scott AR, Leif R, James MT, Roy W, Qiuxin W (2007). Global diversity and distribution of macrofungi. Biodiversity and Conservation 16(1):37-48.
Crossref
|
|
|
|
|
Nabubuya JH, Muyonga JD, Kabasa (2010). Nutritional and hypocholesterolemic properties of Termitomyces microcarpus mushrooms. African Journal of Food, Agriculture, Nutrition and Development 10(3):2236-2257.
Crossref
|
|
|
|
|
Nakalembe JD, Kabasa D, Olila (2015). Comparative nutrient composition of selected wild edible mushrooms from two agro-ecological zones, Uganda. Springer Plus 4:433.
Crossref
|
|
|
|
|
Osarenkhoe O, John O, Theophilus D (2014). Ethno mycological Conspectus of West African Mushrooms: An Awareness Document. Advances in Microbiology 4(1):9-54.
Crossref
|
|
|
|
|
Osemwegie OO, Eriyaremu EG, Abdulmalik J (2006). A survey of macrofungi in Edo/Delta region of Nigeria, their morphology and uses. Global Journal of Pure Applied Sciences 12(2):149-157.
Crossref
|
|
|
|
|
Ouzouni PK, Veltsistas PG, Paleologos EK, Riganakos KA (2007). Determination of metal content in wild edible mushroom species from regions of Greece. Journal of Food Composition and Analysis 20(6):480-486.
Crossref
|
|
|
|
|
Palazzolo E, Gargano ML, Venturella G (2012). The nutritional composition of selected wild edible mushrooms from Sicily (southern Italy). International Journal of Food Sciences and Nutrition 63(1):79-83.
Crossref
|
|
|
|
|
Ragunathan R, Swaminathan K (2003). Nutritional status of Pleurotus spp. grown on various agro-wastes. Food Chemistry 80(3):371-375.
Crossref
|
|
|
|
|
Saiqa S, Haq NB, Muhammad AH (2008). Studies on chemical composition and Nutritive evaluation of wild edible mushrooms. Iranian Journal of Chemistry and Chemical Engineering 27(3):151-154.
|
|
|
|
|
Sanmee R, Dell B, Lumyong P, Izumori K, Lumyong S (2003). Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chemistry 82(4):527-532.
Crossref
|
|
|
|
|
Shashirekha MN, Rajarathnam S (2011). Mushroom nutraceuticals. In: Rajarathnam S, Ramteke RS Eds., Advances in preservation and processing technologies of fruits and vegetables, New India Publishing Agency, New Delhi, India pp. 605-656.
|
|
|
|
|
Teke NA, Kinge TR, Bechem E, Mih AM, Kyalo M, Stomeo F (2017). Macro-fungal diversity in the Kilum-Ijim forest, Cameroon. Studies in Fungi 2(1):47-58.
Crossref
|
|
|
|
|
Teke NA, Kinge TR, Bechem E, Nji TM, Ndam LM, Mih AM (2018). Ethnomycological study in the Kilum-Ijim mountain forest, Northwest Region, Cameroon. Journal of Ethnobiology and Ethnomedicine 14(25):1-12.
Crossref
|
|
|
|
|
Tuzen M (2003). Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchemical Journal 74(3):289-297.
Crossref
|
|
|
|
|
Usha S, Saguna V (2014). Investigation on the nutritional value of edible mushrooms viz., Auricularia polytricha and Pleurotus ostreatus. Asian Journal of Science and Technology 5(8):497-500.
|
|
|
|
|
Varo P, Lähelmä O, Nuurtamo M, Saari E, Koivistoinem P (1980). Mineral element composition of Finished Food. Acta Agriculturae Scandinavica 22:89-113.
|
|
|
|
|
Wani BA, Bodha RH, Wani AH (2010). Nutritional and medicinal importance of mushrooms. Journal of Medicinal Plants Research 4(24):2598-2604.
Crossref
|
|
|
|
|
Witte V, Maschwitz U (2008). Mushroom harvesting ants in the tropical rain forest. Naturwissenschaften 95(11):1049-1054.
Crossref
|
|
|
|
|
Yang ZL (2002). On wild mushroom resources and their utilization in Yunnan province, Southwestern China. Journal of Natural Resources 17(4):464-469.
|
|
|
|
|
Yorou SN, De Kesel A (2002). Connaissances ethnomycologiques des peuples Nagot du centre du Bénin (Afrique de l'Ouest). Proceedings of the XVIth AETFAT Congress, Brussels 2000. Systematics and Geography of Plants 71:627-637.
Crossref
|
|